Abstract
We have previously shown that overexpression of the Glud1 (glutamate dehydrogenase 1) gene in neurons of C57BL/6 mice results in increased depolarization-induced glutamate release that eventually leads to selective neuronal injury and cell loss by 12 months of age. However, it is known that isogenic lines of Tg (transgenic) mice produced through back-crossing with one strain may differ in their phenotypic characteristics from those produced using another inbred mouse strain. Therefore, we decided to introduce the Glud1 transgene into the Balb/c strain that has endogenously lower levels of GLUD1 (glutamate dehydrogenase 1) enzyme activity in the brain as compared with C57BL/6. Using an enzyme-based MEA (microelectrode array) that is selective for measuring glutamate in vivo, we measured depolarization-induced glutamate release. Within a discrete layer of the striatum, glutamate release was significantly increased in Balb/c Tg mice compared with wt (wild-type) littermates. Furthermore, Balb/c mice released approx. 50–60% of the amount of glutamate compared with C57BL/6 mice. This is similar to the lower levels of endogenous GLUD1 protein in Balb/c compared with C57BL/6 mice. The development of these Glud1-overexpressing mice may allow for the exploration of key molecular events produced by chronic exposure of neurons to moderate, transient increases in glutamate release, a process hypothesized to occur in neurodegenerative disorders.
Keywords: amperometry, biosensor, electrochemistry, excitotoxicity, neurodegeneration, neurotransmission
Abbreviations: CNS, central nervous system; DLAR, Division of Animal Laboratory Resources; DV, dorsoventral; GLUD1, glutamate dehydrogenase 1; MEA, microelectrode array; m-POD, micro-peroxidase; 1-MPMS, 1-methoxy-5-methylphenazinium methyl-sulfate; Pt, platinum; Tg, transgenic; wt, wild-type
INTRODUCTION
In an effort to generate experimental animals that might exhibit structural and functional changes in the brain as a result of life-long, moderately excessive release of glutamate from neurons, we created Tg (transgenic) mice that express the gene for Glud1 (glutamate dehydrogenase 1) in CNS (central nervous system) neurons (Bao et al., 2009). Based on our observations with these Tg mice (Glud1 mice), GLUD (glutamate dehydrogenase) appears to be a key enzyme in regulating the amount of glutamate synthesized and released from CNS neurons (Bao et al., 2009). This is an important observation, because the Glud1 gene has been identified as a memory-related gene in the hippocampus (Cavallaro et al., 1997) and dysregulation of a related Glud gene expressed in the human brain, Glud2, has been associated with early onset of Parkinson's disease (Plaitakis et al., 2010). The reason for focusing on excess glutamate synthesis in CNS neurons is based on the fact that glutamate is not only the most prevalent excitatory neurotransmitter in the vertebrate CNS, but that it also has long-lasting effects on neuronal function, such as the establishment of long-term potentiation or depression of synaptic transmission (Artola and Singer, 1987; Cotman et al., 1988; Ito, 1989), and on neuronal structure, such as the initiation of neuronal migration, axonal growth and synapse formation during development (Mattson et al., 1988; Brewer and Cotman, 1989; Komuro and Rakic, 1993). Interest in the endogenous activity of glutamate goes beyond the role of this neurotransmitter in learning and memory. Acute treatment of neurons with high concentrations of glutamate leads to neurotoxicity, thus offering a possible link to neurodegenerative diseases (Choi and Rothman, 1990).
In our previous study of heterozygous Glud1 Tg mice, we found that chronic, moderate excess release of glutamate in these mice as compared with wt (wild-type) littermates, led to selective neuronal injury and moderate cell loss with advancing age (Bao et al., 2009). Overexpression of Glud1 only in CNS neurons significantly increased synaptic glutamate release, decreased synaptic long-term potentiation and decreased synapse numbers in selective brain regions. The Tg mice used in these studies were mice that were back-crossed to the C57BL/6 strain, a strain of mice frequently used for biochemical, physiological and behavioural studies. However, it is known that isogenic lines of Tg mice produced through back-crossing with one strain may differ in their phenotypic characteristics from those produced using another inbred mouse strain (Crawley et al., 1997). The diversity of phenotypic characteristics of Tg mice may result from behavioural, physiological or biochemical differences among such strains. For example, inbred strains of C57 mice, such as C57BL/6, have high levels of exploratory activity and low ‘emotional’ or anxiety levels, whereas albino inbred mice, such as the Balb/c strain, exhibit low levels of exploratory activity and high levels of anxiety (Crabbe, 1986; Mathis et al., 1994). These two strains of mice differ also in several neurochemical parameters (i.e. endophenotype), including brain serotonin, the neurosteroid 17-OH-pregnenolone, and the formation of reactive oxygen species in hippocampal neurons in response to glutamate-induced stress (Zhang et al., 2004; Tagawa et al., 2006; Palumbo et al., 2010). Other examples of endophenotypic variation among several strains of mice have been documented and include significant differences in the levels of gene and protein expression in the hippocampus (Fernandes et al., 2004; Pollak et al., 2006).
It is possible to select different strains of mice to generate isogenic lines based on the expected effects that a transgene or targeted gene disruption would have on specific behaviours. However, in a situation such as the Glud1 Tg mice for which the expected behavioural manifestations of the transgene are unknown, a choice of a specific strain on behavioural grounds is not possible. In such a situation, it is important to use more than one strain of mice as the background strain, especially strains that may significantly differ in terms of their behaviours and their physiological or biochemical characteristics. Therefore we decided to introduce the Glud1 transgene into a second strain, one that we determined had endogenously lower levels of GLUD1 (glutamate dehydrogenase 1) enzyme activity in brain as compared with the C57BL/6 mice.
In the present study, we demonstrated that Balb/c mice had lower protein levels of GLUD1, as well as lower neuronal GLUD activity, therefore, fulfilling the criteria for a mouse strain with low endogenous GLUD1 expression that may be used for the generation of a new isogenic line of mice bearing the transgene for Glud1. Following back-crossing of the Tg mice to the Balb/c strain, we measured the effects that introduction of the Glud1 gene had on basal extracellular glutamate levels and on depolarization-induced glutamate release from striatal neurons in these mice and compared such release with that observed in littermate wt mice and C57BL/6 mice overexpressing the same transgene. We decided to study striatal glutamate release based on previous results indicating dendrite and cell body injury (decreases in MAP2A labelling) but relatively well-preserved nerve endings (no changes in synaptophysin labelling) in the striatum of C57BL/6 Glud1 mice (Bao et al., 2009). Unlike the striatum, the hippocampus of Glud1 mice, and especially the CA1 region of the hippocampus, suffered dendrite and nerve terminal losses. Therefore glutamate release measurements in the striatum would not be compromised by substantial nerve terminal losses. In order to study glutamate release in these strains of mice, we utilized an enzyme-based MEA (microelectrode array) selective for measuring glutamate with low limits of detection (<0.2 μM), high spatial resolution (333×15 μm), fast temporal resolution (500 ms) (Burmeister and Gerhardt 2001; Burmeister et al., 2000, 2002, 2004) and minimal damage to surrounding brain tissue (50–100 μm) (Hascup et al., 2009).
