Abstract
Species differ widely with regard to parental investment strategies and mechanisms underlying those strategies. The passing of benefits to likely offspring can be instantiated with a number of different computational and behavioral systems. We report results from an agent-based model in which offspring maintain proximity with parents and parents transmit benefits to offspring without the capacity of either parent or offspring to ‘recognize’ one another. Instead, parents follow a simple rule to emit benefits after reproducing and offspring follow a simple rule of moving in the direction of positive benefit gradients. This model differs from previous models of spatial kin-based altruism in that individuals are modeled as having different behavioral rules at different life stages and benefits are transmitted unidirectionally from parents to offspring. High rates of correctly directed parental investment occur when mobility and sociality are low and parental investment occurs over a short period of time. We suggest that strategies based on recognition and bonding/attachment might serve to increase rates of correctly directed parental investment under parameters that are shown here to otherwise lead to high rates of misdirected and wasted parental investment.
Keywords: conditional movement, parental investment, kin recognition, parental care, agent-based model
INTRODUCTION
A wide range of taxonomic groups show evidence of parental investment (Kleiman and Malcom 1981; Clutton-Brock 1991; Gubernick and Klopfer 1981). In these taxa, parental investment takes a variety of forms, and the nature and pattern of these forms has been fairly well characterized (Gubernick and Klopfer 1981; Ross 2003; Kolliker 2007; Quinlan 2007; Steinegger and Taborsky 2007; Maurer 2008; Wolovich et al. 2008; Fernandez-Duque et al. 2009; Mattle and Wilson 2009). For some taxa there is also solid evidence suggesting that the behavioral tendencies leading to the transmission of benefits to offspring have a genetic basis and will therefore be favored by natural selection (Kappeler and Schaik 2005; Chapais and Berman 2004; Kokko and Jennison 2008; Charpentier et al. 2008). This is in support of some of the initial formulations of parental investment theory proposing that natural selection will favor the passing of benefits to offspring and kin when the costs of doing so are relatively low, the benefits relatively high and the likelihood of relatedness high enough (Hamilton 1964a; Hamilton 1964b; Trivers 1972; Maynard Smith 1977).
Even when ultimate evolutionary explanations for the existence of parental investment may be satisfying for a given taxon, it is usually the case that we lack a similar understanding of the mechanisms and processes regulating parental investment. Any instance of parental investment must be somehow instantiated by proximate cognitive and behavioral mechanisms that enable parents to transmit benefits to offspring. For example, kin recognition mechanisms such as phenotypic matching, the presence of recognition alleles and learning of kin characteristics could enable selective transmission of benefits to offspring (Hepper 1991). Each of these requires relatively complex cognitive and behavioral capacities. Still, it is possible that under certain circumstances, parental investment can be correctly directed to offspring in the absence of recognition abilities or other complex cognitive processes. In other words, there may be simple mechanisms leading to adaptive parental investment that do not require kin recognition.
Proximity and parental investment
Spatial proximity between parents and offspring has the potential to function as a relatively simple mechanism for adaptive parental investment. The transmission of benefits from parents to offspring typically requires spatial proximity between them. Incubation, lactation, regurgitation, food sharing, carrying, and protection are all forms of parental investment requiring that offspring and parents maintain proximity (Kleiman and Malcom 1981; Clutton-Brock 1991).
The recognition of the importance of spatial proximity in parental behavior is not new. The maintenance of spatial proximity between offspring and caretaker is the central concept in attachment theory and is a well-described phenomenon. In attachment theory, one of the main benefits of maintaining spatial proximity is thought to be increased protection from predators (Bowlby 1969; Mason and Mendoza 1998). It has also been noted that spatial proximity can enable the selective transmission of benefits to offspring, such as food, warmth and various kinds of sensory stimulation (Gubernick and Klopfer 1981).
However, offspring might not always be near, and the indiscriminate provision of benefits to those who happen to be nearby can lead to misdirected or wasted parental investment. Among group living species and those with high mobility, there may be more opportunities for ‘mistakes’ in the transmission process so that benefits are transmitted to non-offspring. It has been suggested that the long-term social recognition associated with ‘attachment’ might function as a mechanism to reduce those potential errors (Gubernick and Klopfer 1981). Likewise, in species with lower mobility or those that are less social, mechanisms of social attachment might be unnecessary for the effective and accurate transmission of benefits to offspring.
Here we ask whether the ability to maintain spatial proximity can enable high levels of correctly directed parental investment, even in the absence of cognitive complexity. It is true that proximity can be maintained through a variety of complex behavioral and cognitive mechanisms including recognition systems, bonding, attachment, clinging/carrying abilities and other capacities, but these complex systems may not be necessary for accurate parental investment. We explore this possibility here.
In this model, we instantiate a very simple decision rule that maintains parent-offspring proximity: offspring follow the gradient of benefits emitted by their parents. Our model allows us to explore whether this very simple rule that does not involve kin recognition or memory can lead to correctly directed parental investment. Further, we explore the limits of this rules’ viability by exploring its performance under a variety of ecological and social conditions. By identifying conditions under which this simple rule does not lead to correctly directed parental investment, we gain some understanding of the ecological and social conditions that are likely to favor the evolution of more complex rules for parental investment.
