Abstract
Acetaminophen (APAP) overdose is the leading cause of acute liver failure in the US and UK. Recent studies implied that APAP-induced injury is partially mediated by interleukin-1β (IL-1β), which can activate and recruit neutrophils, exacerbating injury. Mature IL-1β is formed by caspase-1, dependent on inflammasome activation. The objective of this investigation was to evaluate the role of the Nalp3 inflammasome on release of damage associated molecular patterns (DAMPs), hepatic neutrophil accumulation and liver injury (ALT, necrosis) after APAP overdose. Mice deficient for each component of the Nalp3 inflammasome (Caspase-1, ASC and NALP3) were treated with 300 mg/kg APAP for 24 h; these mice had similar neutrophil recruitment and liver injury as APAP-treated C57Bl/6 wildtype animals. In addition, plasma levels of DAMPs (DNA fragments, keratin-18, hypo- and hyper-acetylated forms of high mobility group box-1 protein) were similarly elevated with no significant difference between wildtype and gene knockout mice. In addition, aspirin treatment, which has been postulated to attenuate cytokine formation and the activation of the Nalp3 inflammasome after APAP, had no effect on release of DAMPs, hepatic neutrophil accumulation or liver injury. Together these data confirm the release of DAMPs and a sterile inflammatory response after APAP overdose. However, as previously reported minor endogenous formation of IL-1β and the activation of the Nalp3 inflammasome have little impact on APAP hepatotoxicity. It appears that the Nalp3 inflammasome is not a promising therapeutic target to treat APAP overdose.
INTRODUCTION
Acetaminophen (APAP) is a widely used safe analgesic and antipyretic drug. However, an overdose can cause hepatotoxicity and even liver failure in animals and humans (Larson et al., 2005). Early mechanistic studies identified formation of a reactive metabolite, glutathione depletion and covalent binding to cellular proteins as critical initiating events in the toxicity (Cohen et al., 1997; Nelson, 1990). More recent studies showed the central role of mitochondrial dysfunction, including oxidant stress and peroxynitrite formation, the mitochondrial membrane permeability transition pore opening (MPT) and nuclear DNA fragmentation as propagation events in APAP-induced cell death in the liver (Jaeschke et al., 2003; Jaeschke and Bajt, 2006).
In recent years the new concept emerged that APAP-induced cell death triggers a neutrophilic inflammatory response, which has the potential to further aggravate the existing injury (Jaeschke, 2005; Liu and Kaplowitz, 2006). A neutrophil-mediated injury component has been identified in a variety of experimental conditions including hepatic ischemia-reperfusion injury, endotoxemia, alcoholic hepatitis, obstructive cholestasis and in several drug-induced liver injury models (Jaeschke, 2006; Jaeschke and Hasegawa, 2006). It has been recognized that the initial cell death triggers formation of inflammatory mediators including activated complement factors (Jaeschke et al., 1993), cytokines and chemokines (Okaya and Lentsch, 2003). A variety of molecules released from necrotic cells were identified that could induce cytokine formation through stimulating toll-like receptors (TLRs) (Schwabe et al., 2006). These molecules collectively termed damage-associated molecular patterns (DAMPs) include high mobility group box-1 (HMGB1) protein, heat shock proteins (HSPs), and DNA fragments (Bianchi, 2007). Similar DAMPs are also released and correlate with the degree of hepatic damage observed during APAP-induced liver injury (Antoine et al., 2009, 2010; Jahr et al., 2001; Martin-Murphy et al., 2010; Scaffidi et al., 2002) and are responsible for hepatic neutrophil accumulation (Scaffidi et al., 2002).
