Abstract
We reported previously that dietary isoflavones modulate arterial blood pressure in vivo and that the daidzein metabolite equol rapidly activates endothelial NO synthase (eNOS) via Akt and extracellular signal–regulated kinase 1/2– dependent signaling. In this study, we report the first evidence in human endothelial cells that acute stimulation of mitochondrial superoxide generation by equol (100 nmol/L) is required for eNOS activation. Scavengers of superoxide (superoxide dismutase and manganese [III] tetrakis[1-methyl-4-pyridyl]porphyrin) abrogated equol stimulated Akt and eNOS phosphorylation, and the mitochondrial complex I inhibitor rotenone inhibited Akt, extracellular signal–regulated kinase 1/2, and eNOS phosphorylation, as well as NO-mediated increases in intracellular cGMP. Equol also induced rapid alterations in F-actin fiber distribution, with depolymerization of F-actin with cytochalasin D abrogating equol-stimulated mitochondrial superoxide generation. Treatment of cells with pertussis toxin or inhibition of GPR30/epidermal growth factor receptor kinase transactivation prevented equol-induced activation of extracellular signal–regulated kinase 1/2 via c-Src, Akt, and eNOS. Moreover, inhibition of epidermal growth factor receptor kinase activation with AG-1478 abrogated equol-stimulated mitochondrial reactive oxygen species generation and subsequent kinase and eNOS activation. Our findings suggest that equol-stimulated mitochondrial reactive oxygen species modulate endothelial redox signaling and NO release involving transactivation of epidermal growth factor receptor kinase and reorganization of the F-actin cytoskeleton. Identification of these novel actions of equol may provide valuable insights for therapeutic strategies to restore endothelial function in cardiovascular disease.
Keywords: equol, isoflavones, endothelium, eNOS, mitochondria, cytoskeleton, redox signaling
Dietary soy products contain significant amounts of genistein and daidzein,1,2 which are structurally similar to estrogen, with a preferential affinity for estrogen receptor (ER)-β.3 Plasma concentrations of genistein and other isoflavones are as low as 40 nmol/L in humans consuming low-isoflavone diets but can reach ≈4 μmol/L in Japanese consuming a traditional soybean-rich diet.4,5 The isoflavone equol is produced by metabolism of daidzein by intestinal gut microflora in only 40% to 50% of individuals,6 but equol is more bioavailable than either genistein or daidzein and reaches plasma concentrations of ≈100 nmol/L in vivo.5
Cardiovascular benefits of supplementation with genistein and tetrahydrodaidzein have been observed,7,8 and we reported previously that dietary isoflavones (genistein, daidzein, and equol) can modulate blood pressure in vivo, antioxidant and endothelial NO synthase (eNOS) gene expression, and intracellular glutathione levels in male rats.9 We further demonstrated that feeding aging male rats an isoflavone-enriched diet improves agonist-mediated endothelium-derived hyperpolarizing factor production in small resistance arteries.10 A recent study in healthy postmenopausal women established that an isoflavone-enriched, low-fat meal increases endothelium-dependent relaxation in vivo within 5 to 7 hours.11 Notably, genistein and dehydroequol elicit rapid endothelium-dependent increases in forearm blood flow in vivo,12,13 and equol relaxes preconstricted rat aortic rings.14 In human fetal endothelial cells, equol acutely stimulates endothelial NO release at basal cytosolic Ca2+ levels via activation of extracellular signal–regulated kinase (ERK) 1/2 and protein kinase B (Akt) but independent of classic ER signaling.14
ERs, aside from their classic function as transcription factors, mediate rapid activation of second messenger systems and intracellular kinases.15 Recently, nonclassic membrane-localized ERs have been identified and appear to mediate the rapid actions of 17β-estradiol on intracellular signaling cascades.16,17 The orphan G protein– coupled receptor (GPR30) is a 7-transmembrane spanning G protein–coupled receptor18,19 that binds both 17β-estradiol and genistein.20 GPR30 has been studied primarily in cancerous cells21; however, vascular cells also express the receptor.22 Activation of GPR30 induces transactivation of the epidermal growth factor receptor (EGFR) via Gβγ. Once activated, EGFR stimulates ERK1/2 via activation of c-Src and the phosphoinositide 3-kinase (PI3K)/Akt pathway.23,24
In addition to GPR30 activation, emerging evidence implicates reactive oxygen species (ROS) as second messengers in the activation of PI3K, ERK1/2, c-Src, and EGFR tyrosine kinase.25–27 Although the mitochondrial respiratory chain represents a major source of ROS in the endothelium,28 to our knowledge there are no reports linking activation of eNOS by equol with increased mitochondrial ROS generation. We hypothesized that modulation of GPR30/EGFR, F-actin cytoskeleton, and mitochondrial ROS generation by equol may account for the acute activation of eNOS.