Models of parental investment and altruism
A variety of primarily qualitative theoretical models of the evolution of parental investment have been proposed which have focused on the viability of parental investment and strategic concerns associated with bi-parental care (Trivers 1972; Maynard Smith 1977; Dawkins and Carlisle 1976; Lancaster and Lancaster 1983; Hamilton 1984; Kurland and Gaulin 1984; Kokko and Jennison 2008; Kokko, Jennions, and Brooks 2006). Qualitative and mathematical models such as these have provided traction for understanding the cost-benefit tradeoffs inherent in parental investment decisions, but are less effective for exploring the viability of various mechanisms that might underlie parental investment. On the other hand, simulations provide a way of exploring and testing the functional and evolutionary viability of various mechanisms that can underlie behaviors such as parental investment.
Despite a relative dearth of spatial simulations of the viability of parental investment per se (Lion and van Baalen 2007), a number of models of the evolution of cooperation/altruism have addressed topics that are relevant to the evolution of parental care. Simulations have, for example, investigated the viability of cooperative behavior in populations with various kinds of mobility (Aktipis 2004; Brauchli, Killingback, and Doebeli 1999; Enquist and Leimar 1993; Ferriere and Michod 1996; Marshall and Rowe 2003; Mitteldorf and Wilson 2000) and in situations where individuals can use social recognition memory (Vos and Zeggelink 1994; Cox, Sluckin, and Steele 1999; Aktipis 2006). If parental investment were considered a type of altruism, models such as these could be used to draw conclusions about the viability of parental investment. For instance, cooperative/altruistic strategies tend to be successful in stable population structures and when individuals can use recognition memory. If these same principles were valid in relationship to the passing of benefits from parents to offspring, mobility and recognition would play important roles in parental investment strategies.
Modeling parental investment as a form of altruism or cooperation requires certain considerations and assumptions. Offspring are typically the recipients of benefits and might never ‘reciprocate’ by passing benefits back to a parent. This unidirectional passing of benefits is not captured by traditional models of the evolution of cooperation, and other potential differences between parents and offspring are abstracted away in most models. The maintenance of spatial proximity is also an important component of parental investment strategies that is only peripherally captured by spatial (e.g., Brauchli, Killingback, and Doebeli 1999; Nowak and May 1992; Ifti, Killingback, and Doebeli 2004) and network models (e.g., Ohtsuki et al. 2006; Lehmann, Keller, and Sumpter 2007; Taylor, Day, and Wild 2007) of the evolution of cooperation. Models have also explored the ways in which spatial structure and proximity emerge from the interactions of organisms with each other and with a shared environment (Ramos-Fernández, Boyer, and Gómez 2006).
In the following pages, we present an agent-based model that investigates the effectiveness of a parental investment system that does not make use of social recognition, but relies instead on relatively simple rules. Using an agent-based model based on simple rules that promote proximity maintenance between parents and offspring, we explore whether those rules lead to correctly directed parental investment under certain social and ecological conditions. We use a spatial agent-based model in order to allow for spatial interactions between parents and offspring. This model allows us to explore the viability of simple proximity maintenance mechanisms that can promote correctly directed parental investment. By exploring parameters under which these simple rules for parent/offspring behavior can generate effective parental investment and those under which it falters, it becomes possible to outline the ecological and social factors that might favor more complex systems for directing parental investment.
Specifically, offspring use conditional movement rules to maintain proximity to entities (parents) emitting benefits. It has been suggested that conditional movement may be considered one of the fundamental building blocks of behavior, operating on the most basic types of information that are available in an organism’s environment (Aktipis 2008). Here we apply those principles to the examination of parent-offspring behavior and we provide an agent based model that shows how certain aspects of parent-offspring behavior can be instantiated with conditional movement and benefit transmission rules.
In this model we explore the effects of mobility, sociality, density and length of parental investment on correctly directed, misdirected and wasted parental investment. We predict that increased mobility, sociality and density will decrease the amount of correctly directed parental investment by decreasing the likelihood that parents and offspring will remain in proximity. Similarly, long periods of parental investment should decrease the likelihood that parents and offspring will remain in proximity, decreasing the amount of correctly directed parental investment.
MODEL DESCRIPTION
The description follows the standardized ODD protocol for describing individual and agent based models (Grimm et al. 2006; Grimm and Railsback 2005). This protocol, widely used, has been established to standardize the presentation of agent-based models.
Purpose
This model was developed to explore the parameters under which simple rules promoting parent-offspring proximity can lead to correctly directed parental investment (PI). Parents simply emit benefits and offspring follow positive benefit gradients, leading to proximity maintenance under some conditions. The model explores the effects of mobility, sociality, density and length of PI. We can then speculate that strategies based on recognition and bonding/attachment might serve to increase rates of correctly directed PI under parameters that are shown here to otherwise lead to high rates of misdirected and wasted PI.
State variables and scales
Time and space are both represented discretely. Space is represented as discrete locations in a one-dimensional line made of 201 lattice locations or ‘patches.’ Movement of parent agents along this line is determined by their movement propensity, offspring movement is determined by their benefit approach rule. During each time step, agents and patches execute the commands described in the schedule.
As time advances, the system moves down, as in a standard cellular automaton model (Figure 1). This enables visualization of the behavior of the system over time.
Figure 1.