Despite extensive evidence against a direct involvement of neutrophils in APAP hepatotoxicity (Bauer et al., 2000; Cover et al., 2006; James et al., 2003; Lawson et al., 2000; Welty et al., 1993; Williams et al., 2010a), several recent reports suggested a critical role of interleukin-1α (IL-1α) (Chen et al., 2007) and in particular IL-1β, in the pathophysiology (Imaeda et al., 2009). It was shown that stimulation of TLR9 by DNA fragments during early APAP-induced cell death can lead to the transcriptional activation of the IL-1β gene resulting in the formation of pro-IL-1β (Imaeda et al., 2009). The pro-form of IL-1β has to be proteolytically cleaved by activated caspase-1 (interleukin-1 converting enzyme) to yield the active cytokine (Sims and Smith, 2010). Caspase-1 activation is regulated by the assembly of the inflammasome, which consists of Nalp3 (NACHT, LRR, and pyrin domain-containing protein 3), ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1 (Kanneganti et al., 2007; Lamkanfi and Dixit, 2009). Imaeda et al. (2009) showed reduced APAP-mediated injury in gene knockout mice of each individual component of the inflammasome, suggesting an important role of inflammasome activation and IL-1β formation in the pathophysiology of experimental APAP hepatotoxicity. Moreover, inhibition of inflammasome activation by aspirin resulted in protection from APAP hepatotoxicity (Imaeda et al., 2009). These intriguing results open up new avenues of research, provide further opportunities for therapeutic strategies to treat APAP hepatotoxicity and can potentially lead to the identification of human susceptibility factors for drug-induced liver injury. However, there are conflicting data associated with this concept that require reconciliation. One of the most critical observations arguing against a significant role played by the inflammasome is the fact that IL-1 signaling cannot directly induce cell death in vivo (Sims and Smith, 2010). The only way IL-1α or -1β can cause relevant liver injury is through activation of inflammatory cells, i.e. neutrophils (Bajt et al., 2001), and exacerbation of acetaminophen-induced hepatocyte damage. However, neutrophils are unlikely to cause liver injury after APAP overdose (Bauer et al., 2000; Cover et al., 2006; James et al., 2003; Lawson et al., 2000; Welty et al., 1993; Williams et al., 2010a). In addition, we have shown that a potent pan-caspase inhibitor prevented mature IL-1β formation during APAP-induced liver injury (Williams et al., 2010b). However, various pan-caspase inhibitors, despite their high efficacy to inhibit caspases in vivo, had consistently no effect on APAP-mediated neutrophil accumulation or liver injury (Antoine et al., 2009; Lawson et al., 1999; Williams et al., 2010b). Thus, evaluation of the role of the Nalp3 inflammasome and caspase-1 in APAP toxicity in vivo resulted in opposing findings when investigated through pharmacological interventions to inhibit caspase-1 activity compared to a genetic approach to eliminate this enzyme (Imaeda et al., 2009, Williams et al., 2010b). In order to reconcile these contradicting results, the objective of this investigation was to further re-evaluate the contribution of activation of the Nalp3 inflammasome in the pathogenesis of APAP hepatotoxicity by using gene knockout mice of all components and by using aspirin treatment.
MATERIALS AND METHODS
Animals
Eight to twelve week old male ASC-/-, Caspase-1-/-, Nalp3-/- and C57BL/6 control mice with an average weight of 18 to 24 g were bred and maintained at St. Jude Children's Research Hospital (Memphis, TN). Nalp3-/-, ASC-/- and Caspase-1-/- mice backcrossed to C57Bl/6 background for at least 10 generations have been described previously (Shaw et al., 2010). Additionally, C57BL/6 mice were bred and housed at the University of Liverpool (Liverpool, UK). All animals were housed in environmentally controlled rooms with 12 h light/dark cycle and allowed free access to food and water. Experiments followed the criteria of the National Research Council for the care and use of laboratory animals in research and guidelines set forth by the University of Liverpool Animal Ethics Committee, respectively. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
Experimental design
Mice were intraperitoneally (i.p.) injected with 300 mg/kg APAP (dissolved in warm saline) after overnight fasting or with an equivalent volume of saline. Additionally, some mice were given aspirin via drinking water (0.06 mg/mL or 1.0 mg/mL) for three days and fasted overnight prior to 530 mg/kg APAP (i.p.). All animals were sacrificed 24 h after APAP. Blood was drawn into heparinized syringes for measurement of alanine aminotransferase (ALT) activity (Pointe Scientific, Canton, MI) and then stored at -80°C. The liver was removed and was rinsed in cold saline; liver sections were fixed in 10% phosphate buffered formalin for histological analyses. The remaining liver lobes were snap-frozen in liquid nitrogen and stored at -80°C.