We report the first evidence that inhibition of mitochondrial ROS abolishes equol-induced activation of Akt, ERK1/2, eNOS phosphorylation, and NO production. Our findings link equol-stimulated mitochondrial ROS generation with EGFR transactivation, because inhibition of EGFR activity inhibits kinase activation and eNOS phosphorylation. Furthermore, depolymerization of the F-actin cytoskeleton, known to interact with both EGFR and mitochondria, abrogates mitochondrial ROS generation. Our study provides a novel link between equol-mediated EGFR activation and downstream signal transduction involving mitochondrial ROS in the activation of eNOS.
Methods
For a more detailed Methods description for immunoblotting, quantitative RT-PCR, and cGMP ELSIA, as well as chemicals and reagents, please see the online Data Supplement at http://hyper.ahajournals.org.
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated by collagenase digestion (1 mg/mL) and cultured in low phenol red M199 containing 10% (v/v) FCS, 10% FCS (v/v) newborn calf serum, and 5 mmol/L of l-glutamine and endothelial cell growth factor (20 μg/mL).14
Chemiluminescence Detection of ROS Generation in Intact Endothelial Cells
Confluent HUVEC monolayers were incubated in low serum (1% FCS) M199 for 4 hours and then preincubated for 30 minutes in Krebs buffer (in mM: NaCl 118.0, KCl 6.0, NaHCO3 25.0, NaH2PO4 1.2, HEPES 5.0, d-glucose 10.0, CaCl2 1.6, and MgSO4 1.2 [pH 7.4]) containing l-arginine (100 μmol/L) in the absence or presence of superoxide (O2·−) dismutase (SOD; 200 U/mL), polyethylene glycol-SOD (PSOD; 50 U/mL), polyethylene glycol-catalase (PCAT; 200 U/mL), manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin (Mn; 100 μmol/L), or rotenone (2 μmol/L). Cells were then incubated in Krebs buffer containing lucigenin (5 μmol/L) and NADPH (100 μmol/L) and challenged with equol (100 nmol/L) or vehicle (0.01% dimethyl sulfoxide [DMSO]) in the absence or presence of inhibitors. Luminescence was immediately recorded in a microplate luminometer (Chameleon V, Hidex) at 37°C after the addition of lucigenin.29 Maximal luminescence values obtained over a 20- to 40-minute interval (see Figure 1A) were averaged for each treatment condition, and values from 3 to 4 independent cell cultures were expressed as mean light units per milligram of protein.
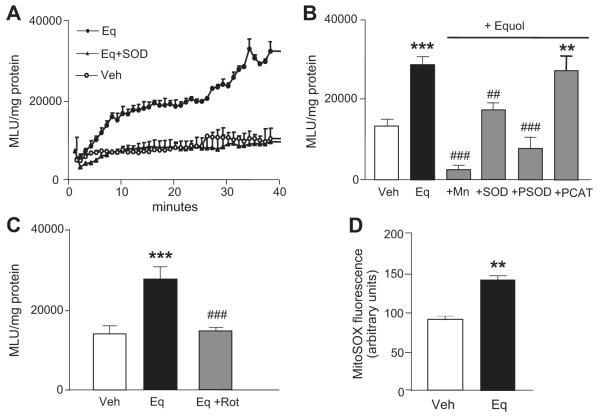
Figure 1.
Equol stimulates intracellular ROS generation in endothelial cells. A, HUVECs were preincubated for 30 minutes in Krebs buffer containing l-arginine (100 μmol/L) and either vehicle (0.01% DMSO) or SOD (200 U/mL) and cells then treated for 2 minutes with vehicle (○) or equol (Eq; 100 nmol/L, ●) in the continued absence or presence of SOD (▲), lucigenin (5 μmol/L), and NADPH (100 μmol/L). ROS generation was measured by enhanced chemiluminescence over 40 minutes and expressed as mean light units (MLUs) per milligram of protein averaged over 20 to 40 minutes. B, Effects of Mn (100 μmol/L), SOD (200 U/mL), PSOD (50 U/mL), and PCAT (200 U/mL) on equol-stimulated ROS generation. C, Effects of rotenone (Rot; 2 μmol/L) on equol-stimulated ROS generation. D, HUVECs were preloaded for 30 minutes with MitoSOX Red (5 μmol/L) and then treated for 20 minutes with vehicle (0.01% DMSO) or equol (100 nmol/L) in serum-free medium. Cells were subsequently fixed with 4% paraformaldehyde and fluorescence measured by confocal microscopy, as described in the Methods section. Mean±SEM of measurements in cultures from 3 to 5 different donors, **P<0.01 and ***P<0.001 vs vehicle alone; ##P<0.01 and ###P<0.001 vs equol.