Screenshot of a simulation using the default parameters. The simulation begins at the top and the world (a horizontal line with 201 locations) and agents move down as time progresses, leaving a visual record of their movement over time. Offspring are initially placed in the patch immediately to the right of their parents (at the top of the figure), and after that parents and offspring move according to the schedule and rules described below. Parents emit energy every time period in the patch immediately to their right, and offspring consume this energy. Remaining energy diffuses over time, and patches with energy (resources) on them are indicated by a lighter pink hue.
There are four kinds of entities: global variables which are associated with the overall state of the system (e.g., the number of agents), patches (unique lattice locations) and two types of agents (parents and offspring). Table 1 provides a full description of the state variables associated with each of the entities.
Table 1.
Overview of state variables associated with each type of entity. Bold indicates the independent variable and arrows indicate dependent variables.
| Entity | State variable | Description |
|---|---|---|
| Global | • Number of pairs | Number of parent-offspring pairs included in simulation. Higher values indicate higher density. |
| • Sociality | Likelihood that an agent will enter an already occupied patch | |
| • Diffusion | Rate at which benefits diffuse to neighboring patches | |
| ➢ Correct PI | Number of time periods in which a parent places benefits on a patch occupied by offspring | |
| ➢ Misdirected PI | Number of time periods in which a parent places benefits on a patch occupied by non-offspring | |
| ➢ Wasted PI | Number of time periods in which a parent places benefits on an unoccupied patch | |
| Patches | • Location | Coordinates of the patch |
| • Resource | Amount of energy on current patch | |
| Agent-parent | • Mobility | Percentage likelihood of moving 1 unit each time step |
| • Turn propensity | Percentage likelihood agent changes direction | |
| • Benefit provision | Amount of energy parent places on patch immediately to the right per time step | |
| • Heading | Direction of agent movement | |
| • Location | Coordinates of agent | |
| Agent-offspring | • Consumption amount | Amount of energy offspring consume from current patch per time step |
| • Heading | Direction of agent movement | |
| • Location | Coordinates of agent | |
Process overview and scheduling
The model proceeds in discrete time steps, and entities execute procedures in the following order (a more detailed schedule is provided in Appendix A):
Resources diffuse between neighboring patches.
Parents produce energy, adding to the resources on the current patch.
-
Agents move
Parents move according to mobility, and change heading according to turn propensity.
Offspring follow benefit approach rule.
Offspring consume resources from the current patch.
Design concepts
Emergence
Parent-offspring proximity emerges from the simple individual-level rules being used by the agents. When this proximity is achieved, it results in correctly directed PI, also an emergent phenomenon.
Adaptation
No evolutionary adaptation occurs.
Fitness
Although fitness is not explicitly modeled, the level of correct PI may correlate with fitness in certain ecological and social circumstances in which the model may be applied.
Prediction
Agents lack the ability to predict outcomes of future interactions or integrate information across time steps.
Sensing
Offspring have the ability to sense the resource level on the current patch and the patch immediately ahead.
Interaction
Parents and offspring interact indirectly through the shared environment (i.e., the passing of benefits from parents to offspring through patches).
Stochasticity
Parental mobility, turn propensity, and sociality are modelled probabilistically.
Collectives
Parent-offspring pairs can be considered collectives, but they do not have aggregate variables associated with them.
Observation
In the first three experiments, 100 independent runs (of length 2000 time steps) took place for each parameter value being explored (Table 2). The amount of correctly directed, misdirected and wasted PI is reported at the end of each run and averaged across all 100 runs. The fourth experiment reports correctly directed, misdirected and wasted PI every time step for 2000 time steps, averaged across 30 runs.
Table 2.
Initial and default values for all variables. Bold indicates the independent variable and arrows indicate dependent variables.
| Entity | State variable | Initial/Default Value | Units |
|---|---|---|---|
| Global | • Number of pairs | 10 | count |
| • Sociality | 1% | % likelihood | |
| • Diffusion | .5 | rate | |
| ➢ Correct PI | 0 | count | |
| ➢ Misdirected PI | 0 | count | |
| ➢ Wasted PI | 0 | count | |
| Patches | • Location | (−25 – 25, −25 – 25) | coordinates |
| • Resource | 0 | energy | |
| Agent-parent | • Mobility | 10% | % likelihood |
| • Turn propensity | 5% | % likelihood | |
| • Benefit provision | .05 | energy | |
| • Heading | 90° | degrees | |
| • Location | Random along line | coordinates | |
| Agent-offspring | • Consumption amount | .05 | energy |
| • Heading | 90° | degrees | |
| • Location | To the right of parent | coordinates | |
Initialization
All runs were initialized according to default parameters as shown in Table 2.
Input
The model has been designed as a general model of PI that may apply across a variety of species. Parents emitted benefits locally and offspring consumed benefits locally, so spatial proximity of parents-offspring pairs resulted in PI. This is consistent with the observations that spatial proximity can enable the selective transmission of benefits to offspring including food, warmth and various kinds of sensory stimulation (Gubernick and Klopfer 1981). It could also be generalized to include the benefits associated with protection from predators (Bowlby 1969; Mason and Mendoza 1998). These observations about PI in various species provided a basis for the assumptions of this model.
EXPERIMENTS AND RESULTS
Each experiment reported below is a set of runs investigating a particular set of parameter values. More details regarding the data collection can be found in the ‘observation’ section in design concepts in the model description.
