Abstract
Purpose
Management of empyema has been debated in the literature for decades. Although both primary video-assisted thoracoscopic surgery (VATS) and tube thoracostomy with pleural instillation of fibrinolytics have been shown to result in early resolution when compared to tube thoracostomy alone, there is a lack of comparative data between these modes of management. Therefore, we conducted a prospective, randomized trial comparing VATS to fibrinolytic therapy in children with empyema.
Methods
After Institutional Review Board approval, children defined as having empyema by either loculation on imaging or more than 10,000 white blood cells/μL were treated with VATS or fibrinolysis. Based on our retrospective data using length of postoperative hospitalization as the primary end point, a sample size of 36 patients was calculated for an α of .5 and a power of 0.8. Fibrinolysis consisted of inserting a 12F chest tube followed by infusion of 4 mg tissue plasminogen activator mixed with 40 mL of normal saline at the time of tube placement followed by 2 subsequent doses 24 hours apart.
Results
At diagnosis, there were no differences between groups in age, weight, degree of oxygen support, white blood cell count, or days of symptoms. The outcome data showed no difference in days of hospitalization after intervention, days of oxygen requirement, days until afebrile, or analgesic requirements. Video-assisted thoracoscopic surgery was associated with significantly higher charges. Three patients (16.6%) in the fibrinolysis group subsequently required VATS for definitive therapy. Two patients in the VATS group required ventilator support after therapy, one of whom required temporary dialysis. No patient in the fibrinolysis group clinically worsened after initiation of therapy.
Conclusions
There are no therapeutic or recovery advantages between VATS and fibrinolysis for the treatment of empyema; however, VATS resulted in significantly greater charges. Fibrinolysis may pose less risk of acute clinical deterioration and should be the first-line therapy for children with empyema.
Keywords: Fibrinolysis, VATS, Empyema, Children
Empyema, the purulent pleural manifestation of complicated pneumonia, is a difficult clinical problem with an often protracted course. The traditional treatment of pleural drainage via chest tube has been associated with a high failure rate because of the inability to drain loculated areas. These patients subsequently required mechanical debridement of all solid components, which is a simple and effective solution, albeit with the morbidity of a thoracic operation. The minimally invasive video-assisted thoracoscopic surgery (VATS), with low operative morbidity, has become the gold standard for the operative management for fibropurulent pleural space disease [1-6].
Given that fibrin deposition is the backbone of the matrix upon which solid septations develop in an empyema, infusion of fibrinolytic solution has been shown to be effective in promoting resolution of disease in multiple studies [8-23].
Video-assisted thoracoscopic surgery has been clearly shown to result in earlier and more complete resolution of empyema than chest tube drainage alone [6-8]. Fibrinolysis has been shown to be superior to chest tube drainage alone, by both direct comparison and when used in patients who failed chest drainage only [9,11-15,18-20,23]. The delineation of whether VATS or fibrinolysis should be the initial treatment in patients with empyema requires a prospective, randomized comparison. Therefore, we initiated this trial in children diagnosed with empyema.
1. Methods
Approval was obtained from the Children’s Mercy Hospital (Kansas City, Mo) Institutional Review Board (IRB) before enrolling patients in this study (IRB no. 06 01-019). Patients were subsequently enrolled only after obtaining consent from the patient’s legal guardian. The consent forms and consent process were carefully evaluated by the IRB on a continual basis. The trial was registered with clinicaltrials.gov (ID: NCT00323531). All persons obtaining consent participated in specific IRB training in the consent process.
1.1. Participants
The study population consisted of patients younger than 18 years with a diagnosis of empyema.
Inclusion criteria required the diagnosis of empyema. Empyema was defined as septations or loculations identified in the pleural space by computed tomography or ultrasound. In addition, patients with a frankly purulent tap with white blood cell count greater than 10,000 cells/μL were included.