Mitochondrial ROS Production Measured Using MitoSOX Red Fluorescence
Mitochondrial ROS production was measured using the fluorogenic dye MitoSOX Red, a mitochondrially targeted derivative of hydroethidine.30 HUVECs on glass cover slips were loaded with MitoSOX Red (5 μmol/L) for 30 minutes. Cells were subsequently treated in duplicate for 20 minutes with equol (100 nmol/L) or vehicle (0.01% DMSO), and fluorescence was detected in 4% paraformaldehyde-fixed cells by confocal microscopy (560/625 nm). Fluorescence images were obtained from a total of 200 cells per cover slip in each of 4 cultures from 4 different donors. In other experiments, cells were pretreated with the cytoskeletal disrupting agent cytochalasin D (2.5 μmol/L) or EGFR tyrosine kinase inhibitor AG-1478 (5 μmol/L) and then stimulated acutely with equol (100 nmol/L) and monitored with MitoSOX Red fluorescence.
F-Actin Staining With Rhodamine-Phalloidin
Alterations in F-actin cytoskeletal distribution were visualized in fixed cells stained with rhodamine-phalloidin, as described previously.31 Cells were treated with control, vehicle (0.01% DMSO), or equol (100 nmol/L) for 20 minutes, fixed, polymerized F-actin fibers stained with rhodamine-phalloidin (500 nmol/L) for 2 hours at room temperature, and nuclei counterstained with Hoechst (10 μg/mL−1) for 1 minute. Fluorescence was detected by confocal microscopy with sequential acquisition at wavelengths of 560/625 nm and 375/450 nm used to visualize F-actin and nuclei staining, respectively. In other experiments, cells were pretreated for 30 minutes with cytochalasin D (2.5 μmol/L) before cotreating cells for 20 minutes with equol (100 nmol/L) in the continued absence or presence of cytochalasin-D (2.5 μmol/L).
Statistical Analysis
Data are expressed as mean±SEM of measurements in 3 to 5 different HUVEC cultures obtained from different donors, unless stated otherwise. Statistical analyses were performed using a Student 2-tailed t test or 1-way ANOVA followed by Dunnett multiple comparison, with P<0.05 considered statistically significant.
Results
Equol Stimulates Intracellular ROS Generation in Intact Endothelial Cells
To investigate whether equol stimulates ROS generation, HUVECs were treated with vehicle (0.01% DMSO) or equol (100 nmol/L), and ROS generation was monitored over a 20- to 40-minute assay using lucigenin chemiluminescence. Equol-stimulated ROS production was abrogated by pretreatment with 200 U/mL of SOD (Figure 1A). To confirm the generation of O2·−, cells were preincubated with the cell-permeable H2O2 and O2·− scavenger Mn (100 μmol/L), PSOD (50 U/mL), or H2O2 metabolizing enzyme catalase (PCAT; 200 U/mL). Equol-mediated increases in lucigenin chemiluminescence were significantly inhibited by Mn, PSOD, and SOD, whereas PCAT failed to inhibit equol-stimulated ROS generation (Figure 1B). To determine whether mitochondria were responsible for equol-induced O2·− generation, endothelial cells were pretreated in the absence or presence of the mitochondrial complex I inhibitor rotenone (2 μmol/L) and then challenged with equol. Rotenone abrogated equol stimulated O2·− production (Figure 1C), and, furthermore, treatment with equol (100 nmol/L) enhanced cellular fluorescence in HUVECs loaded with the mitochondrial-targeted ROS indicator MitoSOX Red (Figure 1D).
Effects of O2·− Scavengers on Equol-Stimulated eNOS, Akt, and ERK1/2 Phosphorylation
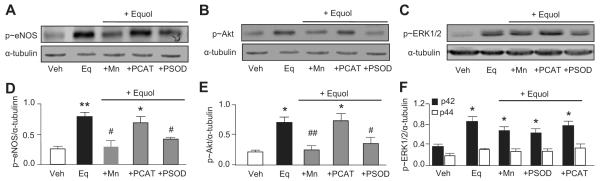
We reported previously that equol (100 nmol/L)-stimulated eNOS phosphorylation depends on the activation of Akt and ERK1/214 and here provide evidence that equol elicits concentration- and time-dependent increases in eNOS phosphorylation (please see Figure S1 and S2 in the online Data Supplement, available at http://hyper.ahajournals.org). To determine whether inhibition of equol-induced ROS generation affects activation of eNOS and upstream kinases, HUVECs were pretreated with Mn (100 μmol/L), PSOD (50 U/mL), or PCAT (200 U/mL) and challenged acutely with equol (100 nmol/L for 2 minutes). Cell lysates were probed for phosphorylated eNOS, phosphorylated Akt, and phosphorylated ERK1/2, and notably Mn and PSOD, but not PCAT, abrogated equol-stimulated phosphorylation of eNOS (Figure 2A and 2D) and Akt (Figure 2B and 2E), whereas phosphorylated ERK1/2 was unaffected by these ROS scavengers (Figure 2C and 2F).