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for blinded evaluation of the areas of necrosis by the pathologist. The percent of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared to the cross sectional area. Additional sections were stained for neutrophils using the antimouse neutrophil allotypic marker antibody (AbD Serotec, Raleigh, NC) as previously described (Williams et al., 2010a). Positively stained neutrophils consistent with cellular morphology were quantified in 15 high power fields (HPF). Some sections were also stained for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell death assay (Roche, Indianapolis, IN) as previously described (Lawson et al., 1999).
mRNA expression
Quantification of mRNA expression was performed by real-time PCR (RT-PCR) analysis as described previously (Bajt et al., 2008). cDNA was made by reverse transcription of total tissue RNA by M-MLV reverse transcriptase in the presence of random primers (Invitrogen, Carlsbad, CA). Forward and reverse primers for the genes were designed using Primer Express software (Applied Biosystems, Foster City, CA). After cDNA concentration normalization, SYBR green PCR Master Mix (Applied Biosystems) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the ABI 7900 instrument (Applied Biosystems). All genes evaluated were first normalized to the β-actin gene and then expressed as a fold increase relative to control arbitrarily set as 1.0. Calculations are made by the 2^(-ddCt) formula.
Glutathione quantification
Glutathione (GSH) and glutathione disulfide (GSSG) were measured from liver homogenate using the Tietze method as previously described in detail (Jaeschke and Mitchell, 1990). Briefly, frozen tissue was homogenized in sulfosalicylic acid/EDTA. For total GSH determination samples were assayed using dithionitrobenzoic acid. Similarly, measurement of GSSG was performed using the same method after trapping and removal of GSH with N-ethylmaleimide.
Plasma DNA fragments
To quantify the release of nuclear DNA fragments into plasma the cell death detection ELISA (Roche, Indianapolis, IN) was used according to the manufacturer's instructions.
Plasma HMGB1 and Keratin-18
Quantification of plasma HMGB1 and Keratin-18 were previously described in detail (Antoine et al. 2009, 2010). Briefly, serum proteins were immunoprecipitated and subsequently analyzed by LC-MS/MS.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. If the data were not normally distributed, the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn's Multiple Comparisons Test was used. P < 0.05 was considered significant.
RESULTS
Liver injury and inflammation in Nalp3 inflammasome-deficient mice
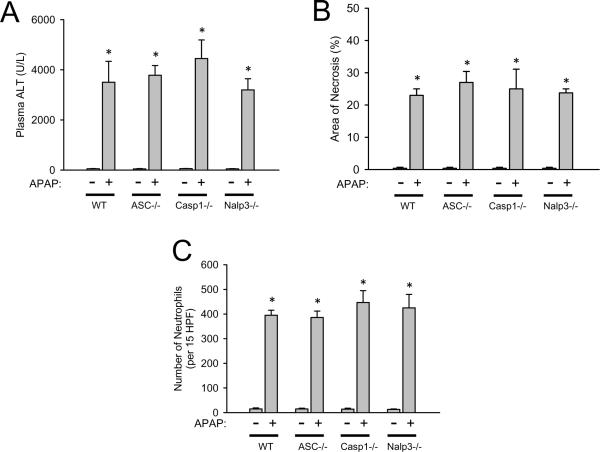
To determine if the Nalp3 inflammasome is critical for APAP-induced toxicity, inflammasome-deficient mice (ASC-/-, Caspase-1-/- and Nalp3-/-) and C57BL/6 wildtype mice were given APAP and sacrificed 24 h later. Liver injury was determined by plasma ALT (Figure 1A) and histological quantification of liver necrosis (Figure 1B and Figure 2). In contrast to previously published results (Imaeda et al., 2009), the injury in all genotypes was similar demonstrating the Nalp3 inflammasome does not appear to be a critical factor in murine APAP-induced liver injury. Additionally, it was hypothesized that APAP-induced sterile inflammation and IL-1β production mediates immune cell infiltration, potentiating injury. To evaluate if Nalp3 inflammasome activation is important for neutrophil recruitment during APAP overdose, hepatic neutrophils were quantified. Consistent with the injury, total hepatic neutrophil counts were not different between genotypes (Figure 1C and Figure 2), which agrees with previously published results demonstrating IL-1β does not participate in neutrophil recruitment during APAP-overdose (Williams et al., 2010b). To further evaluate inflammation, hepatic mRNA levels for various cytokines, chemokines and adhesion molecules were compared (Table 1). Interestingly, IL-18, a cytokine which also requires caspase-1 processing, was down-regulated after APAP overdose and IL-1β was only slightly increased similar to previously published results (Williams et al., 2010b). Despite the absence of each Nalp3 inflammasome component, there was no alteration in gene expression between genotypes of any cytokine (IL-1β, IL-18, IL-10, IL-6, TNF-α), chemokines (MIP-1α, MIP-1β, MIP-2α, MCP-1), or adhesion molecule (ICAM-1) measured (Table 1). This demonstrates a nearly identical inflammatory response, which appears to be independent of the Nalp3 inflammasome, during APAP overdose.