METHODS
Generation of Balb/c GLUD Tg mice
All experimental procedures related to animals followed the procedures of the Institutional Animal Care and Use Committees of the University of Kansas and the University of Kentucky, as well as those of the National Institutes of Health. Efforts were made for minimizing the number of animals used. On arrival from the University of Kansas, mice (10–12 months of age) were quarantined for a minimum of 1 week in the DLAR (Division of Laboratory Animal Resources) at the University of Kentucky. Mice were group housed under a 12 h light/12 h dark regime with food and water ad libitum. All appropriate animal care (food, water, bedding, cage cleaning, etc.) was performed by DLAR staff. Following experimentation, the mice were killed with an overdose of anaesthetic followed by decapitation.
As previously described (Bao et al., 2009), Tg mice for Glud1 were generated by microinjecting oocytes from hyper-ovulating C57BL6/SJL hybrid mice with linearized DNA containing the cDNA for mouse Glud1 under the control of the promoter for neuron-specific enolase. The construct of the cDNA for Glud1, the oocyte microinjection procedure and the identification of the four founder mice carrying the transgene were described previously (Bao et al., 2009). The founder Tg mice were each cross-bred to Balb/c mice and heterozygous Tg mice identified by PCR methods (Bao et al., 2009). Cross-breeding with Balb/c mice has been carried out for more than ten generations.
Measurement of GLUD activity and GLUD1 protein levels in brain homogenates and synaptosomes
Whole brains from adult C57BL/6, Balb/c and CD-1 mice were obtained following decapitation under anaesthesia (CO2) and were homogenized in ice-cold buffer (0.32 M sucrose, 0.5 mM MgSO4, 10 mM amino caproic acid, 0.1 mM EGTA, 0.1 mM benzamide, 0.1 mM benzamidine hydrochloride, in 10 mM Hepes buffer, pH 7.4). The homogenates were rapidly frozen in liquid N2 and stored at −80°C until used. Synaptosomes were isolated from freshly prepared brain homogenates using Ficoll™ gradient centrifugation procedures identical with those previously described (Chen et al., 1988). Protein content in all preparations was measured as described (Michaelis et al., 1983).
The enzyme activity of GLUD was measured in the direction of glutamate oxidation and deamination using a coupled reaction scheme similar to that described (Maeda et al., 1989; Tsuji et al., 1989). In this scheme, the NADH formed during varying incubation time periods was allowed to reduce O2 to O2−• and H2O2 in a coupled reaction with the electron mediator 1-MPMS (1-methoxy-5-methylphenazinium methyl-sulfate). The O2−• and H2O2 formed were detected as light generated by the chemiluminescence reaction with isoluminol in the presence of m-POD (micro-peroxidase) (Maeda et al., 1989; Tsuji et al., 1989). The GLUD enzyme reactions with brain homogenates were performed in 96-well chemiluminescence microplates and were initiated by the addition of 10 μl of homogenate (50 μg/ml final protein concentration) to 90 μl of reaction buffer (final concentrations in mM: 125.0 ammonium acetate, 1.0 EDTA, 0.9 NAD, 1.0 ADP, 1.0 leucine, 20.0 l-glutamate and 50.0 triethanolamine, pH 7.5). Following incubation at 23°C for 15 min, the NADH formed was estimated by adding to the wells 100 μl of 50 μM 1-MPMS and after 30 s, 100 μl of a 1:1 mixture of 0.24 mM isoluminol in 0.8 M carbonate buffer, pH 9.5, and 1 μM m-POD. The chemiluminescence signal was measured for 5 s at 15 s after the addition of isoluminol and m-POD. Different concentrations of NADH in the absence of any enzyme were used to measure the relationship of light emitted to NADH concentration and these values were used to estimate the specific activity of GLUD in brain homogenates. When purified liver GLUD1 enzyme activity was being measured for the standardization of the assay method, the protein added to the reaction buffer was 1 μg/ml final concentration.
The protein levels for GLUD1 in brain homogenates were estimated by two different procedures, immunoblot assays following SDS/PAGE of brain proteins and ELISA of Glud1 in brain homogenates. These procedures were performed as previously described (Babcock et al., 1996; Pal et al., 2003; Bao et al., 2009) using affinity-purified anti-GLUD1 antibody (1:1000 dilution, Sigma–Aldrich).
Glutamate measurements using MEAs
Enzyme-based MEAs with Pt (platinum) recording surfaces were fabricated, assembled and calibrated for in vivo mouse recordings according to published literature (Hascup et al., 2006; Burmeister et al., 2000). For selective glutamate recordings, two of the MEA recording sites were coated with an l-glutamate oxidase (EC 1.4.3.11) (Seikagaku America, East Falmouth, MA, U.S.A.) coating solution as previously described (Nickell et al., 2005). The remaining two MEA recording sites (self-referencing or sentinel sites) were coated similar to the glutamate recording sites, with the exception that the coating solution did not contain l-glutamate oxidase. When the recorded sentinel site current was subtracted from the glutamate recording site current, the resulting signal represented the neuronal extracellular glutamate concentration (Burmeister and Gerhardt, 2001; Burmeister et al., 2002).
All Pt recording surface were electroplated with mPD (1,3-phenylenediamine) (5 mM; Acros Organics; Morris Plains, NJ, U.S.A.) using the FAST16mkII recording system as previously described (Stephens et al., 2009; Hinzman et al., 2010). This created an exclusion layer that restricts the passage of ascorbic acid, dopamine and 3,4-dihydroxyphenylacetic acid. Each MEA was calibrated prior to implantation as previously described (Nickell et al., 2005). A total of nine MEAs were used in the present study and they had an average (±S.E.M.) glutamate sensitivity of 7.5±0.3 pA/μM, selectivity ratio of 127±27 to 1, and LOD (limit of detection) of 0.6±0.2 μM based on a signal-to-noise ratio of 3.