Exclusion criteria were an existing contraindication to either thoracoscopy or to the fibrinolytic agent. In addition, patients with additional foci of infection or a diagnosis of an immunocompromised state were excluded. Patients with comorbid conditions that would require hospitalization beyond the course of the empyema were excluded.
1.2. Interventions
After diagnosis, patients were randomized to treatment by either video-assisted thoracoscopic debridement or pleural infusion of fibrinolytic solution. The fibrinolytic agent was Activase (alteplase; Genentech Inc, San Francisco, Calif), which is a recombinant version of human tissue plasminogen activator (tPA).
1.3. Sample size
Power calculation was based on length of hospitalization obtained from retrospective data within our institution between patients treated with fibrinolysis and those treated with thoracoscopy. Establishing α at .05 and a power of 0.80, this produced a sample size of 18 patients per arm.
1.4. Assignment
The randomization sequence was computer generated using an individual unit of randomization in an unstratified sequence in blocks of 4. After empyema was diagnosed as described above, the family was approached for informed permission for the patient to participate by one of the named authors of this study. The randomization sequence was accessed to identify the next allotment after the consent form was signed.
1.5. Protocol
Thoracoscopic debridement was performed by 1 of 5 institutional staff surgeons with thoracoscopic experience as dictated by the call schedule. The number and size of ports used were dictated by the patient size and difficulty of debridement. In general, 3 ports were used using one 10-mm port and two 5-mm ports. The initial fluid and/or pieces of the solid debris were sent for culture. In all cases, a single soft suction drain in the form of a 19F round Blake drain (Ethicon Inc, Piscataway, NJ) was left to drain the pleural space.
Patients treated with fibrinolysis had a 12F chest tube placed using the Seldinger technique (Thal-Quick Chest Tubes, Cook Critical Care, Bloomington, Ind). The small size of chest tubes was chosen on the basis that larger drains have been shown to confer no advantage in empyema treatment when fibrinolysis is used [23]. Tubes were placed using local anesthetic with sedation either at the bedside, in a dedicated procedure room, or in the operating room depending on the age of the patient, physiologic status, time of day, and staff available for sedation. The initial drainage fluid was sent for culture, and the tubes were then placed on continuous suction at a pressure of −20 cm H20. Fibrinolysis was performed by mixing 4 mg of tPA into 40 mL of sterile normal saline on the floor by the administering physician. This solution was injected directly into the tube with a dwell time of 1 hour after which the tube was placed back to continuous suction. The tPA solution was administered at the time of tube placement with 2 additional doses each given 24 hours later to comprise 3 treatments for a 48-hour span.
Tubes in both arms were managed by the surgical service, and they were removed when there was no sign of air leakage and drainage output was less than 1 mL/kg per day calculated for the previous 12 hours.
After intervention, discharge criteria consisted of the patient remaining afebrile for 48 hours, tolerating regular diet, and off oxygen therapy. Patients were discharged to home with a minimum 10-day course of oral antibiotics that offered comparable antimicrobial coverage as the intravenous regimen at the time of discharge.
The general antimicrobial plan for both groups consisted of clindamycin (10 mg/kg per dose) every 6 hours and ceftriaxone (25 mg/kg per dose) every 12 hours. If hemodynamic instability existed (hypotension, need for vasoactive medications, or persistent tachycardia), then vancomycin (15 mg/kg per dose) every 6 hours was added. Antibiotic therapy was tailored toward positive culture results and the individual patient’s course. All patients were observed by the infectious disease team who directed outpatient therapy and follow-up.
1.6. Data collection
Upon diagnosis, age, weight, degree of oxygen support, white blood cell count, days of symptoms before diagnosis, and number of doctor visits before intervention were recorded. Results from pleural and blood cultures were recorded.