Experiment 1: Effects of Mobility
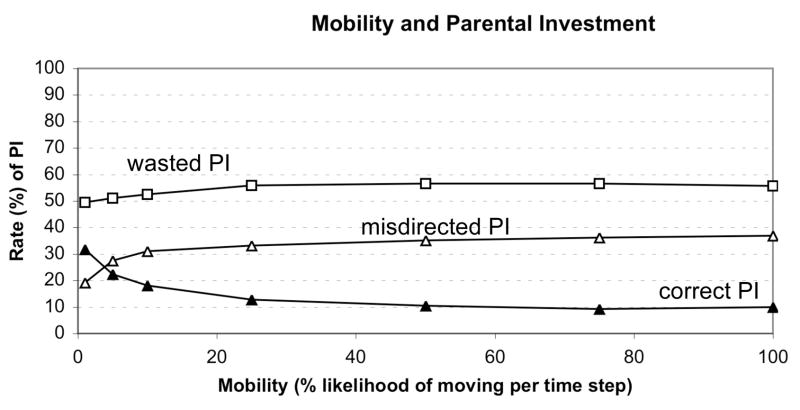
There was a straightforward relationship between parental mobility and PI. As mobility increased, correctly directed PI decreased and misdirected PI increased (Figure 2). Only at very low levels of mobility, was correctly directed PI greater than misdirected PI, suggesting that increases in parental mobility have dramatic effects on the effectiveness of PI. Wasted PI was high for all levels of mobility. However, wasted PI did increase slightly as mobility increased.
Figure 2.
Relationship between mobility and parental investment.
Experiment 2: Effects of Sociality
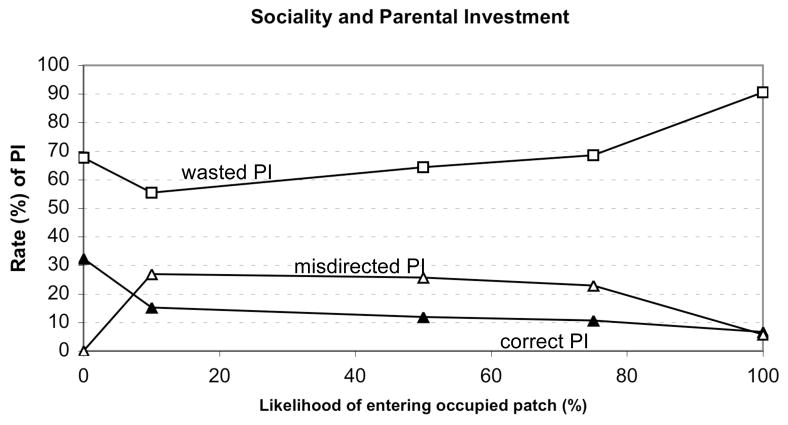
Sociality was also clearly associated with changes in the patterns of PI (Figure 3). When agents did not enter occupied patches (sociality = 0), the rate of misdirected PI was 0 and the rate of correct PI was relatively high. Correctly directed PI occurred only with very low levels of sociality, as sociality increased, the rate of misdirected PI increased dramatically. Misdirected PI increased as sociality increased and then decreased as sociality reached 100%.
Figure 3.
Relationship between sociality (likelihood of entering an occupied patch) and parental investment.
Experiment 3: Effects of Density
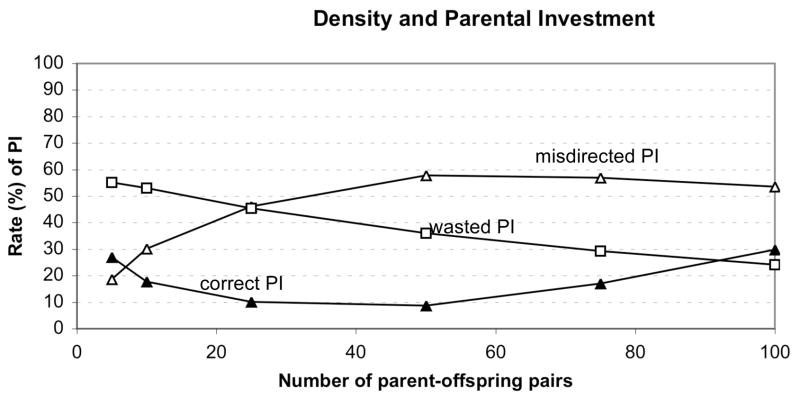
The density of parent-offspring pairs was related to changes in PI (Figure 4). Interestingly, both low and high densities, but not intermediate ones, resulted in high levels of correct PI and low levels of misdirected PI. The highest level of misdirected PI occurred at intermediate densities. Higher densities led to fewer instances of wasted PI, because higher densities made it less likely that the benefits would be placed on an unoccupied patch.
Figure 4.
Relationship between density (number of parent-offspring pairs) and parental investment.