The MEA assemblies for in vivo recordings consisted of an enzyme-based working electrode and a single-barrel glass micropipette (internal tip diameter, 12–15 μm) positioned at an equal distance from the four recording electrode sites (Burmeister and Gerhardt, 2001; Burmeister et al., 2002). Either an isotonic solution of 70 mM KCl (70 mM KCl, 79 mM NaCl and 2.5 mM CaCl2, pH 7.4) or a 5 mM glutamate solution (in physiological saline, pH 7.4) was loaded into the single-barrel glass micropipette. Glud1 and wt mice were anaesthetized (1.25 g/kg urethane, intraperitoneally) and placed in a stereotaxic frame fitted with a mouse adaptor in a manner similar to that described recently (Burmeister et al., 2002; Bao et al., 2009). A 200-μm-diameter Ag/AgCl reference electrode was implanted into the frontal cortex at a site remote from the recording areas. The MEA assemblies were lowered into the striatum in steps of 500 μm (Friedemann and Gerhardt, 1992) using the co-ordinates for mouse brain (from bregma: anteroposterior, +1.3; mediolateral, ±1.5; DV (dorsoventral), −2.25 mm to −3.75 mm; Franklin and Paxinos, 1997). Solutions were ejected from the glass micropipette using a Picospritzer II (Parker Hannifin). The volume ejected was monitored using a stereomicroscope fitted with a reticule (Friedemann and Gerhardt, 1992). Constant voltage amperometry was performed using a Fast Analytical Sensing Technology (FAST16mkII) electrochemistry instrument (Quanteon) and FAST software for four-channel simultaneous recordings (Burmeister and Gerhardt, 2001).
A constant volume of 70 mM KCl (50–150 nl) was used to elicit glutamate release in the striatum of both groups. This stimulus range was chosen since volumes above 150 nl did not increase the amount of measurable glutamate. Therefore, the stimulus was determined to elicit the maximal amplitude of glutamate release (peak concentration in micromolar). After a stable baseline at each recording depth in the striatum was reached (∼10 min), ten signals per recording site were obtained. Stimulations were locally applied 1 min apart to allow sufficient clearance of the released glutamate from the extracellular space. These signals were averaged into a single data point, thus generating one data point for each DV position and a maximum of four data points per pass in the striatum of either hemisphere.
Amperometric data, time and pressure ejection marks for each individual Pt recording site were saved in the recording system. A modified Excel spreadsheet, in conjunction with individual electrode calibration constants, was used to analyse the glutamate curves. For the calculation of uptake rate kinetics (micromolar per second), only amplitudes of exogenously applied 5 mM l-glutamate (in physiological saline; pH 7.4) between 3 and 13 μM were used in the analyses. Uptake followed first-order-rate kinetics; therefore the uptake rate constant (k−1) was the logarithmic slope of glutamate concentration versus time (s−1) estimated by use of regression analyses (R2≥0.9). Multiplication of k−1 with the maximum amplitude of glutamate signal yielded the uptake rate (micromolar per second).
Statistical analyses
Results from all assays were analysed using either one-way ANOVA with post-hoc pairwise analysis (Bonferroni) for multiple group comparisons, or by paired t-test for two group comparisons, as noted. For averaged glutamate release studies, a two-tailed t-test was used to compare Balb/c Glud1 wt and Tg mice. In addition, a two-way ANOVA followed by a Bonferroni post-hoc was used to analyse recording depth variations in resting glutamate, depolarization-induced glutamate release and clearance of glutamate. Results are shown as means±S.E.M. and significance was defined as P<0.05.
RESULTS
Selection of a mouse strain with low endogenous GLUD1 activity
Based on previous behavioural observations that C57BL/6 exhibit very different behavioural characteristics than Balb/c mice, we decided to include these two strains in a direct comparison of relative enzyme activities and levels of the brain GLUD1 protein. As a control for the effects of mouse inbreeding, we also added an outbred strain, the CD-1 mice. In order to enhance the sensitivity of the GLUD assay procedure, we used a chemiluminescence detection method of coupled reactions as described in the Methods section (Maeda et al., 1989; Tsuji et al., 1989). The limits of detection of NADH formed using the chemiluminescence procedure were approx. 1000-fold lower than those established using a fluorescence detection method (results not shown). Prior to the initiation of measurements of brain GLUD1 activity in the three mouse strains, the chemiluminescence procedure for the detection of glutamate oxidation and deamination by GLUD1 was validated using purified liver enzyme. The kinetic characterization of purified liver GLUD1 performed across different glutamate concentrations and in the presence of 0.9 mM NAD+ and the enzyme activators leucine and ADP (1 mM), led to an estimated Km for the enzyme equal to 8.4 mM. This value of the Km for glutamate of the purified liver GLUD1 measured at pH 7.5, was intermediate between the reported Km for the enzyme measured at pH 6 (Km = 22.7 mM) and that at pH 8 (Km = 4.9 mM) (Bailey et al., 1982). Thus, the chemiluminescence procedure appeared to be both sensitive and accurate in the detection of GLUD activity.
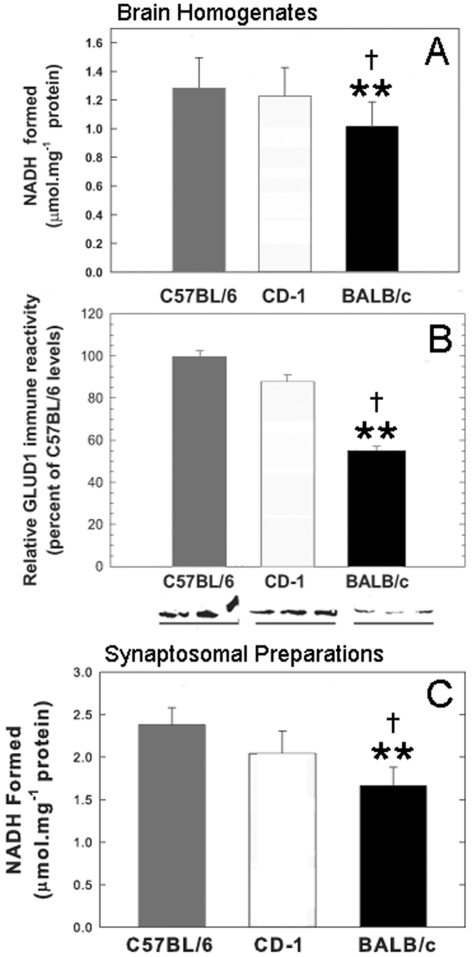
The enzyme activity in whole brain homogenates from C57BL/6, CD-1 and Balb/c, mice was measured at a single glutamate (20 mM) and NAD+ (0.9 mM) concentration, under conditions identical with those used for the liver enzyme, including the GLUD1 activators leucine and ADP. A high glutamate concentration was used so that the enzyme activity would approximate the Vmax for GLUD1 as determined in the studies with purified liver enzyme. The NAD+ concentration used was near that of the estimated Km for liver GLUD1 when measured in the presence of ADP (Bailey et al., 1982). The results from the measurements of GLUD1 activity (glutamate deamination and oxidation) in brain homogenates from the three mouse strains indicated significantly lower enzyme activity in Balb/c as compared with either C57BL/6 or CD-1 mice (Figure 1A). The outbred strain CD-1 had very similar GLUD1 activity to that of C57BL/6 (Figure 1A).