After the intervention, length of hospitalization, days and degree of oxygen therapy, days until afebrile, analgesic doses, requirements for a second procedure, and patient charges were recorded. All complications, readmissions, and recurrences were recorded. Procedure charges were compiled from procedures used for the treatment of the empyema only. This includes both fibrinolysis and the subsequent operation for the fibrinolysis patients who did not respond. General hospital charges including intensive care support measures were not included in the procedure charges.
1.7. Statistical analysis
Continuous variables were compared using an independent sample, 2-tailed Student’s t test. Discrete variables were analyzed with Fisher’s Exact test. Significance was defined as P value of .05 or less. Descriptive statistics are listed at mean ± SD.
2. Results
From March 2006 to November 2007, 36 patients were enrolled in the study.
2.1. Patient characteristics
At diagnosis, there were no differences between groups in age, weight, degree of oxygen support, white blood cell count, days of symptoms, or number of physician visits. Complete data at diagnosis are expressed in Table 1.
Table 1.
Patient characteristics at diagnosis
| VATS (n = 18) |
Fibrinolysis (n = 18) |
P | |
|---|---|---|---|
| Age (y) | 4.8 ± 4.3 | 5.2 ± 4.2 | .77 |
| Weight (kg) | 24.6 ± 22.6 | 20.7 ± 11.8 | .52 |
| White blood cell count | 20.8 ± 10.6 | 19.7 ± 8.6 | .71 |
| Oxygen support (L/min) | 0.81 ± 0.93 | 0.79 ± 0.93 | .96 |
| Days of symptoms | 9.0 ± 2.9 | 10.6 ± 6.2 | .32 |
| No. of physician visits | 2.9 ± 1.9 | 2.7 ± 1.3 | .69 |
A component of pulmonary abscess existed in 2 patients, both of which were in the fibrinolysis group.
2.2. Patient microbiology
Pleural culture was positive in 6 (33%) of the fibrinolysis group and 3 (17%) of the VATS group. Organisms identified in the fibrinolysis group included 3 patients with Staphylococcus aureus, 2 patients with Streptococcus pneumoniae, and 1 with Streptococcus viridans. Of the 3 patients with Staphylococcus aureus, 2 of them had methicillin-resistant strains. Organisms in the VATS group included Streptococcus pneumoniae in 2 patients and Streptococcus angiosus in 1 patient. Blood culture was positive in 3 (17%) patients from the VATS group and 2 patients (11%) from the fibrinolysis group, all of which grew Streptococcus pneumoniae. Only one patient had positive blood and pleural cultures—that patient was in the fibrinolysis arm with Streptococcus pneumoniae as the identified organism from both sources.
2.3. Outcome
There was no difference in length of stay after intervention, days until afebrile, days of oxygen therapy, or number of doses of analgesics. There were significantly higher procedure charges in the VATS group. Outcome data are displayed in Table 2. Three patients (16.6%) in the fibrinolysis subsequently required VATS for definitive therapy. Two patients in the VATS group required ventilator support after therapy, one of whom continued to have progressive sepsis resulting in transient renal failure requiring temporary dialysis. No patients in the fibrinolysis group clinically worsened after initiation of therapy. No patients in either group were readmitted after discharge for ongoing or recurrent pulmonary disease.
Table 2.
Clinical outcomes
| VATS (n = 18) |
Fibrinolysis (n = 18) |
P | |
|---|---|---|---|
| Length of posttherapy hospitalization (d) |
6.9 ± 3.7 | 6.8 ± 2.9 | .96 |
| Posttherapy days of O2 support |
2.3 ± 1.7 | 2.3 ± 2.1 | .90 |
| Days to afebrile after intervention |
3.1 ± 2.7 | 3.8 ± 2.9 | .46 |
| Analgesia doses | 22.3 ± 28.5 | 21.4 ± 12.0 | .90 |
| Hospital charges | $11.7K ± $2.9K | $7.6K ± $5.4K | .02 |
3. Discussion
At the time this study was developed and initiated, there were no published prospective trials comparing intrapleural fibrinolysis to thoracoscopic debridement for empyema. There were an abundance of data on the use of fibrinolysis in children to facilitate earlier resolution of empyema [8-23].A review on the therapeutic options for empyema in children was published shortly before we began this study that concluded with the statement that a prospective, randomized trial comparing VATS to intrapleural fibrinolytic therapy was needed [24].
