INTRODUCTION
Bilateral subthalamic (STN) deep brain stimulation (DBS) provides symptom relief for the majority of well-screened advanced Parkinson’s disease (PD) patients.1 However, we have recently shown that bilateral STN DBS may result in significant declines in cognitive–motor performance of PD patients.2 The spread of current to non-motor areas of the STN may be responsible for cognitive and cognitive–motor declines.
While guidelines exist on stimulation parameter settings that are typically effective, it is not practical to evaluate each of the thousands of stimulation parameter combinations possible. Therefore, the therapeutic benefit achieved with DBS is dependent on the intuitive skill and experience of the programming clinician. To assist the programming process, we developed Windows-based software tools that enable 3D visualisation of the volume of tissue activated (VTA) by DBS.3
The goal of this study was to compare two methods of DBS programming, the typical clinical method and our computational approach, on cognitive–motor performance in an advanced PD patient.
CASE REPORT
A 58-year-old right-handed male with an 8-year history of PD underwent simultaneous bilateral STN-DBS 14 months prior to study participation. His stimulation parameters were optimised by traditional clinical methods and were stable for the 6 months prior to study participation. This patient did not have any history of cognitive deficits or postoperative changes in cognitive function based on neuropsychological testing. Blinded UPDRS-III evaluations were performed Off DBS and On DBS using previously determined clinical DBS parameters while the patient was off antiparkinsonian medication for 12 h. Under these conditions, the patient demonstrated a 45% reduction (improvement) in the UPDRS III with DBS on when compared with off.
Cognitive–motor performance was quantified using a dual-task paradigm.2 The dual-task consists of the simultaneous performance of a working memory task (n-back) and force-tracking task with the dominant limb. The patient completed 15 dual-task trials in each of the three stimulation conditions. Paired t tests were used to assess statistical significance.
A patient-specific computational model of lead location and volume of tissue activation (VTA) for the research subject was developed with Cicerone3 that included coupled integration of magnetic resonance imaging data, intraoperative microelectrode recording data, 3D brain atlases, DBS electrode contact location and VTA predictions all coregistered into the neurosurgical stereotactic coordinate system4,5 (figure 1). The model was created without knowledge of the clinical stimulation parameters. The VTA predictions were used to define stimulation parameter settings for both sides of the brain to maximise current spread into the dorsal STN and white matter dorsal to this region of the STN, areas associated with optimised therapeutic benefit,5 while minimising current spread into non-motor portions of the STN.
Figure 1.
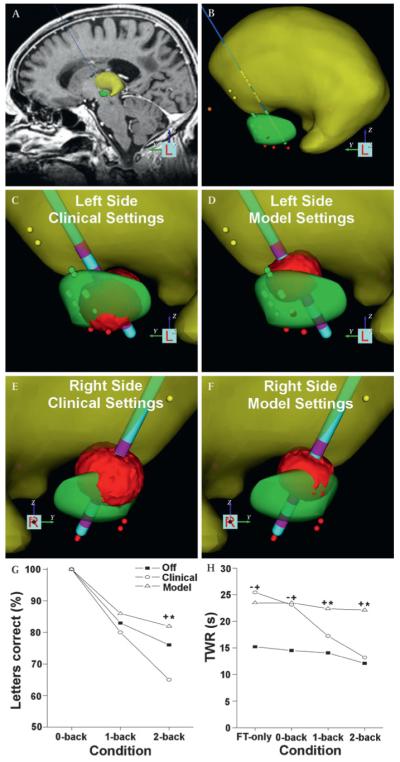
Patient-specific model of deep brain stimulation and dual-task performance. (A) Stereotactic coordinate system was defined using fiducial markers of the neurosurgical frame in the imaging data, and a 3D brain atlas was fitted to match the patient’s neuroanatomy (yellow volume–thalamus; green volume–subthalamic nucleus). (B) Stereotactic locations of intraoperative microelectrode recordings were entered into the model (thalamic cells–yellow dots; subthalamic cells–green dots; substantia nigra cells–red dots). The DBS electrodes were positioned based on stereotactic coordinates and their anatomical locations were verified by postoperative imaging data. (C) Left-side clinical settings: contact 1, 3.2 V, 0.06 ms, 185 Hz. Contact 1 was 13.7 mm lateral (L), 2.2 mm posterior (P) and 3.0 mm ventral (V) to the mid-commisural point (MCP). (D) Left-side model settings: contact 2, 2.4 V, 0.06 ms, 130 Hz. Contact 2 was at 14.5 L, 0.7 P, 0.5 V mm to MCP. (E) Right-side clinical settings: contact 2, 3.2 V, 0.06 ms, 185 Hz. Contact 2 was at 14.8 L, 0.9 P, 0.7 V mm to MCP. (F) Right-side model settings: contact 2, 2 V, 0.06 ms, 130 Hz. (G) Results of the n-back task during dual-task condition while off DBS (filled squares), clinical parameter settings (open circles) and model defined parameter settings (open triangles). (H) Results of the time within ±2% of the target range (TWR) during single task force-tracking only (FT only) and dual-task (FT+n-back task) while off DBS, clinical parameters and model defined parameters. A cross designates a significant difference between model-derived and off DBS, an asterisk marks a significant difference between model and clinical DBS, and a dash represents a significant difference between clinical DBS and off DBS.