Figure 1. GLUD activity in C57BL/6, CD-1 and Balb/c mice.
GLUD activity in brain homogenates and isolated synaptosomes, and immune reactivity in brain homogenates from C57BL/6, CD-1 and Balb/c mice. (A) GLUD activity in brain homogenates from the three inbred strains. The results represent the means (±S.E.M.) of triplicate determinations obtained from five mice of each strain. Group comparison by one-way ANOVA indicated significant differences in GLUD activity of brain homogenates among the three strains (F = 13.41; P = 0.003). Pair-wise multiple comparisons of the three strains indicated significant differences (Bonferroni t-test) between the activity of homogenates from C57BL/6 and those from Balb/c, and between those from CD-1 and from Balb/c. The differences between C57BL/6 and CD-1 homogenates were not statistically significant. In all graphs, **P<0.01 for pair-wise statistical comparisons of Balb/c versus C57BL/6 mice, and †P<0.05 for comparisons of Balb/c versus CD-1 mice. (B) ELISA assays of immune reactivity of anti-GLUD1 antibodies with brain homogenates from the brains of the C57BL/6, CD-1 and Balb/c mice. Results represent the means±S.E.M. of relative immunoreactivity as compared with that of C57BL/6 (set as 100%). The results were from triplicate determinations with three mice in each inbred strain. Pair-wise statistical comparisons (t-test) indicated significant differences between the GLUD immune reactivity of C57BL/6 and Balb/c mice, and the immune reactivity of CD-1 and Balb/c mice. The differences in GLUD immune reactivity between C57BL/6 and CD-1 brain homogenates were not significant. Immunoblot demonstrations of differential levels of GLUD1 protein in the three inbred strains of mice are shown beneath this graph. Brain homogenates (30 μg protein per lane) from three mice from each strain were run on the same electrophoresis gel and labelled with anti-GLUD1 antibodies. Only one band of 60 kDa was labelled in all lanes containing brain samples. (C) GLUD activity in isolated synaptosomes from the brains of the three inbred strains. The results represent the means (±S.E.M.) of triplicate determinations obtained from three mice of each strain. Group comparison by one-way ANOVA indicated significant differences among the three strains (F = 22.24; P = 0.002). Pair-wise multiple comparisons of the three strains indicated significant differences (Bonferroni t-test) between C57BL/6 and Balb/c, and between CD-1 and Balb/c. The differences in GLUD activity between C57BL/6 and CD-1 synaptosomes were not significant.
The differences in GLUD1 activity might have been related to differential levels of the GLUD protein in the brains of Balb/c mice compared with those in C57BL/6 or CD-1. In order to explore this possibility, brain homogenates from C57BL/6, CD-1 and Balb/c mice were analysed by ELISA and immunoblot assays. Determination of the levels of immunoreactive protein by ELISA was indicative of significantly lower levels of GLUD1 protein in Balb/c brain homogenates than either those in C57BL/6 or CD-1 (Figure 1B). Once again, there was no statistically significant difference detected between C57BL/6 and CD-1 GLUD1 levels in brain homogenates. The results from the ELISA assays were confirmed by the observations made in immunoblot assays (Figure 1B). The antibody labelling of the band at 60 kDa corresponding to the GLUD1 protein in these blots was, again, significantly weaker in Balb/c mice compared with either C57BL/6 or CD-1 mice.
Using brain homogenates for the enzyme and immune assays of GLUD1 did not allow for the discrimination between neuronal and glial forms of GLUD1. In order to assess the activity of GLUD in a neuronal compartment, we isolated synaptosomes from brain homogenates of C57BL/6, CD-1 and Balb/c mice. Enzyme activity in synaptosomes was measured under conditions identical with those used for homogenates. Once again, GLUD activity in synaptosomes isolated from Balb/c mice was significantly lower than that of synaptosomes from C57BL/6 or CD-1 mouse brains (Figure 1C). The GLUD activity in the purified synaptosome fraction from C57BL/6 brains (2.38 μmol/mg of protein, Figure 1C) was substantially higher than that in whole brain homogenates from these mice (1.28 μmol/mg of protein, Figure 1A), an indication that GLUD activity was enriched in this fraction that represented the intraneuronal compartment of synapses.
Resting glutamate levels in Balb/c Glud1 Tg mice
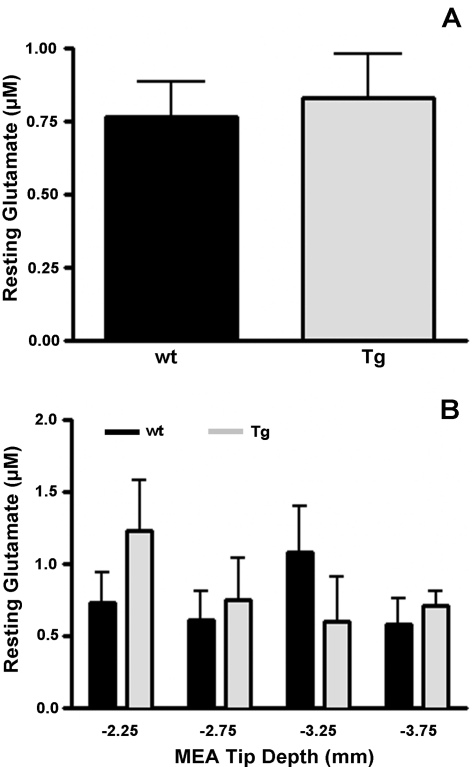
In order to determine changes in tonic levels (i.e., basal levels) of striatal glutamate in Balb/c wt and Tg mice, resting glutamate levels were calculated in each mouse group as previously described (Day et al., 2006; Rutherford et al., 2007; Hascup et al., 2008). Briefly, the self-referencing electrode current was subtracted from the glutamate recording electrode current (and divided by the glutamate recording site slope obtained from in vitro calibration). As shown in Figure 2(A), there was no difference in resting glutamate levels in Balb/c Glud1 wt (0.8±0.1 μM; n = 5) and Tg (0.8±0.2 μM; n = 4) mice. Because of the small size of our recording sites (333×15 μm), we were able to monitor resting glutamate levels in several DV positions in the striatum. As shown in Figure 2(B), this analysis indicated minor differences among the brain regions between Balb/c Glud1 wt and Tg mice, and a two-way ANOVA showed no effect of the mouse genotype at any recording depth.