The development of this study was borne out the fact that referring and treating physicians typically held 1 of 2 opposing mind frames. Proponents for VATS were concerned about fibrinolytic therapy because of discomfort with instillation, prolonged therapy before resolution, and failure of loculation dissolution. As evident from the data generated by this trial, there was no difference in discomfort, no difference in time to defervescence, or time to discharge after treatment. The failure rate was not only modest but very similar to previous studies investigating the use of fibrinolysis [19,24,25]. On the other hand, proponents of fibrinolysis felt most patients could be easily treated without the resource use and morbidity of a formal thoracic operation. Indeed, more resources were mobilized in the delivery of VATS without obvious therapeutic advantages. The fact that 2 patients became significantly more ill after VATS is not a point that we are willing to disregard as a statistically insignificant finding as there is physiologic rationale for this finding. Patients are taken to the operating room under general anesthesia during the acute febrile stage of their illness arising from an affected lung. The unaffected lung is then selectively ventilated with high pressure, whereas the affected lung is physically manipulated and debrided. This results in an expected increase in parenchymal or intraalveolar fluid on the affected side, whereas there is some degree of barotrauma to the unaffected lung. The debridement may facilitate release of inflammatory mediators as the picture of sepsis acutely worsened in a few of the patients who underwent VATS. Furthermore, the 3 patients who subsequently required VATS after fibrinolysis had no difficulty with their operative course, augmenting the argument for primary fibrinolysis in all patients.
During the conduction of this study, another trial comparing intrapleural fibrinolysis to VATS was published [26]. That trial was conducted in London with a nearly identical study design with the exception that the authors used urokinase as the fibrinolytic agent in contrast to our use of tPA. Their study concluded no therapeutic differences between intrapleural fibrinolysis with urokinase and VATS. The only difference detected was the higher procedure cost with VATS. Our study therefore represents the second prospective trial comparing fibrinolysis to VATS and the first to use tPA as the fibrinolytic substance. As our study matured, we were not surprised to see that our results and conclusions were effectively the same as the urokinase study done in London given that there were no major methodology discrepancies between the protocols. However, comparison of data was striking in that our numbers were eerily similar to those from the other study in nearly every common variable. The failure rate for fibrinolysis in their study was 16%, the same rate we found.
Levels of evidence are graded from the lowest grade of 5 to the highest grade of 1. The strongest level 1 evidence is provided when more than one prospective, randomized trial produces congruent results. Therefore, with 2 prospective trials now demonstrating extraordinary congruence, we feel confident and safe in recommending the therapeutic approach outlined in Fig. 1.
Fig. 1.
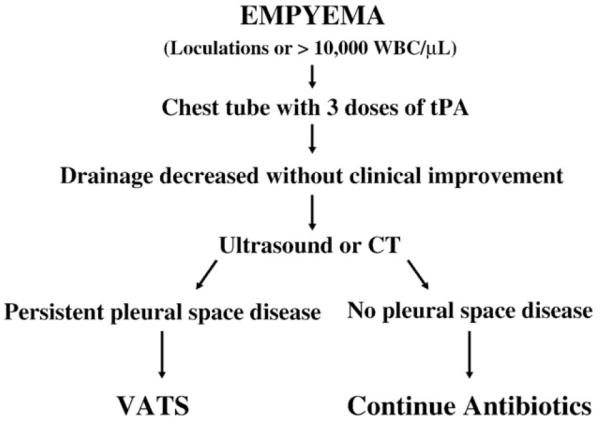
Treatment algorithm for empyema in children dictated by level 1 evidence.