Figure 1. Liver injury and hepatic neutrophil recruitment after APAP-overdose in Nalp3 inflammasome-deficient mice.
Liver injury in C57BL/6 (WT), ASC-/-, Caspase-1-/- and Nalp3-/- mice was measured by plasma ALT (A) and by histological scoring of liver necrosis (B) 24 hours after APAP overdose. Hepatic neutrophil recruitment was also quantified at 24 h by immunohistochemistry and expressed as total liver neutrophils counted in 15 high power fields (x400 magnification) (C). *P < 0.05 compared to untreated control.
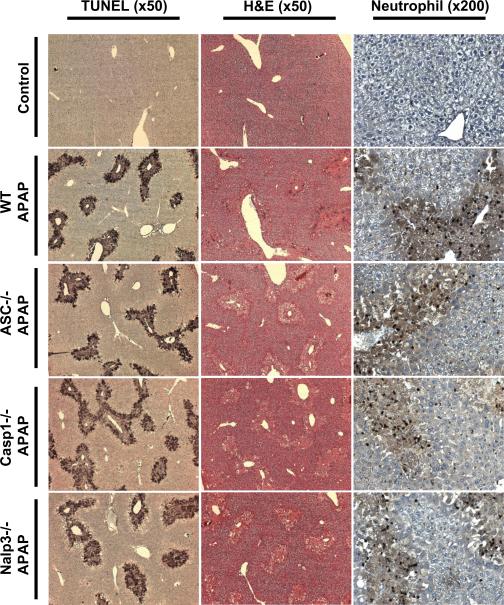
Figure 2. TUNEL, H&E and neutrophil staining in Nalp3-inflammasome deficient mice.
Liver tissue sections were stained with the TUNEL assay (x50 magnification) to demonstrate equivalent DNA damage in all genotypes [C57BL/6 (WT), ASC-/-, Caspase-1-/- and Nalp3-/- mice]. Area of necrosis was determined by H&E staining (x50 magnification). Neutrophil recruitment was identified by immunohistochemistry (x200 magnification). Control panels shown are C57BL/6 saline treated mice; knockout saline-treated mice look morphologically identical (not shown).
Table 1.
Hepatic mRNA induction after acetaminophen overdose in C57BL/6, ASC-/-, Caspase-1-/- and Nalp3-/- mice.