Figure 2. Resting glutamate in the striatum of Balb/c Glud1 mice.
Resting glutamate levels were determined in wt (n = 5) and Tg (n = 4) Balb/c mice at four DV positions (−2.25 to −3.75 mm in 0.5 mm increments) prior to 70 mM KCl depolarization-induced glutamate release studies. No differences in resting glutamate levels were observed across the entire striatum (A) or at different DV positions within the striatum (B).
Depolarization-induced glutamate release
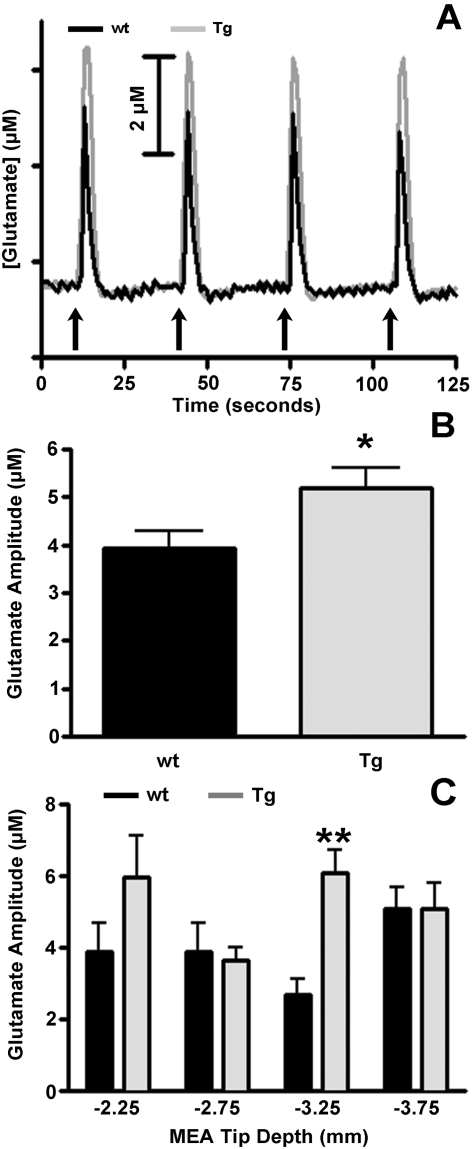
Local application of 70 mM KCl for 1–2 s in the striatum of Tg and wt mice produced robust, reproducible changes in extracellular glutamate that were consistently higher in Tg mice (Figure 3A). For consistency in data analysis, only glutamate signals elicited by ejections of 50–150 nl of 70 mM KCl were analysed to obtain the average maximum amplitude of glutamate release in the striatum of wt and Tg mice. Ejection volumes of KCl eliciting these signals were, on average, nearly identical in wt (111±4 nl) and Tg (116±3 nl) mice, yet the average maximum amplitude for glutamate release was significantly (P<0.05) greater in Tg (5.2±0.4 μM) than wt (3.9±0.4 μM) Balb/c mice (Figure 3B). Again, a depth analysis profile was performed and regional variations were observed between Balb/c wt and Tg mice (Figure 3C). Analysis of the data by two-way ANOVA indicated a statistically significant effect of genotype (F = 6.39, P = 0.01) as well as a significant effect of interaction (F = 2.85, P = 0.04). Bonferroni post-hoc analysis revealed that at DV −3.25 striatal glutamate release was significantly (P<0.01) greater in Balb/c Tg (6.1±0.7 μM) compared with wt (2.7±0.4 μM) mice.
Figure 3. Depolarization-induced glutamate release.
(A) Stimulus-evoked glutamate release in Balb/c wt (black) and Tg (grey) mice following pressure ejection of 50–150 nl of 70 mM KCl (pH 7.4) in the striatum as indicated by the arrows. Reproducible signals of glutamate release were obtained from both mouse groups, Signals of glutamate release from striatal neurons of Balb/c Glud1 Tg mice were consistently greater than those recorded from neurons of wt mice. (B) The average volumes of 70 mM KCl used to elicit depolarization-induced striatal glutamate release were: wt = 111±4 nl and Tg = 116±3 nl. These values did not differ significantly. The average maximal amplitude of KCl-induced glutamate release was significantly greater (*P<0.05) in the striatum of Balb/c Glud1 Tg (n = 4) mice compared with wt Balb/c mice (n = 5). (C) Depth-related alterations in average amplitude of stimulus-evoked glutamate release in the same two mouse groups as in (B). Analysis of data using two-way ANOVA with Bonferroni post-hoc indicated a significant (**P<0.01) increase in stimulus-evoked glutamate release at DV −3.25 in the Balb/c Glud1 Tg (n = 4) compared with glutamate release recordings at the same depth in wt littermates (n = 5).