Figure 2.
Inhibition of ROS generation abrogates equol-stimulated eNOS and Akt phosphorylation. HUVECs were preincubated for 30 minutes in Krebs buffer containing l-arginine (100 μmol/L) in the absence or presence of Mn (100 μmol/L), PSOD (50 U/mL), or PCAT (200 U/mL) before acute stimulation with vehicle (Veh; 0.01% DMSO) or equol (100 nmol/L, 2 minutes) in the continued absence or presence of the inhibitors. A through C, Cell lysates were immunoblotted for phosphorylated (p≈)eNOS, p≈Akt, and p≈ERK1/2. Representative immunoblots are shown with densitometric analyses of results from 4 to 5 different cultures summarized in D through F. Mean±SEM of measurements in cultures from 4 to 5 different donors; *P<0.05, **P<0.01 vs vehicle alone; #P<0.05, ##P<0.01 vs equol.
Mitochondrial ROS Generation Is Required for Equol-Induced Kinase and eNOS Phosphorylation
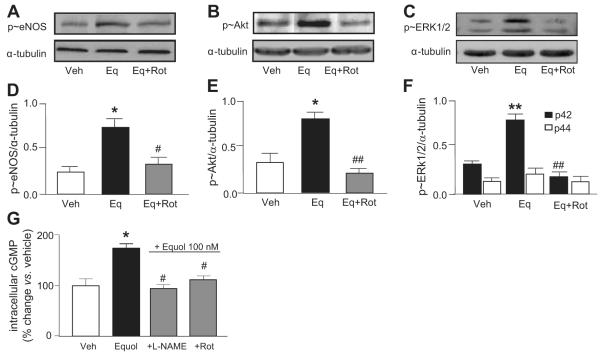
To establish whether mitochondrial O2·− plays a role in equol-stimulated eNOS activation, HUVECs were preincubated with rotenone (2 μmol/L for 30 minutes) and then stimulated acutely with vehicle (0.01% DMSO) or equol (100 nmol/L for 2 minutes) in the continued absence or presence of rotenone. Rotenone blocked the acute phosphorylation of eNOS (Figure 3A and 3D), Akt (Figure 3B and 3E), and ERK1/2 (Figure 3C and 3F) by equol, implicating mitochondrial ROS in the upstream activation of kinases.
Figure 3.
Mitochondrial ROS generation is required for equol-induced kinase and eNOS phosphorylation and NO production. HUVECs were preincubated for 30 minutes in Krebs buffer containing l-arginine (100 μmol/L) in the absence or presence of rotenone (Rot; 2 μmol/L) before acute stimulation with equol (100 nmol/L, 2 minutes) in the continued absence or presence of rotenone. Cell lysates were immunoblotted for phosphorylated (p≈)eNOS (A), p≈Akt (B), and p≈ERK1/2 (C) and densitometric analyses are shown in D to E. Mean±SEM of measurements in cultures from 4 to 5 different donors; *P<0.05, **P<0.01 vs vehicle (0.1% DMSO); #P<0.05, ##P<0.01 vs equol. G, HUVECs were preincubated for 30 minutes in Krebs buffer containing l-arginine (100 μmol/L) in the absence or presence of NG-nitro-l-arginine ester (100 μmol/L) or rotenone (2 μmol/L) and then treated with vehicle (Veh; 0.01% DMSO) or equol (100 nmol/L, 2 minutes). Intracellular accumulation of cGMP in HUVEC monolayers was expressed as percentage of change versus vehicle. Mean±SD of triplicate measurements in HUVEC cultures derived from 2 different donors; *P<0.05 vs vehicle; #P<0.05 vs equol.
Mitochondrial Complex I Inhibition Abolishes eNOS-Dependent cGMP Formation
To confirm that activation of kinases and eNOS by mitochondrial O2·− influences endothelial NO production, effects of rotenone on equol-induced intracellular cGMP accumulation were measured in HUVECs preincubated with an eNOS inhibitor (NG-nitro-l-arginine ester; 100 μmol/L) or rotenone (2 μmol/L) and then stimulated for 2 minutes with equol (100 nmol/L) in the continued absence or presence of inhibitors. NG-Nitro-l-arginine ester prevented equol-stimulated increases in cGMP levels, confirming intracellular cGMP as a reliable measure NO production (Figure 3G).14 Consistent with rotonene-mediated inhibition of ROS production and phosphorylation of eNOS, Akt, and ERK1/2, rotenone abrogated equol-stimulated cGMP levels.