| Liver mRNA expression (fold change) | ||||
|---|---|---|---|---|
| Gene |
C57BL/6 |
ASC-/- |
Caspase1-/- |
Nalp3-/- |
| IL-1β | 2.5 ± 0.4 | 3.5 ± 0.8 | 2.1 ± 0.3 | 1.8 ± 0.7 |
| IL-18 | -1.6 ± 0.2 | -1.5 ± 0.1 | -1.4 ± 0.3 | -1.6 ± 0.1 |
| IL-10 | 9.4 ± 3.3 | 7.9 ± 2.3 | 9.8 ± 2.0 | 11.9 ± 4.8 |
| IL-6 | 3.9 ± 0.7 | 3.9 ± 0.6 | 4.1 ± 1.0 | 3.3 ± 1.0 |
| ICAM-1 | 4.4 ± 0.3 | 4.6 ± 0.4 | 4.5 ± 0.7 | 3.8 ± 0.3 |
| MIP-1α | 15.6 ± 2.6 | 20.0 ± 2.8 | 24.7 ± 5.1 | 14.6 ± 3.4 |
| MIP-1β | 7.8 ± 0.5 | 10.3 ± 1.9 | 12.6 ± 2.9 | 11.3 ± 2.5 |
| MIP-2α | 26.2 ± 8.5 | 27.8 ± 5.3 | 19.2 ± 2.8 | 13.3 ± 4.6 |
| MCP-1 | 70.9 ± 16.9 | 68.8 ± 12.6 | 73.9 ± 19.1 | 58.5 ± 12.7 |
| TNF-α | 14.8 ± 3.1 | 14.9 ± 2.1 | 11.8 ± 3.0 | 9.7 ± 4.0 |
Hepatic mRNA levels 24 h after administration of 300 mg/kg APAP in C57BL/6 wild type animals or Nalp3 inflammasome gene knockout mice. mRNA levels are calculated as the cytokine mRNA-to-β-actin mRNA ratio. For mRNA calculations the values of untreated controls were set as 1 and the fold change of the APAP-treated animals is shown. Data represent means ± SE of n = 5 animals per group. All data shown are significant as compared to untreated genotype-matched controls (P<0.05). No gene from APAP-treated knockout animals was significantly different as compared to APAP-treated C57BL/6 controls.
APAP-induced oxidant stress in Nalp3 inflammasome-deficient mice
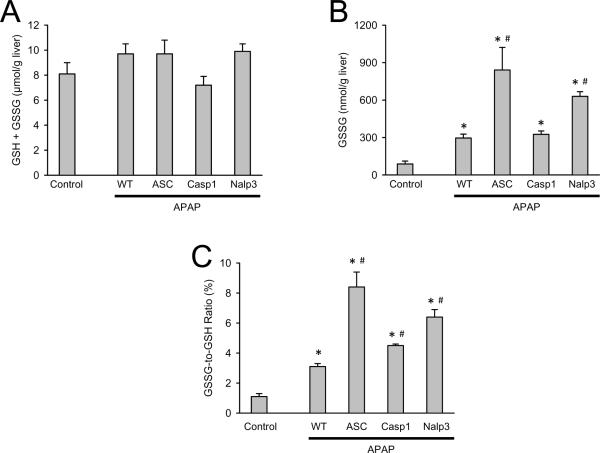
To evaluate liver glutathione recovery and oxidant stress, hepatic GSH and GSSG levels were measured. All genotypes had equivalent GSH recovery at 24 h after APAP (Figure 3A) and untreated control mice had equivalent hepatic GSH levels between genotypes (data not shown). In addition, all genotypes had increased GSSG levels over controls with ASC-/- and Nalp3-/- groups having further elevated GSSG levels versus wildtype APAP-treated animals (Figure 3B). Despite significantly increased GSSG to GSH ratios in all Nalp3 inflammasome-deficient mice compared to wildtype controls (Figure 3C) there was no alteration in injury (Figure 1A and 1B). These data show increased and somewhat variable levels of oxidative stress between genotypes but this variability did not impact liver injury.
Figure 3. Liver glutathione and GSSG in Nalp3-inflammasome deficient mice.
Total GSH was measured in liver tissue homogenate of saline treated C57BL/6 (control) and C57BL/6 (WT), ASC-/-, Caspase-1-/- and Nalp3-/- at 24 h after APAP (A). GSSG was measured at 24 h after APAP (B) and the ratio of GSSG to total GSH is shown (C). Knockout saline-treated control mice are not different from C57BL/6 saline-treated controls (not shown). *P < 0.05 compared to saline control. #P < 0.05 compared to APAP-treated C57BL/6 mice.