Experiment 4: Effects of Length of Investment Period
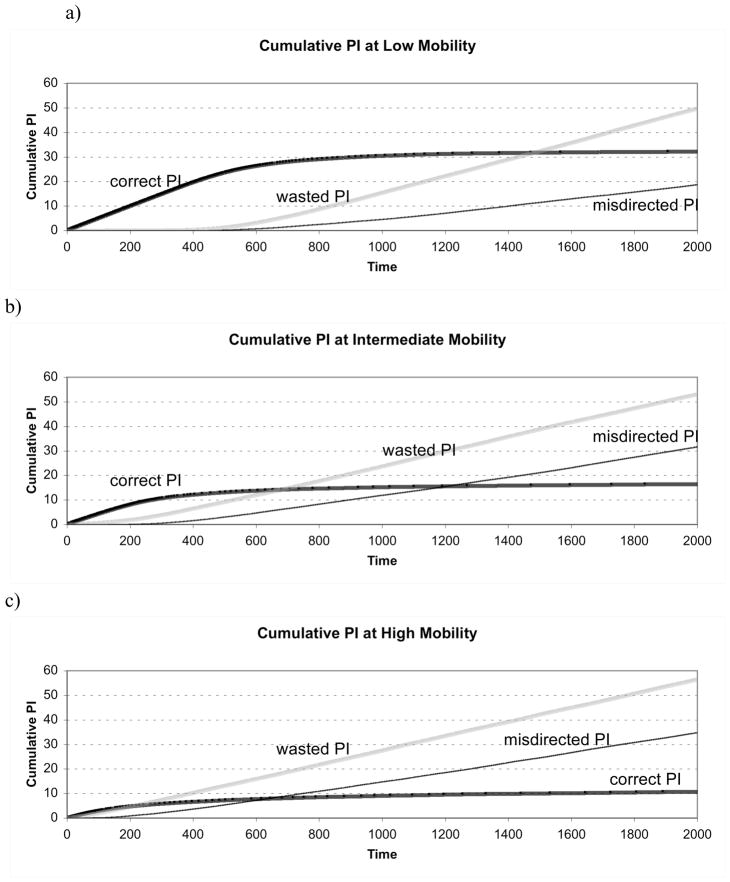
Figure 5 show the cumulative rates of each type of PI (Figure 5a–c) averaged over 30 runs. The slope of each line represents the rate of change in instances of correct, misdirected and wasted PI as time increases. When mobility was low (1%), cumulative correct PI increased for approximately 800 time steps (5a). At intermediate mobility (10 % chance of moving each time step) cumulative correct PI increased for only about 400 time steps (5b). Finally, at high levels of mobility (50% chance of moving each time step), cumulative correct PI began to plateau after only about 200 time steps (5c), and misdirected and wasted PI increased quickly.
Figure 5.
Cumulative parental investment at a) low mobility (1%) b) intermediate mobility (10%) and c) high mobility (50%).
These results indicate that PI over shorter time scales was more accurate than reflected by the data presented in experiments 1–3. By examining the change in cumulative PI over time, we determined that correctly directed PI had an initial period of linear increase, then reached an asymptote as offspring became separated from parents. Wasted PI and misdirected PI were initially zero, but began to increase as correctly directed PI reached the asymptote. The pattern described for correctly directed PI was observed for three different levels of mobility, with lower levels of mobility corresponding to longer periods of correct PI.
DISCUSSION
Our results demonstrate that parents can preferentially transmit benefits to offspring without necessarily having mechanisms for kin recognition. It is known that kin discrimination, or differential treatment of conspecifics based on correlates of genetic relatedness, can occur in the absence of processes for assessing genetic relatedness (Holmes 2004). For example, Komdeur and Hatchwell suggest that spatially-based mechanisms such as ‘feed anything in my nest or territory’ may be the simplest way to instantiate discriminative provisioning to offspring (1999). In the present model, parents simply emit benefits locally and offspring maintain proximity to parents by following the benefit gradient. These simple rules led to correctly directed parental investment in certain ecological and social conditions.
In the model we presented, the parameters that we explored affected parental investment in ways that are largely consistent with the literature. Mobility, sociality, density and the length of parental investment were all influential in the outcomes of the model. The model generated high rates of correct parental investment with low mobility, low sociality, high and low density, and short periods of parental investment. Overall, the model generated the highest rates of parental investment under conditions that resulted in a relatively close proximity between parents and offspring.
Our operationalization of parental investment fits Trivers’ (1972) definition of parental investment as a rivalrous investment in offspring. This makes our model applicable to situations that meet these criteria, rather than a broader and more poorly defined class of parent-offspring interactions (e.g., parental behavior or parental care).
Mobility and parental investment
Low levels of mobility resulted in higher rates of correctly directed parental investment, lower rates of misdirected investment and lower rates of wasted investment. Reduced mobility of parents and/or offspring makes the location of either more predictable, increasing the probability of correctly directed parental investment. The existence of nests, dens, burrows in a broad range of taxa illustrate how a predictable location associated with reduced mobility can be adaptive. Thus, one expects that low rates of parental mobility should increase the effectiveness of parental investment in the natural world. And there is good evidence supporting this. For example, a phylogenetic examination of the evolution of parental care in cockroaches found that the appearance of parental care was positively associated with ovoviviparity because the retention of the eggs until hatching allows for the proximity of the adults and neonates both in time and space (Nalepa 1997). Among traditional human hunter-gatherer societies, it has been suggested that mobility of parents may influence infant survival. In the Aché of Paraguay, young children increased proximity to the mother and spent more time in tactile contact with her during mobile foraging trips (Kaplan 1996). At a more proximate level, decreased motor activity has been shown to be associated with higher levels of oxytocin (Uvnäs-Moberg et al. 1994), a hormone that has been implicated in parturition and parental behavior.