It would be ideal to identify predictors of failure for patients treated with fibrinolysis. That is not feasible from our data with only 3 patients who failed fibrinolysis. Furthermore, there were no identifiable distinguishing features from those 3 patients. Only 1 of the 3 patients who failed fibrinolysis had positive pleural cultures (Streptococcus pneumoniae). Of note, the 2 patients with methicillin-resistant Staphylococcus aureus–positive pleural cultures responded well to fibrinolysis as did the 2 patients with intraparenchymal abscesses. Although these small numbers do not support bold statements, they do suggest one should not expect failure of fibrinolysis on the basis of radiographic or microbiological data. Identification of predictors will require a much larger population of responses and failures before statistically important findings will emerge. As more institutions use the treatment algorithm in Fig. 1, uncovering predictors of failure may be possible.
This study was not designed as an equivalence trial but as a definitive trial based on data obtained in our hospital from patients treated with both methods. The difference at the time of study conduction (7 days of hospitalization after therapy for fibrinolysis vs 5 days for VATS) was substantial and consistent, which is why the sample size generated was not large. The prospective trial comparing urokinase to VATS was designed using the same parameter from their retrospective data. That both studies subsequently showed no difference in length of hospitalization when all patients were managed under the same protocol nicely depicts the importance of prospective data in settling controversial management issues. The provider effect, which cannot be separated out in retrospective studies, is the most likely explanation for the outcome equivalence seen in both institutions despite a clear outcome difference in the retrospective data. At the point that our prospective trial recruited the calculated sample size, the primary outcome variable (length of hospitalization after therapy) was truly the same between groups (P = .96). Therefore, there was no justification for further enrollment with a difference too small to ever be clinically relevant and with the results otherwise showing complete congruence with another prospective, randomized trial.
Discussion.
Dr Philip Guzzetta (Washington, DC): First of all, I would like to commend you for doing this prospective study. It is very nicely done and I think does answer one aspect of the question. A problem I have with this is that those of us who lived pre-VATS and then currently post-VATS recognize that doing this procedure under the ideal circumstances in the OR, controlled environment, perfect situation, never a question of sedation, and doing it once, I think outweighs the financial benefits. You mentioned that the chest tubes were placed on sedation. Frequently that is done in our experience in the emergency department, and I would like to know when you talk about sedation, how that was done, and in addition, when you do the taps you also do that under sedation. The advantage to me is that the VATS is a one-time intervention in a pain-free situation and that is never the situation for me anyway when you do it with a chest tube.
Dr St Peter (response): That is a good point and that is why we measured analgesic doses. There was no difference in analgesic doses between the two. They literally got the exact same number of doses. Even though it is not painful to do VATS during the procedure, it of course is painful afterwards when they have incisions and a chest tube in place. Coming into the study, I fully expected that the VATS would have less pain because you are not instilling fibrinolysis each day, but we were surprised to find out that was not the case.
As far as where the tubes are placed, often they would have come in and had a pleural tap done by interventional radiology before there was a surgical consultation when they find loculations or the tap is positive. The sedation is typically Versed (Roche Laboratories, Burlington, NC) and fentanyl. When we were consulted and the patient did not have a tube already in place, we placed Thal-quick chest tubes on the floor with a sedation nurse, also Versed and fentanyl. When you place these tubes, where you put in the needle, the wire, the dilator, and then the tube, it is remarkable how quick and painless it is because really you just need enough sedation to inject a local anesthetic and then from there you are home free. I have done this in patients on the floor with no sedation and just local when they are old enough to understand what is going on. The problems that we did run into are when you want to get a sedation nurse to come to the bedside. They may say the patient is too tachypneic or they do not want to come in on a weekend, so we would transfer the patient down to the pediatric intensive care unit so that we could do the sedation ourselves.