Clearance of 5 mM l-glutamate
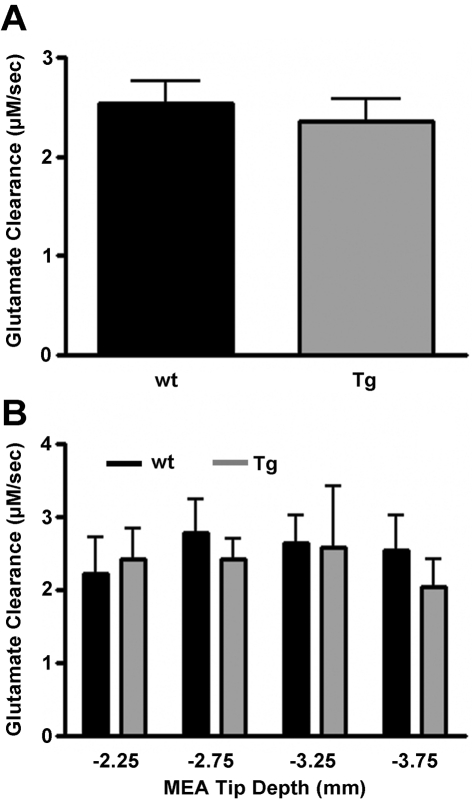
In order to assess the role that glutamate transport might play in the differential levels of glutamate release in striatum following depolarization, glutamate transport into neurons or glia in the vicinity of the MEAs was also estimated by measuring glutamate clearance after local application of nanolitre volumes of 5 mM l-glutamate. Since neurotransmitter uptake follows Michaelis–Menten kinetics (Nicholson, 1995) and is known to be affected by the amount of available substrate, glutamate clearance was analysed by selecting a subset of signals with maximum release values in the range of 3–13 μM (amplitude matched). This concentration range was chosen because pressure ejection peak glutamate values in this range were quite reproducible in both Balb/c wt (7.8±0.3 μM, n = 5) and Tg (8.3±0.3 μM, n = 4) mice. The volume of 5 mM l-glutamate solution ejected from the glass micropipette was also similar in Balb/c Glud1 wt (26±1 nl) and Tg (28±3 nl) mice. Within the subset of signals representing the concentration range selected, clearance rates of exogenously applied 5 mM l-glutamate were similar for wt (2.5±0.2 μM/s) and Tg (2.4±0.2 μM/s) mice (Figure 4A). Also, a depth analysis profile revealed no regional variation in the clearance of exogenously applied glutamate in the striatum (Figure 4B).
Figure 4. Clearance rate of exogenously applied 5 mM glutamate.
(A) The clearance rate of exogenously applied 5 mM l-glutamate to the mouse striatum was analysed by selecting a subset of signals with maximum release values in the range of 3–13 μM. Peak values were similar in both Balb/c wt (7.8±0.3 μM, n = 5) and Tg (8.3±0.3 μM; n = 4) mice as was the rate of clearance. (B) When a depth profile was performed, no differences were observed in the clearance rate of exogenously applied 5 mM l-glutamate between wt and Tg Glud1 Balb/c mice.
Depolarization-induced glutamate release in Balb/c compared with C57BL/6 mice
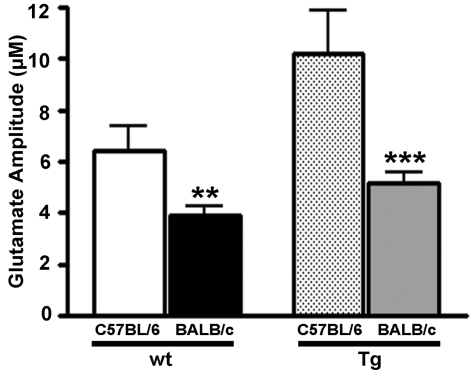
Since the main focus of the present study was to determine whether the background strain of mice to which the founder Glud1 Tg mice were back-crossed had any effects on synaptic glutamate formation and release in vivo, we directly compared depolarization-induced glutamate release in wt and Tg Balb/c and C57BL/6 mice. We previously showed that overexpression of the Glud1 gene in mice back-crossed to C57BL/6 mice significantly increases depolarization-induced glutamate release in these Tg mice compared with wt littermates (Bao et al., 2009). Again, for consistency in data analysis, only glutamate signals elicited by ejections of 50–150 nl of 70 mM KCl in the striatum of Balb/c and C57BL/6 Tg and wt mice were analysed to obtain the average maximum amplitude of glutamate release in all groups (volume matched). Volumes of the KCl stimulus were similar in Balb/c wt (111±4 nl) to those in C57BL/6 wt (117±4 nl), and the same was true for Balb/c Tg (116±3 nl) and C57BL/6 Tg (120±6 nl) mice. The C57BL/6 mice released significantly more glutamate compared with the Balb/c mice (Figure 5). A two-tailed t-test indicated that depolarization-induced glutamate release was significantly lower in the Balb/c mice, either wt or Tg (results presented above) when compared with the glutamate release values of the respective genotype of C57BL/6 mice: wt, 6.4±1.0 μM, n = 9; P<0.01; and Tg: 10.2±1.7 μM; n = 5; P<0.001. In other words, the Balb/c wt and Glud1 Tg mice released 51–61% of the amount of glutamate released following stimulation of striatal neurons in C57BL/6 wt and Glud1 Tg mice respectively.
Figure 5. Depolarization-induced glutamate release in Balb/c versus C57BL/6 Glud1 mice.
The average volume of 70 mM KCl used to elicit striatal glutamate release was similar in Balb/c wt (111±4 nl; n = 5) and Tg (116±3 nl; n = 4) mice, as well as in C57BL/6 wt (117±4 nl; n = 9) and Tg (120±6 nl; n = 5). Depolarization-induced glutamate release was decreased in Balb/c Glud1 wt (3.9±0.4 μM) and Tg (5.2±0.4 μM) mice compared with C57BL/6 Glud1 wt (6.4±1.0 μM) and Tg (10.2±1.7 μM) mice. **P<0.01, ***P<0.001.
DISCUSSION
The observations made in our current studies that the Balb/c mouse neurons had significantly lower endogenous levels of GLUD enzyme activity and GLUD1 protein in comparison with either C57BL/6 or an outbred strain, CD-1, are consistent with the results of a recent proteomic analysis of differential protein levels in the hippocampus of five inbred strains of mice (Pollak et al., 2010). In that study, it was shown that Balb/c mice had the lowest GLUD1 protein levels of all strains examined (Pollak et al., 2010). Thus the differences we detected between Balb/c and C57BL/6 in the levels of GLUD1 protein in whole brain homogenates or synaptosomes appear to be a molecular characteristic of this strain of mice. Our findings were indicative of a diminished function of total GLUD enzyme activity in whole brain and in synaptosomes of Balb/c versus C57BL/6 or CD-1 mice. The low GLUD activity and low GLUD1 protein levels thus led us to select the Balb/c mouse strain for the back-crossing of the Glud1 Tg mouse founders. The procedures used for back-crossing and genotyping of mice were identical for both the C57BL/6 and the Balb/c mice (Bao et al., 2009). The endophenotypic effects of overexpression of the Glud1 gene on brain function in the Glud1 Tg Balb/c mice were assessed as the change in glutamate release following KCl depolarization of neurons.