Sociality
Low levels of sociality generated higher rates of correctly directed parental investment. When sociality was 0 (i.e. agents could not pass over one another), there were no instances of misdirected parental investment, although rates of wasted investment could be high. It is possible to imagine that low mobility (see earlier discussion) will be associated with low sociality in a number of taxa. If the probability of encountering other conspecifics is relatively low, this should result in an increased probability of being in close contact with ones offspring when parents and offspring have low mobility.
On the other hand, high levels of sociality generated high rates of misdirected investment. However, this high rate of misdirected investment with high sociality need not imply evolutionary disadvantages. In species that breed in highly related groups (e.g., cooperative breeders and eusocial insects), high rates of ‘misdirected’ investment might still promote inclusive fitness because beneficiaries are highly related even if they are not the individual’s offspring. In evolutionary terms, the costs and benefits of ‘correct’ and ‘misdirected’ benefit transmission will be based on the relatedness of the recipient of the ‘misdirected’ investment.
Density
High rates of correct investment resulted at both very high and very low densities of agents. Low densities enabled offspring to maintain proximity by decreasing the likelihood they would encounter benefit gradients generated by other parents. When there was low density (5 parent-offspring pairs) there were fewer nearby agents whose proximity might disrupt the parent-offspring pairs, leading to high rates of correct parental investment and low rates of misdirected parental investment.
High densities, on the other hand, increased the level of correct parental investment for other reasons. At high density (100 parent-offspring pairs), parents were unlikely to place benefits on unoccupied patches, leading to high rates of correct parental investment and low rates of wasted parental investment. Also, under very high densities, parent-offspring pairs were tightly packed and ‘held together’ by neighbors on either side. Because the likelihood that agents would enter an occupied patch (sociality parameter) was very low, the result was that tightly packed parent-offspring pairs were unlikely to get separated.
Unsurprisingly, high densities led to lower levels of wasted parental investment because offspring were less likely to wander off. As mentioned in the above section on sociality, in certain types of organisms (eusocial species), low levels of wasted parental investment may be more important than correct parental investment because of high relatedness among individuals that are part of a group. In fact, one of the benefits of grouping together tightly may be dramatic decreases in wasted parental investment.
Length of parental investment
We demonstrated that shorter lengths of parental investment result in higher levels of correct investment and lower levels of misdirected and wasted parental investment. These results suggest that, ceteris paribus, longer periods of parental investment might be more viable in species with relatively low mobility. Of course, the addition of more complex strategies for parental investment might allow long periods of parental investment to occur with fewer errors. Furthermore, the kin structure of the social world can influence the viability of long periods of parental investment (Galef; Kleiman and Malcom 1981). As suggested above, when all potential recipients are highly related, misdirected investment might not be costly in terms of inclusive fitness.
The optimal length of parental investment is a question of interest in a number of topic areas within evolutionary biology including work on life history theory (MacArthur and Wilson 1967; Daly and Wilson 1983; Kaplan and Gangestad 2005), parental investment theory (Trivers 1972; Maynard Smith 1977; Wade and Shuster 2002)and parent-offspring conflict (Pugesek 1990; Queller 1994; Godfray 1995; Maestripieri 2002). The present simulations can potentially be applied to questions from these areas. For example, questions in life history theory could be investigated by determining effective rules for when to switch from parental investment to having additional offspring. Parental investment theory and parent-offspring conflict involve strategic elements that originate in the conflict over who will invest in offspring and for how long. These components could be added to future models for the purpose of investigating the nature of biparental care and strategic components of parent-offspring interactions.
A role for memory, recognition and bonding
We have demonstrated that simple rules can result in viable parental investment, and that recognition is not necessary for correctly directed parental investment. However, high mobility and high sociality, the simple mechanisms explored here, can result in relatively high rates of misdirected and wasted parental investment when the period of investment is longer. These findings suggest that highly social and mobile species with long periods of parental investment are likely to have developed other ‘solutions’ to the problem of minimizing misdirected and wasted parental investment. These might include offspring caching, recognition memory on the part of parents and/or offspring, and mutual or unidirectional bonding between parents and offspring. We consider these below as they may apply to mammals.
Mammals are characterized by long periods of parental investment and relatively high mobility (Galef 1981, Kleiman, 1981). The present model shows that when mobility is high, the rate of correct parental investment decreases quickly as the length of parental investment increases (see Figure 5c). This suggests that mammals might use other strategies to decrease the rates of misdirected and wasted parental investment. For example, strategies based on recognition or bonding/attachment might play important roles in long-investing species (including, but not limited to mammals).
The rates of correct, misdirected and wasted parental investment can be explored in future simulations that include abilities other than the simple ones investigated in the present model. For example, the effectiveness of offspring caching could be examined by enabling parents to use spatial memory to return to a location in which immobile offspring were placed. The potential benefits of social recognition can be investigated by endowing parents and/or offspring with social memory for one another. Bonding and attachment can be explored by enabling parents and/or offspring to maintain proximity with a particular individual (including the mate in the case of biparental care). Future work along these lines might illuminate the nature of differences in parental behavior and parental cognition among species and the role of ecological factors in shaping selection for various parental investment strategies.