Mitochondrial ROS Generation Occurs Downstream of EGFR Activation
ROS generation is known to occur downstream of EGFR activation32 and to also potentiate EGFR transactivation.33 To establish a relationship between equol-induced EGFR activation and mitochondrial O2·− generation, cells were pretreated for 30 minutes with the EGFR kinase inhibitor AG-1478 (5 μmol/L) and then stimulated with equol (100 nmol/L for 20 minutes) before measuring mitochondrial ROS generation using MitoSOX Red. EGFR inhibition abrogated mitochondrial O2·− generation (Figure 4), suggesting that mitochondrial ROS generation occurs downstream of EGFR activation.
Figure 4.
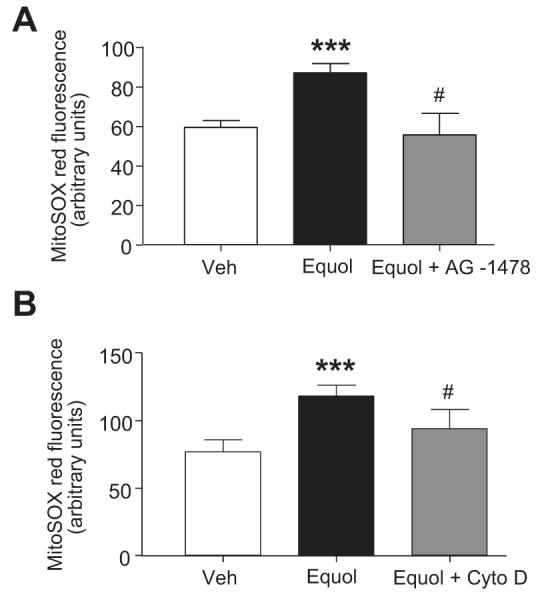
Inhibition of EGFR transactivation or depolymerization of F-actin abrogates equol-stimulated mitochondrial ROS generation. HUVECs were loaded with MitoSOX Red (5 μmol/L) for 30 minutes in the absence or presence of AG-1478 (5 μmol/L) (panel A) or cytochalasin D (2.5 μmol/L) (panel B). Cells were then treated for 20 minutes with vehicle (0.01% DMSO) or equol (100 nmol/L) in the continued absence or presence of these agents. Mean±SEM of duplicate measurements of arbitrary MitoSOX Red fluorescence (200 cells per cover slip) in cultures from 4 different donors; ***P≤0.001 vs vehicle; #P≤0.05 vs equol.
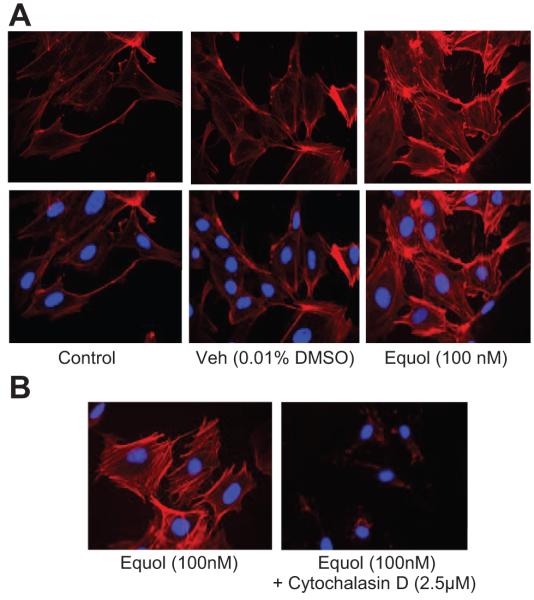
Because F-actin has been shown to modulate mitochondrial ROS production34,35 and to potentiate EGFR dimerization by clustering of EGFRs,36 we hypothesized that F-actin may provide a link between EGFR activation and downstream mitochondrial ROS generation. HUVECs treated with equol (100 nmol/L, 20 minutes) were fixed in 4% paraformaldehyde, polymerized F-actin fibers stained with rhodamine-phalloidin (250 nmol/L), nuclei counterstained with Hoechst (10 μg/mL), and confocal images of phalloidin with Hoechst staining overlaid. We found that equol induced acute alterations in the arrangement of F-actin, with a thickening of cortical F-actin and the appearance of internal stress fibers (Figure 5A). Depolymerization of F-actin after treatment with cytochalasin-D (2.5 μmol/L; Figure 5B) was associated with an inhibition of mitochondrial ROS production (Figure 5C), confirming that F-actin may provide a link between EGFR activation and mitochondrial ROS generation.
Figure 5.