Resting glutamate levels in Balb/c Glud1 wt and Tg mice were nearly identical, suggesting that the overexpression of neuronal Glud1 had no effect on the tonic levels of extracellular glutamate. Unfortunately, these values may be misleading owing to the potential effects of urethane anaesthesia on tonic levels of glutamate in vivo. Urethane exerts its effects predominantly through neurotransmitter-gated ion channels to enhance inhibitory synaptic neurotransmission as well as inhibit excitatory neurotransmission (Hara and Harris, 2002). Our laboratory has observed that resting glutamate levels in brain are attenuated by 60–80% in urethane-anaesthetized animals compared with freely moving rats (Rutherford et al., 2007). In fact, the tonic levels of glutamate reported in the present study were reduced by ∼80% compared with previously reported resting glutamate values in awake, freely moving mice (∼5 μM; Hascup et al., 2008). These results are indicative of the need to exercise caution when interpreting tonic levels of glutamate (or other neurotransmitters) in anaesthetized animals.
Pressure ejection of 70 mM KCl elicited robust and reproducible glutamate release in the striatum of both Balb/c and C57BL/6 mice. This release was significantly increased in the Balb/c Glud1 Tg versus wt mice, a finding that was similar to the previously observed increases in depolarization-induced glutamate release in Glud1 Tg C57BL/6 mice (Bao et al., 2009). A depth analysis profile revealed that this increase in glutamate release occurred at a particular location within the striatum, at DV −3.25 mm. Importantly, at this same recording depth we did not observe any differences in the clearance of exogenously applied 5 mM l-glutamate between Glud1 Balb/c wt and Tg mice. This indicates that the increase in depolarization-induced glutamate release was not the result of altered clearance characteristics in the Tg mice.
When we compared the depolarization-induced glutamate release in Balb/c mice with previously reported values for the C57BL/6 Glud1 mice (Bao et al., 2009), an interesting trend emerged. Using similar volumes of stimulus, the average maximal amplitude of depolarization-induced glutamate release in Balb/c wt and Glud1 Tg mice was 50–60% of the amount released in C57BL/6 mice. This lower level of evoked glutamate release corresponded to the decreased endogenous GLUD1 protein levels and GLUD activity that we observed in Balb/c compared with C57BL/6 mice.
One of the most intriguing findings from the current studies was the observation that depolarization-induced glutamate release was localized to the DV layer −3.25 within the striatum, even though GLUD1 overexpression occurred in all neurons in the brain (Bao et al., 2009). Unfortunately, limited data exist for the neuroanatomical connections of the mouse brain (Bohland et al., 2009), particularly a region that lacks clear cytoarchitecture such as the striatum (Zeiss, 2005), thus making interpretation of the results obtained from this specific sub-region difficult. In mouse brain, it has previously been shown that glutamatergic afferents to the striatum originate in either the cortex (60%) or the thalamus (40%) and may form synaptic contacts on the same medium spiny neurons, yet it is the thalamostriatal neurons that have a higher release probability as compared with corticostriatal inputs (Ding et al., 2008). At DV −3.25, we may have recorded from a unique proportion of these thalamostriatal neuronal terminals and that these terminals might be the ones that were selectively altered in the Balb/c Glud1 Tg mice, resulting in the increased glutamate release.
A particular strength of using MEAs, especially compared with other in vivo recording techniques such as microdialysis, is the capability afforded by these electrodes to investigate glutamate temporal dynamics at several recording depths of a layered structure, such as the striatum. The heterogeneity of multi-layered brain structures has been well characterized in a number of studies using electrochemical methods. Using carbon-fibre electrodes, Friedemann and Gerhardt (1992) demonstrated a DV striatal gradient with respect to the amount of potassium stimulation needed to evoke dopamine release. Nickell et al. (2007) showed that the glutamate clearance time was increased in the dorsal versus ventral striatum as a function of aging in the Fischer 344 rat. More recently, Stephens et al. (2009) showed significant changes in stimulus-evoked release and re-uptake of glutamate in different hippocampal subregions with respect to aging in the Fischer 344 rat. Collectively, these results support the idea that nerve terminal projections within a specific brain structure are not homogeneous in terms of distribution and/or excitability, and it is necessary to analyse multiple depths and not simply infer that results obtained from a single layer are representative of the structure as a whole (Nickell et al., 2007).
The primary finding of our study was that Balb/c Glud1 Tg mice had significantly increased depolarization-induced glutamate release compared with wt littermates, which was similar to the results obtained with the C57BL/6 mice (Bao et al., 2009). These observations were a clear indication that introduction of the Glud1 transgene in neurons of phenotypically and endophenotypically different strains of mice had very similar effects on neuronal release of glutamate. Furthermore, these results provide strong evidence for the importance of the Glud1 gene in synaptic glutamate neurotransmission. The development of this Glud1-overexpressing mouse model may allow for the exploration of issues such as how chronic exposure of neurons to elevated glutamate levels can affect the control of various behavioural processes and of key molecular events that are hypothesized to occur during aging and in neurodegenerative disorders. While no behavioural changes were observed with the mice used in the present study, further experiments are being conducted to determine any behavioural phenotypes elicited by overexpression of Glud1 with respect to age.
ACKNOWLEDGEMENTS
We thank A. Benremouga for the conduct of the glutamate dehydrogenase assays. Dr Greg A. Gerhardt is the sole proprietor of Quanteon.
Footnotes
This work was supported by the NSF (National Science Foundation) [grant number EEC-0310723]; NIH/NIDA (National Institutes of Health/National Institute on Drug Abuse) [grant number DA017186]; CEBRA, Phase II, NIA, [grant number AG12993]; NIAAA (National Institute of Alcohol Abuse and Alcoholism) [grant numbers AA11419, AA04732, AA12276]; NSF [grant numbers DBI-9987807, DBI-0352848]; NIDA [grant number DA017186]; NINDS (National Institute of Neurological Disorders and Strokes) [grant number NS39787]; NIMH (National Institute of Mental Health) [grant number MH58414]; NIDA Training [grant number DA022738]; NIDA [grant number DA015088], The Kansas Technology Enterprise Corporation, The Miller, Hedwig and Wilbur Fund, and The University of Kansas Research Development Fund.