In conclusion, our results demonstrate that complex recognition or bonding abilities are not a prerequisite for effective parental investment. In other words, that the ability to recognize kin is not necessary for effective transmission of benefits to kin. In our simulations, parents simply emit benefits in an environment in which unconsumed benefits diffuse and offspring follow a benefit approach rule: to move in the direction of a positive benefit gradient. The results presented here demonstrate that simple strategies used by parents and offspring can lead to correctly directed parental investment under certain parameter values without the need for complex strategies such as those involving social recognition and bonding. Our findings have implications for understanding the nature of parental investment in various species and the selection pressures that might favor more complex decision rules underlying parental investment strategies. Although we did not directly explore the viability of more complex systems, such as those underlying recognition or attachment, our model suggests that certain ecological and social influences are more likely to lead to stronger selection pressures for such systems.
Acknowledgments
We would like to thank the lab groups of Eduardo Fernandez-Duque, Bruce Ellis and Lee Cronk for helpful comments and suggestions regarding this manuscript. Fernandez-Duque was supported by the Argentinean CONICET and both Aktipis and Fernandez-Duque were supported by the University of Pennsylvania during the analysis of the data and preparation of the manuscript. Aktipis was supported by a NSF Graduate Research Fellowship and National Cancer Institute grants F32CA144331 and R01CA140657. We thank Liz Dong for editorial assistance.
APPENDIX A: SUBMODELS
This section provides additional detail regarding simulation schedule and the subprocesses.
Resources diffuse between neighboring patches: half of the diffusion amount to patch immediately to right, and other half to patch immediately to left
Parents produce energy, adding to the resources on the current patch
-
Agents move
Parents move according to mobility, change heading with turn propensity
-
Offspring follow benefit approach rule
Where R = resource level on the present patch, R1 = the resource level on the patch ahead, FD1 is the movement command, ‘forward 1,’ and TRN is the movement command ‘turn around’ (allowing the organism to repeat this rule facing a different direction in the next time step).
Offspring consume resources from the current patch
Contributor Information
C. Athena Aktipis, Department of Ecology and Evolutionary Biology, University of Arizona.
Eduardo Fernandez-Duque, Department of Anthropology, University of Pennsylvania.
References
- Aktipis CA. Know when to walk away: contingent movement and the evolution of cooperation in groups. J Theor Biol. 2004;231:249–260. doi: 10.1016/j.jtbi.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Aktipis CA. Recognition memory and the evolution of cooperation: how simple strategies succeed in an agent-based world. Adapt Behav. 2006;14:239–247. [Google Scholar]
- Aktipis CA. Dissertation. University of Pennsylvania; 2008. When to Walk Away and when to stay: cooperation evolves when agents can leave unproductive partners and groups. [Google Scholar]
- Bowlby J. Attachment and Loss. Hothgarth Press; London: 1969. [Google Scholar]
- Brauchli K, Killingback T, Doebeli M. Evolution of cooperation in spatially structured populations. J Theor Biol. 1999;200:405–417. doi: 10.1006/jtbi.1999.1000. [DOI] [PubMed] [Google Scholar]
- Chapais B, Berman CM. Kinship and Behavior in Primates. Oxford University Press; USA: 2004. [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multimale primate society. Proc Natl Acad Sci. 2008;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. 1. Princeton University Press; Princeton: 1991. [Google Scholar]
- Cox SJ, Sluckin TJ, Steele J. Group size, memory and the interaction rate in the evolution of cooperation. Curr Anthropol. 1999;40:369–377. [Google Scholar]
- Daly M, Wilson M. Sex, Evolution and Behavior. Wadsworth Publishing Company; Belmont, CA: 1983. [Google Scholar]
- Dawkins R, Carlisle TR. Parental investment, mate desertion and a fallacy. Nature. 1976;262:131–133. [Google Scholar]
- Enquist M, Leimar O. The evolution of cooperation in mobile organisms. Anim Behav. 1993;45:747–757. [Google Scholar]
- Ferriere R, Michod R. The evolution of cooperation in spatially heterogeneous populations. Am Nat. 1996;147:692–717. [Google Scholar]
- Galef BG. The Ecology of Weaning. Parasitism and the Achievement of Independence by Altricial Mammals. In: Gubernick DG, Klopfer PH, editors. Parental Care in Mammals. Plenum Press; New York: pp. 211–241. [Google Scholar]
- Godfray HCJ. Evolutionary theory of parent–offspring conflict. Nature. 1995;376:133–138. doi: 10.1038/376133a0. [DOI] [PubMed] [Google Scholar]
- Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss-Custard J, Grand T, Heinz S, Huse G. A standard protocol for describing individual-based and agent-based models. Ecol Model. 2006;198:115–126. [Google Scholar]
- Grimm V, Railsback SF. Individual-based modeling and ecology. Princeton University Press; Princeton: 2005. [Google Scholar]
- Gubernick DJ, Klopfer PH. Parental Care in Mammals. 1. Plenum Press; New York: 1981. [Google Scholar]