Model defined VTAs were compared with the clinically determined VTAs. On the left side, the clinical settings used contact 1 (figure 1C) while the model selected contact 2 (figure 1D). On the right side, both the model and clinical settings used contact 2 (figure 1E,F). For both sides, the model suggested a lower stimulation amplitude and frequency, resulting in smaller VTAs.
Three weeks after the initial study visit, clinical and laboratory testing were repeated using model-derived parameters. Upon arrival, both stimulators were turned off for 2 h. Stimulators were turned on and reprogrammed using model-derived parameters. After 60 min with model-derived parameters, clinical and dual-task tests were repeated. Because Cicerone software is a research tool, following study completion the patient’s stimulators were returned to the clinical settings.
Model-derived parameters resulted in a 43% decrease in the UPDRS-III score compared with off DBS. During the simple dual-task condition (FT+0-back), n-back performance was 100% across stimulation settings (figure 1G). As task difficulty increased, working memory declined significantly while the patient was off DBS and under clinical stimulation parameters compared with model settings (p<0.001). The accuracy of the patient’s force tracking was determined by calculating their time spent within ±2% of the target range (TWR) (figure 1H). Model-derived parameters resulted in significantly better (p<0.001) force tracking during the 1- and 2-back dual-task conditions than clinical DBS. With the clinical settings, TWR during the 2-back dual-task condition was similar to force-tracking while off DBS. With model-derived parameters, motor performance was relatively consistent despite an increase in task difficulty.
DISCUSSION
The aim of this case study was to assess cognitive–motor performance during clinical and model-derived stimulation settings. Both methods of programming resulted in similar improvements in clinical motor ratings of the patient. However, under modestly demanding dual-task conditions, clinical parameters resulted in poorer cognitive (working memory) and motor (force-tracking) performance compared with model-derived parameters. In fact, in the most difficult dual-task condition, force-tracking performance was similar while the patient was on DBS with clinical parameters and off DBS. These cognitive–motor declines observed with clinical parameters may be the result of current spread to non-motor areas of the STN.
Clinical parameter settings from the left electrode generated a VTA that was concentrated on the central STN. This location, while providing clinical benefit, incorporated the ventromedial non-motor portions of the STN. When task demands increased to include a cognitive and motor component, similar to the context in which ADLs are performed, performance deteriorated. Given its small size, stimulation within the STN can result in spread of current to limbic and associative areas as well as to surrounding structures and fibre systems that may affect cognitive function.4 These preliminary findings support the hypothesis that the activation of non-motor pathways during STN DBS may contribute to declines in cognitive functioning which compromise motor performance.
Disruption of information processing in the non-motor regions of STN and adjacent areas may be responsible for the varying levels of decline reported in cognitive functioning during STN DBS. This disruption may not produce a detectable deficit in cognitive function when patients are able to focus their attention on the performance of a single cognitive or motor task, as is the case during typical clinical examinations. As information processing demands increase, patients may attempt to draw on cognitive resources that are compromised as a result of the stimulation induced disruption of non-motor pathways and a loss of redundancy of these circuits with bilateral DBS. Preliminary data suggest that using visualisation software to augment the stimulation parameter selection process can mitigate cognitive–motor declines without compromising clinical improvements.
Acknowledgments
Funding This project was supported by the National Institutes of Health (R03 AG022178, R03 NS037959, R01 NS059736) and the WH Coulter Foundation.
Footnotes
Competing interests CCM authored intellectual property related to the project methodology and holds company shares in IntElect Medical Inc. CCM and AMN are paid consultants for IntElect Medical Inc.
Patient consent Obtained.
Ethics approval Ethics approval was provided by the Cleveland Clinic.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240–9. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 2.Alberts JL, Voelcker-Rehage C, Hallahan K, et al. Bilateral subthalamic stimulation impairs cognitive–motor performance in Parkinson’s disease patients. Brain. 2008;131(Pt 12):3348–60. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miocinovic S, Noecker AM, Maks CB, et al. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97(Pt 2):561–7. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- 4.Butson CR, Cooper SE, Henderson JM, et al. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–70. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maks CB, Butson CR, Walter BL, et al. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry. 2009;80:659–66. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]