Dr Donna Caniano (Columbus, Ohio): Shawn, I alsocongratulate you on a wonderful study. My question relates to the timing to determine treatment failure by fibrinolysis. How long do you wait? In the patients who then come to surgery, are the procedures longer because they have had fibrinolytic therapy? Do they have higher blood loss, and what is their length of stay?
Dr St Peter (response): Good points, both. In the patients that fail fibrinolysis, we really did not see that VATS was substantially more difficult. Their numbers are included in those, so the length of stay being equal between groups included the patients that failed.
The failure is difficult to define. The Great Ormond Street study used 4 days of a fever over 38, and we felt that was a little too strict because it is a clinically ambiguous situation. What we do, as that last algorithm shows, is once the drainage has decreased to where you are effectively meeting chest tube removal criteria, then that tube drainage with the fibrinolysis has done all it can do. You are ready to take out the tube, and so if there is still persistent disease in the pleural space that you have not treated with the fibrinolysis, then the patient should go to VATS if they are still symptomatic. In the patient that has clinical resolution, then the tube comes out and there is nothing to worry about. We need evidence of ongoing pleural space disease with a chest tube that is no longer draining.
Footnotes
Presented at the 39th annual meeting of the American Pediatric Surgical Association, Phoenix, AZ, May 27-June 1, 2008.
References
- [1].Wurnig PN, Wittmer V, Pridun NS, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg. 2006;81:309–13. doi: 10.1016/j.athoracsur.2005.06.065. [DOI] [PubMed] [Google Scholar]
- [2].Hope WW, Bolton WD, Stephenson JE. The utility and timing of surgical intervention for parapneumonic empyema in the era of video-assisted thoracoscopy. Am Surg. 2005;71:512–4. [PubMed] [Google Scholar]
- [3].Olgac G, Fazlioglu M, Kutlu CA. VATS decortication in patients with stage 3 empyema. Thorac Cardiovasc Surg. 2005;53:318–20. doi: 10.1055/s-2005-865604. [DOI] [PubMed] [Google Scholar]
- [4].Cheng G, Vintch JR. A retrospective analysis of the management of parapneumonic empyemas in a county teaching facility from 1992 to 2004. Chest. 2005;128:3284–90. doi: 10.1378/chest.128.5.3284. [DOI] [PubMed] [Google Scholar]
- [5].Tsao K, St Peter SD, Sharp SW, et al. Current application of thoracoscopy in children. J Laparoendosc Adv Surg Tech A. 2008;18:131–5. doi: 10.1089/lap.2007.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coote N, Kay E, Coote N. Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2005:CD001956. doi: 10.1002/14651858.CD001956.pub2. [DOI] [PubMed] [Google Scholar]
- [7].Kurt BA, Winterhalter KM, Connors RH, et al. Therapy of parapneumonic effusions in children: video assisted thoracoscopic surgery vrsus conventional thoracostomy drainage. Pediatrics. 2006;118:e547–53. doi: 10.1542/peds.2005-2719. [DOI] [PubMed] [Google Scholar]
- [8].Gates RL, Hogan M, Weinstein S, et al. Drainage, fibrinolytics, or surgery: a comparison of treatment options in pediatric empyema. J Pediatr Surg. 2004;39:1638–42. doi: 10.1016/j.jpedsurg.2004.07.015. [DOI] [PubMed] [Google Scholar]
- [9].Yao CT, Wu JM, Liu CC, et al. Treatment of complicated parapneumonic pleural effusion with intrapleural streptokinase in children. Chest. 2004;125:566–71. doi: 10.1378/chest.125.2.566. [DOI] [PubMed] [Google Scholar]