- Hamilton WD. The genetical evolution of social behavior I. J Theor Biol. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behavior II. J Theor Biol. 1964b;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ. Significance of paternal investment by primates to the evolution of adult male-female associations. In: Taub DM, editor. Primate Paternalism. Van Nostrand Reinhold; New York: 1984. pp. 309–335. [Google Scholar]
- Hepper PG. Kin Recognition. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Holmes WG. The early history of Hamiltonian-based research on kin recognition. Ann Zool Fenn. 2004;41:691–711. [Google Scholar]
- Ifti M, Killingback T, Doebeli M. Effects of neighbourhood size and connectivity on the spatial Continuous Prisoner’s Dilemma. J Theor Biol. 2004;231:97–106. doi: 10.1016/j.jtbi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A theory of fertility and parental investment in traditional and modern human societies. Am J Phys Anthropol. 1996;101:91–135. [Google Scholar]
- Kaplan HS, Gangestad SW. Life history theory and evolutionary psychology. In: Buss D, editor. Handbook of evolutionary psychology. John Wiley & Sons; Hoboken, New Jersey: 2005. pp. 68–95. [Google Scholar]
- Kappeler PM, Schaik CP. Cooperation in Primates and Humans: Mechanisms and Evolution. 1. Springer-Verlag; Berlin: 2005. [Google Scholar]
- Kleiman DG, Malcom JR. The evolution of male parental investment in mammals. In: Gubernick DG, Klopfer PH, editors. Parental Care in Mammals. Plenum Press; New York: 1981. pp. 347–387. [Google Scholar]
- Kokko H, Jennions MD, Brooks R. Unifying and testing models of sexual selection. Ann Rev Ecol Evol Syst. 2006;37:43–66. [Google Scholar]
- Kokko H, Jennison M. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- Komdeur J, Hatchwell B. Kin recognition: function and mechanism in avian societies. Trends Ecol Evol. 1999;14:237–241. doi: 10.1016/s0169-5347(98)01573-0. [DOI] [PubMed] [Google Scholar]
- Kurland JA, Gaulin SJC. The evolution of male parental investment: Effects of genetic relatedness and feeding ecology on the allocation of reproductive effort. In: Taub DM, editor. Primate Paternalism. Van Nostrand Reinhold; New York: 1984. pp. 259–308. [Google Scholar]
- Lancaster JB, Lancaster CS. Parental investment: the hominid adaptation. In: Ortner DJ, editor. How Humans Adapt: A Biocultural Odyssey; Proceedings of the seventh international Smithsonian symposium; Smithsonian Institution Scholarly Press; 1983. pp. 33–66. [Google Scholar]
- Lehmann L, Keller L, Sumpter DJT. The evolution of helping and harming on graphs: the return of the inclusive fitness effect. J Evol Biol. 2007;20:2284–2295. doi: 10.1111/j.1420-9101.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- Lion S, van Baalen M. From infanticide to parental care: why spatial structure can help adults be good parents. Am Nat. 2007;170:E26–E46. doi: 10.1086/519462. [DOI] [PubMed] [Google Scholar]
- MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton University Press; Princeton: 1967. [Google Scholar]
- Maestripieri D. Parent–offspring conflict in primates. Int J Primatol. 2002;23:923–951. [Google Scholar]
- Marshall JAR, Rowe JE. Viscous populations and their support for reciprocal cooperation. Artif Life. 2003;9:327–334. doi: 10.1162/106454603322392497. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Parental investment: a prospective analysis. Anim Behav. 1977;25:1–9. [Google Scholar]
- Mitteldorf J, Wilson DS. Population viscosity and the evolution of altruism. J Theor Biol. 2000;204:481–496. doi: 10.1006/jtbi.2000.2007. [DOI] [PubMed] [Google Scholar]
- Nalepa CA. Postovulation parental investment and parental care in cockroaches. In: Choe JC, Crespi BJ, editors. Social behavior in insects and arachnids. Cambridge Univeristy Press; Cambridge: 1997. pp. 26–51. [Google Scholar]
- Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. [Google Scholar]
- Ohtsuki H, Hauert C, Lieberman E, Nowak MA. A simple rule for the evolution of cooperation on graphs and social networks. Nature. 2006;441:502–505. doi: 10.1038/nature04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugesek BH. Parental effort in the California gull: tests of parent-offspring conflict theory. Behav Ecol Sociobiol. 1990;27:211–215. [Google Scholar]
- Queller DC. Male-female conflict and parent-offspring conflict. Am Nat. 1994;144:S84–S99. [Google Scholar]
- Ramos-Fernández G, Boyer D, Gómez V. A complex social structure with fission–fusion properties can emerge from a simple foraging model. Behav Ecol Sociobiol. 2006;60:536–549. [Google Scholar]
- Taylor PD, Day T, Wild G. Evolution of cooperation in a finite homogeneous graph. Nature. 2007;447:469–472. doi: 10.1038/nature05784. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago, IL: 1972. pp. 135–179. [Google Scholar]
- Uvnäs-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochm Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Vos HD, Zeggelink E. Reciprocal altruism in human social evolution: the viability of altruism with a preference for “old-helping-partners”. Evol Hum Behav. 1994;18:261–278. [Google Scholar]
- Wade MJ, Shuster SM. The evolution of parental care in the context of sexual selection: a critical reassessment of parental investment theory. Am Nat. 2002;160:285–292. doi: 10.1086/341520. [DOI] [PubMed] [Google Scholar]