DAMP release is unaltered in Nalp3 inflammasome-deficient mice
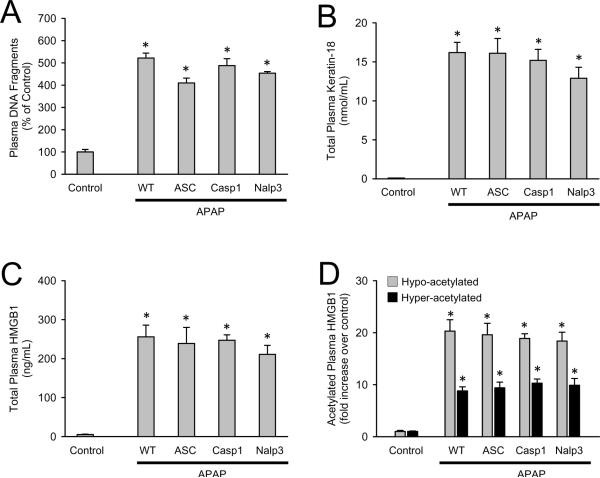
Necrotic cells release damage associated molecular patterns (DAMPs) which can initiate an inflammatory response that was implicated in promoting further injury. DAMPs include high mobility group box-1 (HMGB1) proteins and DNA fragments (Bianchi, 2007). To eliminate the possibility that inflammasome-deficient mice have altered DAMP release, nuclear DNA fragments, keratin-18 and HMGB1 proteins were measured in plasma (Figure 4A-C). Consistent with cellular injury, the increase in plasma DNA fragments was similar in all genotypes (Figure 4A). Total keratin-18 (K-18) is another useful biomarker to demonstrate cellular injury (Antoine et al., 2009). Indeed, there was a significant increase in plasma keratin-18 compared to controls but no differences in plasma concentrations between genotypes (Figure 4B). Another DAMP released after APAP overdose is HMGB1. In addition to total plasma HMGB1 (Figure 4C), the hyper- and hypo-acetylated forms of the protein have been shown to originate from innate immune inflammation and necrotic tissue, respectively (Antoine et al., 2009). Correlating with equivalent inflammation and injury, there were no differences between genotypes of total, hyper- or hypo-acetylated HMGB1 (Figure 4C and 4D), and no differences in plasma DNA fragments, K-18 or HMBG1 were observed between genotypes of saline treated animals (data not shown). These data show equivalent DAMP release and a subsequent equivalent sterile inflammation with or without the presence of functional Nalp3 inflammasome.
Figure 4. Quantification of plasma DAMPs in Nalp3-inflammasome deficient mice.
DNA fragments were measured in plasma of saline treated C57BL/6 (control) and C57BL/6 (WT), ASC-/-, Caspase-1-/- and Nalp3-/- at 24 h after APAP (A) and expressed as percent of control. Similarly, total plasma keratin-18 (B), total plasma HMGB1 (C), and hypo- and hyper-acetylated plasma HMGB1 (D) were measured. Knockout saline-treated mice are not different from C57BL/6 saline-treated controls (not shown). *P < 0.05 compared to saline control.