Equol induces rapid reorganization of the F-actin cytoskeleton in endothelial cells. HUVECs on glass cover slips were equilibrated in M199 containing 1% FCS 4 hours before treatment. A, Cells were treated in duplicate for 20 minutes with serum-free M199 (Control), vehicle (0.01% DMSO), or equol (100 nmol/L); washed with ice-cold PBS; and fixed in 4% paraformaldehyde. Polymerized F-actin was stained with rhodamine-phalloidin (250 nmol/L) and nuclei counterstained with Hoechst (10 μg/mL). Confocal (×40) images from a single culture are representative of results from 4 different cultures. B, HUVECs were pretreated for 30 minutes in the absence or presence of cytochalasin-D (2.5 μmol/L) and then challenged for 20 minutes with equol (100 nmol/L) in the absence or presence of cytochalasin-D. Polymerized F-actin was stained with rhodamine-phalloidin and nuclei counterstained with Hoechst reagent. Confocal images (×40) are representative of similar findings in 4 different cultures.
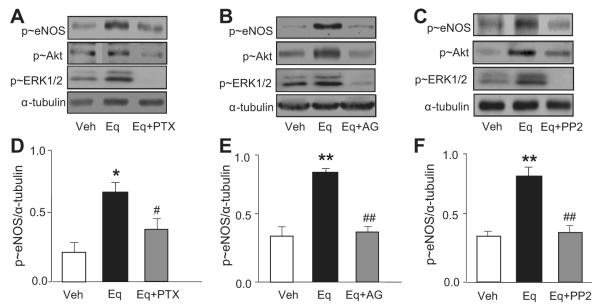
GPR30-Linked Transactivation of EGFR Mediates ERK1/2, Akt, and eNOS Activation
Estradiol binds GPR30 to stimulate kinase activity,21 and, because equol is structurally similar to estrogen,3 we hypothesized a role for GPR30 in Akt and ERK1/2 activation involving G protein–linked EGFR transactivation. Pretreatment of HUVECs with the G-protein inhibitor pertussis toxin (100 ng/mL) or the EGFR kinase inhibitor (AG-1478, 5 μmol/L) for 30 minutes blocked equol-stimulated phosphorylation of ERK1/2, Akt, and eNOS (Figure 6A and 6D and Figure 6B and 6E, respectively). A consistent feature of EGFR transactivation in GPR30 signaling is the recruitment and activation of the protein tyrosine kinase c-Src.37 Thus, HUVECs were preincubated HUVECs for 30 minutes with a c-Src inhibitor (PP2; 10 μmol/L) and then treated acutely for 2 minutes with equol (100 nmol/L). As shown in Figure 6C and 6F, PP2 blocked equol-stimulated eNOS phosphorylation and significantly attenuated ERK1/2 and Akt phosphorylation. Densitometric analysis of phosphorylated Akt and phosphorylated ERK1/2 is summarized in Figure S3.
Figure 6.
GPR30-linked transactivation of EGFR mediates ERK1/2, Akt, and eNOS activation. HUVECs were preincubated for 30 minutes in Krebs buffer containing l-arginine (100 μmol/L) in the absence or presence of pertussis toxin (PTX; 100 ng/mL), EGFR tyrosine kinase inhibitor AG-1478 (AG; 5 μmol/L), or Src kinase inhibitor (PP2; 10 μmol/L) before acute stimulation with equol (Eq; 100 nmol/L, 2 minutes) in the continued absence or presence of inhibitors. Representative immunoblots for phosphorylated (p≈)eNOS, p≈Akt, and p≈ERK1/2 (relative to α-tubulin) are shown in A through C, with densitometric analyses for p≈eNOS summarized in D through F. Mean±SEM of measurements in cultures derived from 4 to 5 different donors; *P<0.05 and **P<0.01 vs vehicle; #P<0.05, ##P<0.01 vs equol.
Discussion
In humans consuming a soy-rich diet, plasma concentrations of equol range between 1 and 100 nmol/L,4,5 depending on “equol producer” status. Because equol producers appear to have improved vascular function, it seems likely that the beneficial impact of soy isoflavones on blood pressure and lipid profiles may be influenced by the ability of subjects to metabolize dietary daidzein.8 Our findings suggest that, in fetal endothelial cells, equol increases mitochondrial ROS, which act as second messengers to induce the rapid stimulation of Akt, ERK1/2, and eNOS activity.