REFERENCES
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Babcock KK, Chen X, Eggeman KT, Kumar KN, Decedue CJ, Michaelis EK. A synaptic membrane glycine-, glutamate- and thienylcyclohexylpiperidine-binding protein: isolation and immunochemical characterization. Neurochem Int. 1996;29:507–519. doi: 10.1016/0197-0186(96)00019-8. [DOI] [PubMed] [Google Scholar]
- Bailey J, Bell ET, Bell JE. Regulation of bovine glutamate dehydrogenase. The effects of pH and ADP. J Biol Chem. 1982;257:5579–5583. [PubMed] [Google Scholar]
- Bao X, Pal R, Hascup KN, Wang Y, Wang WT, Xu W, Hui D, Agbas A, Wang X, Michaelis ML, Choi IY, Belousov AB, Gerhardt GA, Michaelis EK. Transgenic expression of Glud1 (glutamate dehydrogenase 1) in neurons: in vivo model of enhanced glutamate release, altered synaptic plasticity, and selective neuronal vulnerability. J Neurosci. 2009;29:13929–13944. doi: 10.1523/JNEUROSCI.4413-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Wu C, Barbas H, Bokil H, Bota M, Breiter HC, Cline HT, Doyle JC, Freed PJ, Greenspan RJ, Haber SN, Hawrylycz M, Herrera DG, Hilgetag CC, Huang ZJ, Jones A, Jones EG, Karten HJ, Kleinfeld D, Kotter R, Lester HA, Lin JM, Mensh BD, Mikula S, Panksepp J, Price JL, Safdieh J, Saper CB, Schiff ND, Schmahmann JD, Stillman BW, Svoboda K, Swanson LW, Toga AW, Van Essen DC, Watson JD, Mitra PP. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Comput Biol. 2009;5:e1000334. doi: 10.1371/journal.pcbi.1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Cotman CW. NMDA receptor regulation of neuronal morphology in cultured hippocampal neurons. Neurosci Lett. 1989;99:268–273. doi: 10.1016/0304-3940(89)90458-8. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of l-glutamate and other analytes. Anal Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of l-glutamate in the CNS. J Neurosci Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Coates TD, Gerhardt GA. Multisite microelectrode arrays for measurements of multiple neurochemicals. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5348–5351. doi: 10.1109/IEMBS.2004.1404493. [DOI] [PubMed] [Google Scholar]
- Cavallaro S, Meiri N, Yi CL, Musco S, Ma W, Goldberg J, Alkon DL. Late memory-related genes in the hippocampus revealed by RNA fingerprinting. Proc Natl Acad Sci USA. 1997;94:9669–9673. doi: 10.1073/pnas.94.18.9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Cunningham MD, Galton N, Michaelis EK. Immune labeling and purification of a 71-kDa glutamate-binding protein from brain synaptic membranes. Possible relationship of this protein to physiologic glutamate receptors. J Biol Chem. 1988;263:417–426. [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic differences in locomotor activation in mice. Pharmacol Biochem Behav. 1986;25:289–292. doi: 10.1016/0091-3057(86)90267-4. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berlin) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Paya-Cano JL, Sluyter F, D'Souza U, Plomin R, Schalkwyk LC. Hippocampal gene expression profiling across eight mouse inbred strains: towards understanding the molecular basis for behaviour. Eur J Neurosci. 2004;19:2576–2582. doi: 10.1111/j.0953-816X.2004.03358.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos GT. San Diego, CA: Academic Press; 1997. The Mouse Brain in Sterotaxic Coordinates, [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: The effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hascup ER, af Bjerkén S, Hascup KN, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of l-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther. 2008;324:725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Gerhardt GA. Second-by-second measures of l-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. In: Michael A C, Borland L M, editors. In Electrochemical Methods for Neuroscience. Boca Raton, FL: CRC Press; 2006. pp. 407–450. [PubMed] [Google Scholar]
- Hinzman JM, Thomas TC, Burmeister JJ, Quintero JE, Huettl P, Pomerleau F, Gerhardt GA, Lifshitz J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J. Neurotrauma. 2010;27:889–899. doi: 10.1089/neu.2009.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Maeda M, Arakawa H, Tsuji A. Chemiluminescent assay of various enzyme activities and its application to enzyme immunoassays. J Biolumin Chemilumin. 1989;4:140–148. doi: 10.1002/bio.1170040121. [DOI] [PubMed] [Google Scholar]
- Mathis C, Paul SM, Crawley JN. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Genet. 1994;24:171–180. doi: 10.1007/BF01067821. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis EK, Michaelis ML, Chang HH, Kitos TE. High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. J Biol Chem. 1983;258:6101–6108. [PubMed] [Google Scholar]
- Nicholson C. Interaction between diffusion and Michaelis–Menten uptake of dopamine after iontophoresis in striatum. Biophys J. 1995;68:1699–1715. doi: 10.1016/S0006-3495(95)80348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked l-glutamate release in the striatum of Fischer 344 rats. J Neural Transm. 2005;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Pal R, Agbas A, Bao X, Hui D, Leary C, Hunt J, Naniwadekar A, Michaelis ML, Kumar KN, Michaelis EK. Selective dendrite-targeting of mRNAs of NR1 splice variants without exon 5: identification of a cis-acting sequence and isolation of sequence-binding proteins. Brain Res. 2003;994:1–18. doi: 10.1016/j.brainres.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Palumbo ML, Canzobre MC, Pascuan CG, Rios H, Wald M, Genaro AM. Stress induced cognitive deficit is differentially modulated in BALB/c and C57Bl/6 mice: correlation with Th1/Th2 balance after stress exposure. J Neuroimmunol. 2010;218:12–20. doi: 10.1016/j.jneuroim.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Latsoudis H, Kanavouras K, Ritz B, Bronstein JM, Skoula I, Mastorodemos V, Papapetropoulos S, Borompokas N, Zaganas I, Xiromerisiou G, Hadjigeorgiou GM, Spanaki C. Gain-of-function variant in GLUD2 glutamate dehydrogenase modifies Parkinson's disease onset. Eur J Hum Genet. 2010;18:336–341. doi: 10.1038/ejhg.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, John J, Hoeger H, Lubec G. An integrated map of the murine hippocampal proteome based upon five mouse strains. Electrophoresis. 2006;27:2787–2798. doi: 10.1002/elps.200500648. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Bae N, Mostafa G, Hoeger H. Strain-dependent expression of metabolic proteins in the mouse hippocampus. Amino Acids. 2010;39:1451–1462. doi: 10.1007/s00726-010-0609-0. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.009. doi:10.1016/j.neurobiolaging. 2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa N, Sugimoto Y, Yamada J, Kobayashi Y. Strain differences of neurosteroid levels in mouse brain. Steroids. 2006;71:776–784. doi: 10.1016/j.steroids.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Maeda M, Arakawa H. Chemiluminescent assay of co-factors. J Biolumin Chemilumin. 1989;4:454–462. doi: 10.1002/bio.1170040160. [DOI] [PubMed] [Google Scholar]
- Zeiss CJ. Neuroanatomical phenotyping in the mouse: the dopaminergic system. Vet Pathol. 2005;42:753–773. doi: 10.1354/vp.42-6-753. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]