Aspirin does not alter APAP toxicity or DAMP release
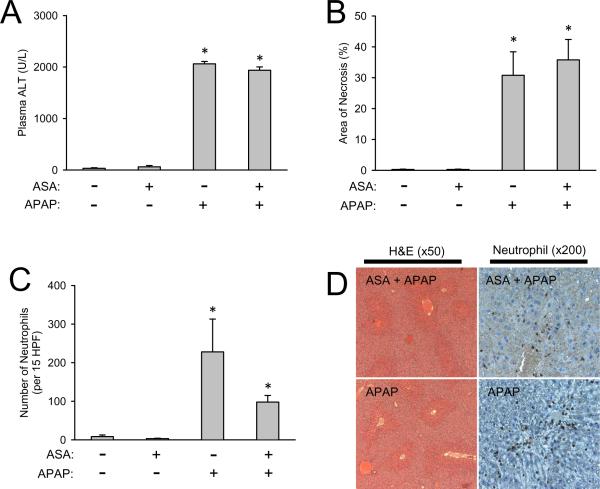
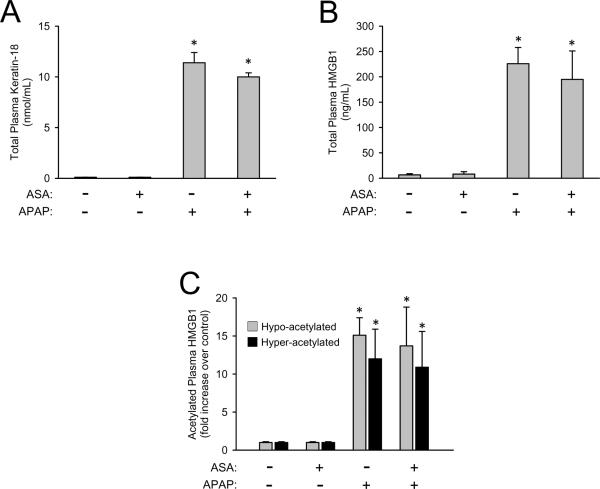
It was hypothesized that aspirin (acetylsalicylic acid, ASA) is capable of inhibiting the Nalp3 inflammasome and it was reported that pretreatment with ASA in drinking water reduced APAP-induced injury (Imaeda et al., 2009). This study was repeated with the ASA dose previously published (0.06 mg/mL in drinking water) as well as a higher dose (1.0 mg/mL in drinking water). Data presented are for only the high dose of ASA because no effect on injury was observed for either dose. ASA in the drinking water of mice caused no liver injury nor was it capable of reducing the APAP-induced liver injury as measured by plasma ALT activities (Figure 5A) or the area of necrosis (Figure 5B and 5D). ASA pretreatment did not alter hepatic GSH levels nor did it cause differences is GSH recovery after overdose indicating it did not affect APAP metabolism (data not shown). ASA pretreatment caused a trend in decreasing hepatic neutrophil recruitment after APAP overdose, but the reduction was not statistically significant (Figure 5C and 5D). In addition, ASA pretreatment caused no alterations in DAMP release which is consistent with the results observed in Nalp3 inflammasome knockout mice. The APAP-induced increase in plasma levels of keratin-18 (Figure 6A), total HMGB1 (Figure 6B) and hyper- and hypo-acetylated HMGB1 (Figure 6C) were not significantly affected by ASA treatment. As a whole the genetic elimination of the Nalp3 inflammasome and pharmacologic inhibition of the Nalp3 inflammasome with ASA did nothing to alter APAP-induced injury.
Figure 5. Liver injury and hepatic neutrophil recruitment in aspirin-pretreated mice after APAP-overdose.
ALT (A) was measured in the plasma of C57BL/6 mice pretreated with or without aspirin (ASA) in the drinking water and then subjected to APAP-overdose or saline injection. Similarly, histological area of necrosis (B), and neutrophil accumulation were quantified (C) were measured as previously described. Representative sections with H&E and neutrophil staining are shown (D). *P < 0.05 compared to the respective control with or without ASA.
Figure 6. Quantification of plasma DAMPs in aspirin-pretreated mice.
Total keratin-18 (A) was measured in the plasma of C57BL/6 mice pretreated with or without aspirin (ASA) in the drinking water and then subjected to APAP-overdose or saline injection. Similarly, total plasma HMGB1 (B), and hypo- and hyper-acetylated HMGB1 (C) were measured. *P < 0.05 compared to the respective control with or without ASA.