- [10].Ekingen G, Guvenc BH, Sozubir S, et al. Fibrinolytic treatment of complicated pediatric thoracic empyemas with intrapleural streptokinase. Eur J Cardiothorac Surg. 2004;26:503–7. doi: 10.1016/j.ejcts.2004.05.032. [DOI] [PubMed] [Google Scholar]
- [11].Misthos P, Sepsas E, Konstantinou M, et al. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. Eur J Cardiothorac Surg. 2005;28:599–603. doi: 10.1016/j.ejcts.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [12].Krishnan S, Amin N, Dozor AJ, et al. Urokinase in the management of complicated parapneumonic effusions in children. Chest. 1997;112:1579–83. doi: 10.1378/chest.112.6.1579. [DOI] [PubMed] [Google Scholar]
- [13].Kornecki A, Sivan Y. Treatment of loculated pleural effusion with intrapleural urokinase in children. J Pediatr Surg. 1997;32:1473–5. doi: 10.1016/s0022-3468(97)90566-2. [DOI] [PubMed] [Google Scholar]
- [14].Barbato A, Panizzolo C, Monciotti C, et al. Use of urokinase in childhood pleural empyema. Pediatr Pulmonol. 2003;35:50–5. doi: 10.1002/ppul.10212. [DOI] [PubMed] [Google Scholar]
- [15].Kiliξ̧ N, Celebi S, Gürpinar A, et al. Management of thoracic empyema in children. Pediatr Surg Int. 2002;18:21–3. doi: 10.1007/s003830200004. [DOI] [PubMed] [Google Scholar]
- [16].Wells RG, Havens PL. Intrapleural fibrinolysis for parapneumonic effusion and empyema in children. Radiology. 2003;228:370–8. doi: 10.1148/radiol.2282020486. [DOI] [PubMed] [Google Scholar]
- [17].Rosen H, Nadkarni V, Theroux M, et al. Intrapleural streptokinase as adjunctive treatment for persistent empyema in pediatric patients. Chest. 1993;103:1190–3. doi: 10.1378/chest.103.4.1190. [DOI] [PubMed] [Google Scholar]
- [18].Cochran JB, Tecklenburg FW, Turner RB. Intrapleural instillation of fibrinolytic agents for treatment of pleural empyema. Pediatr Crit Care Med. 2003;4:39–43. doi: 10.1097/00130478-200301000-00007. [DOI] [PubMed] [Google Scholar]
- [19].Ulku R, Onat S, Kiliξ̧ N. Intrapleural fibrinolytic treatment of multiloculated pediatric empyemas. Minerva Pediatr. 2004;56:419–23. [PubMed] [Google Scholar]
- [20].Bouros D, Antoniou KM, Chalkiadakis G, et al. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc. 2002;16:151–4. doi: 10.1007/s00464-001-9028-3. [DOI] [PubMed] [Google Scholar]
- [21].Stringel G, Hartman AR. Intrapleural instillation of urokinase in the treatment of loculated pleural effusions in children. J Pediatr Surg. 1994;29:1539–40. doi: 10.1016/0022-3468(94)90209-7. [DOI] [PubMed] [Google Scholar]
- [22].Ozcelik C, Inci I, Nizam O, et al. Intrapleural fibrinolytic treatment of multiloculated postpneumonic pediatric empyemas. Ann Thorac Surg. 2003;76:1849–53. doi: 10.1016/s0003-4975(03)01012-9. [DOI] [PubMed] [Google Scholar]
- [23].Thomson AH, Hull J, Kumar MR, et al. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax. 2002;57:343–7. doi: 10.1136/thorax.57.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jaffe A, Balfour-Lynn IM. Management of empyema in children. Pediatr Pulmonol. 2005;40:148–56. doi: 10.1002/ppul.20251. [DOI] [PubMed] [Google Scholar]
- [25].Doski JJ, Lou D, Hicks BA, et al. Management of parapneumonic collections in infants and children. J Pediatr Surg. 2000;35:265–8. doi: 10.1016/s0022-3468(00)90022-8. [DOI] [PubMed] [Google Scholar]
- [26].Sonnappa S, Cohen G, Owens CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med. 2006;174:221–7. doi: 10.1164/rccm.200601-027OC. [DOI] [PubMed] [Google Scholar]