We have obtained novel insights into the cellular mechanisms linking equol-stimulated mitochondrial ROS with activation of eNOS and NO production in endothelial cells. The involvement of ROS in the activation eNOS and upstream kinases was established by observing that inhibition of ROS generation with scavengers of O2·−, but not H2O2 (Figure 1B), abrogated equol-stimulated Akt and eNOS phosphorylation (Figure 2A and 2B). A surprising feature of equol-mediated signaling in endothelial cells is that, although this isoflavone has antioxidant properties in endothelial cells,38 we observed an increase in mitochondrial O2·− production in response to nanomolar concentrations of equol (Figure 1D). Although ROS are elevated in cardiovascular and other diseases associated with sustained oxidative stress, under physiological conditions ROS can act as “second messengers” in the regulation of redox-sensitive kinases and transcription factors.25–28
Previous studies reported that activation of eNOS by structurally related polyphenols involves ROS-mediated activation of Akt39,40; however, the intracellular sources and species of ROS were not determined. Mitochondria and NADPH oxidase represent 2 major sources of endothelial ROS generation.28 Notably, rapid stimulation of ROS generation in endothelial cells by 17β-estradiol is inhibited by rotenone but unaffected by inhibitors of NADPH oxidase.35 These studies, together with our present findings, strongly suggest that equol acutely stimulates mitochondrial O2·− generation. Because equol-induced ROS generation was completely inhibited by rotenone and equol-enhanced MitoSOX Red fluorescence, it seems unlikely that Nox2 and Nox4, localized predominantly to the plasma membrane and endoplasmic reticulum,41,42 modulated eNOS activity. In endothelial cells, NADPH oxidase can also generate extracellular O2·−, which, in turn, may affect intracellular signaling pathways by entering cells through membrane chloride channels.43 In this context, estrogen downregulates NADPH oxidase subunit expression in endothelial cells after 8 hours,44 and equol rapidly inhibits NADPH oxidase activity in macrophages.45
Mitochondria generate ROS via respiratory complexes I and III; however, ROS generation via complex III may play a key role in modulating cytosolic signaling pathways.46 Inhibition of mitochondrial ROS generation in active cells by rotenone suggests that cells were in state 3. Although elevation of intracellular Ca2+ results in mitochondrial Ca2+ loading and ROS generation,47 we reported previously that genistein, daidzein, and equol fail to elicit Ca2+ transients in human endothelial cells,14 suggesting an alternate mechanism for isoflavone-stimulated ROS generation.
Our findings suggest that equol-induced mitochondrial ROS and eNOS activation may be mediated by GPR30-linked transactivation of the EGFR. Treatment with pertussis toxin or AG-1478 abolished phosphorylation of eNOS and the upstream kinases Akt and ERK1/2, with ERK1/2 activity dependent on c-Src activation (Figure 6). Similarly, treatment with AG-1478 inhibited mitochondrial ROS production (Figure 4), indicating that mitochondrial ROS generation occurs downstream of EGFR activation and is unlikely to be attributed to direct binding of equol to the mitochondrial respiratory complexes. EGFR-induced PI3K activation has been suggested previously to mediate mitochondrial ROS production via alterations in mitochondrial ATP-activated potassium channel activity.32 In contrast, our data indicate that kinase activation occurs downstream of mitochondrial ROS production. Several studies have reported that ROS potentiate EGFR transactivation and, thus, kinase activation.33,48 Furthermore, PI3K/Akt and ERK1/2 kinase pathways are redox sensitive, potentially enabling kinase activation by equol-induced mitochondrial ROS generation.
To our knowledge, we report the first evidence that the isoflavone equol induces rapid alterations in cytoskeletal F-actin distribution (Figure 5). We propose that the mechanism linking EGFR activation and mitochondrial ROS production involves equol-induced alterations in F-actin distribution, because disruption of the cytoskeleton inhibits equol-stimulated mitochondrial ROS generation (Figure 4B). It is unlikely that our findings reflect an artifactual disruption of mitochondrial integrity by cytochalasin-D, because previous studies have demonstrated that mitochondria retain their ability to respond to mitochondrial inhibitors, such as antimycin A.34 Recent findings indicate that F-actin may directly bind to the EGFR49 and partition EGFR receptors to enhance receptor dimerization, which could, in turn, potentiate mitochondrial ROS and kinase activation.36
The present study highlights a potential protective role for equol in cardiovascular disease. We propose that equol and other isoflavones evoke mitochondrial O2·− generation in endothelial cells, leading to transactivation of the EGFR; activation of c-Src, ERK1/2, PI3K/Akt, and eNOS; and rapid NO release (please see Figure S4).
Perspectives.
In view of the importance of the developmental origins of health and disease,50 our study provides novel insights into the mechanisms by which isoflavones acutely regulate eNOS activity, as well as eNOS mRNA and protein expression (please see Figure S5) in fetal endothelial cells. It is worth noting that exposure to dietary soy during fetal development and early life may reduce the susceptibility to cardiovascular disease and obesity in adulthood.51 By influencing developmental plasticity in utero and in postnatal development, isoflavones may not only alter the expression of genes encoding eNOS9 but also others associated with metabolism and antioxidant defenses.52 Thus, based on our previous studies in rodents in vivo15 and the present findings in fetal endothelial cells, equol and other isoflavones may improve endothelial function and lower blood pressure in the adulthood via fetal programing.50
Supplementary Material
Acknowledgments
We gratefully acknowledge the midwives of St Thomas’ Hospital labor ward and thank Dr Vladimir Snetkov (Division of Asthma, Allergy and Lung Biology, King’s College London, London, United Kingdom) for assistance in fluorescence measurements of mitochondrial ROS generation.
Sources of Funding This work was supported in part by the Biotechnology and Biological Sciences Research Council (BBS/S/K/2004/11207), British Heart Foundation (FS/99075), Heart Research United Kingdom (RG2542 and RG2588), and European Union Cooperation in Science and Technology (COST) Action B35.
Footnotes
Disclosures None.
References
- 1.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 2.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans: I–review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptorβ. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 4.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 5.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-equol, a potent ligand for estrogen receptorβ, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 7.Altavilla D, Crisafulli A, Marini H, Esposito M, D’Anna R, Corrado F, Bitto A, Squadrito F. Cardiovascular effects of the phytoestrogen genistein. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:179–186. doi: 10.2174/1568016043477297. [DOI] [PubMed] [Google Scholar]
- 8.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81:189–195. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- 9.Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Vina J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 10.Knock GA, Mahn K, Mann GE, Ward JP, Aaronson PI. Dietary soy modulates endothelium-dependent relaxation in aged male rats: increased agonist-induced endothelium-derived hyperpolarising factor and basal nitric oxide activity. Free Radic Biol Med. 2006;41:731–739. doi: 10.1016/j.freeradbiomed.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall WL, Formanuik NL, Harnpanich D, Cheung M, Talbot D, Chowienczyk PJ, Sanders TA. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J Nutr. 2008;138:1288–1292. doi: 10.1093/jn/138.7.1288. [DOI] [PubMed] [Google Scholar]
- 12.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation. 2001;103:258–262. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- 13.Chin-Dusting JP, Fisher LJ, Lewis TV, Piekarska A, Nestel PJ, Husband A. The vascular activity of some isoflavone metabolites: implications for a cardioprotective role. Br J Pharmacol. 2001;133:595–605. doi: 10.1038/sj.bjp.0704088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joy S, Siow RC, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, Mann GE. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006;281:27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 15.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 17.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 18.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J. 2010;57:101–107. doi: 10.1507/endocrj.k09e-332. [DOI] [PubMed] [Google Scholar]
- 20.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 21.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17β-estradiol on function and expression of estrogen receptorα, estrogen receptorβ, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 23.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 24.Shah BH, Neithardt A, Chu DB, Shah FB, Catt KJ. Role of EGF receptor transactivation in phosphoinositide 3-kinase-dependent activation of MAP kinase by GPCRs. J Cell Physiol. 2006;206:47–57. doi: 10.1002/jcp.20423. [DOI] [PubMed] [Google Scholar]
- 25.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 29.He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis. doi: 10.1016/j.numecd.2009.12.008. In press. [DOI] [PubMed] [Google Scholar]
- 30.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 31.Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- 32.Krieg T, Cui L, Qin Q, Cohen MV, Downey JM. Mitochondrial ROS generation following acetylcholine-induced EGF receptor transactivation requires metalloproteinase cleavage of proHB-EGF. J Mol Cell Cardiol. 2004;36:435–443. doi: 10.1016/j.yjmcc.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 34.Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L486–L496. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- 35.Felty Q. Estrogen-induced DNA synthesis in vascular endothelial cells is mediated by ROS signaling. BMC Cardiovasc Disord. 2006;6:16. doi: 10.1186/1471-2261-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 37.Quinn JA, Graeber CT, Frackelton AR, Jr, Kim M, Schwarzbauer JE, Filardo EJ. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30. Mol Endocrinol. 2009;23:1052–1064. doi: 10.1210/me.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimbach G, De Pascual-Teresa S, Ewins BA, Matsugo S, Uchida Y, Minihane AM, Turner R, VafeiAdou K, Weinberg PD. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica. 2003;33:913–925. doi: 10.1080/0049825031000150444. [DOI] [PubMed] [Google Scholar]
- 39.Anselm E, Chataigneau M, Ndiaye M, Chataigneau T, Schini-Kerth VB. Grape juice causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of eNOS. Cardiovasc Res. 2007;73:404–413. doi: 10.1016/j.cardiores.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282:13736–13745. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- 41.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 42.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner AH, Schroeter MR, Hecker M. 17β-Estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 2001;15:2121–2130. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 45.Hwang J, Wang J, Morazzoni P, Hodis HN, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic Biol Med. 2003;34:1271–1282. doi: 10.1016/s0891-5849(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 46.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 47.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 48.Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 49.Song W, Xuan H, Lin Q. Epidermal growth factor induces changes of interaction between epidermal growth factor receptor and actin in intact cells. Acta Biochim Biophys Sin (Shanghai) 2008;40:754–760. [PubMed] [Google Scholar]
- 50.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 51.Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T, Mori C. Fetal exposure to phytoestrogens–the difference in phytoestrogen status between mother and fetus. Environ Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Siow RC, Mann GE. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31:468–477. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.