DISCUSSION
Role of the Nalp3 inflammasome in APAP-induced liver injury
The main objective of this investigation was to reconcile apparently contradictory results regarding the importance of the Nalp3 inflammasome in APAP hepatotoxicity obtained by different groups using a genetic versus a pharmacological approach. Imaeda et al. (2009) reported significantly reduced liver injury and improved survival in ASC-/-, Nalp3-/- and caspase1-/- mice. Although it was only shown for Nalp3-/- mice that the APAP-induced increase in IL-1β protein formation was prevented, the assumption was that all knockout mice were partially protected because processing of pro-IL-1β to the active, pro-inflammatory cytokine IL-1β should have been prevented when caspase-1 as part of the Nalp3 inflammasome was nonfunctional (Imaeda et al., 2009). These conclusions are consistent with a previous report showing that IL-1 receptor-deficient mice were almost completely (95%) protected against APAP-induced liver injury (Chen et al., 2007). It was assumed that inflammatory cells played a deleterious role in the pathogenesis (Imaeda et al., 2009; Chen et al., 2007). In contrast to these reports, our current findings indicate very clearly that ASC-/-, Nalp3-/- and caspase1-/- knock-out mice are neither protected (ALT, area of necrosis, oxidant stress, DNA damage) nor do these animals show any evidence of a reduced inflammatory response (cytokine and chemokine formation, hepatic neutrophil accumulation). These data are fully consistent with our previous report where we found not only increases of IL-1β mRNA after APAP overdose but also moderate increases in caspase-dependent mature IL-1β protein formation (Williams et al., 2010b). However, the caspase inhibitor had no protective effect on liver injury or hepatic neutrophil accumulation, a concept consistent with other published findings (Antoine et al., 2009). It is notable that the 300% increase of IL-1β formation after APAP as reported in our study (Williams et al., 2010b) is actually higher than the 13% increase measured by Imaeda et al. (2009). The mechanistic basis of this still requires further investigation. Independent of these differences in pathological outcome, both studies clearly show that the absolute plasma levels of IL-1β protein are very low even after APAP overdose (Imaeda et al., 2009; Williams et al., 2010b). Thus, in order to assess if levels of IL-1β that have been shown to activate and recruit neutrophils into the liver (Bajt et al., 2001), can impact APAP toxicity, animals were treated with pharmacological levels of murine recombinant IL-1β (about 3-4 orders of magnitude above endogenous concentrations). Although this indeed caused more recruitment of activated neutrophils into the liver, it still had no effect on liver injury (Williams et al., 2010b). In agreement with these findings, IL-1R-/- mice were not protected against APAP hepatotoxicity (Williams et al., 2010b). These data together indicate that despite the fact that some IL-1β is generated in a caspase-dependent manner after APAP overdose, it is highly unlikely that this minor, endogenously formed cytokine has any major impact on the inflammatory response induced during APAP hepatotoxicity. In addition, neither our previous study (Williams et al., 2010b) nor our present experiments showed any evidence for increased formation of IL-18, another cytokine that is processed by caspase-1, after APAP overdose. Moreover, theoretically, any pathophysiological effect of both cytokines would have to involve cytotoxicity mediated by inflammatory cells. However, there is extensive experimental evidence to suggest that neutrophils are not involved in APAP-induced liver injury (Bauer et al., 2000; Cover et al., 2006; James et al., 2003; Lawson et al., 2000; Welty et al., 1993; Williams et al., 2010a). Thus, based on the currently reported data from our study activation of the Nalp3 inflammasome does not play a critical role in the exacerbation of APAP hepatotoxicity.
In addition to playing a critical role in the formation of the Nalp3 inflammasome, recent reports have suggested that ASC is involved in inflammasome-independent cellular functions such as lymphocyte survival (Shaw et al., 2010), antigen-induced T cell activation by dendritic cells (Ippagunta et al., 2010) and antigen-specific humoral immunity (Ellebedy et al., 2011). The mechanism(s) underlying the critical role of ASC in these functions is still unknown, but it is possible ASC is involved in other inflammasome-independent functions in various cell types. However, ASC-/- mice developed APAP-induced liver injury to the same degree as wildtype animals suggesting that all inflammasome-dependent and inflammasome-independent functions of ASC do not play a relevant role in APAP hepatotoxicity.
The role of aspirin in APAP hepatotoxicity
Imaeda et al. (2009) also reported that a low dose of aspirin (N-acetylsalicylic acid) can reduce APAP hepatotoxicity independent of its effect on platelet aggregation or cyclooxygenase-1 or -2. It was concluded that aspirin protected due to inhibition of the transcriptional activation of cytokine formation (IL-1β, IL-18, TNF-α and interferon-γ) and also inhibition of the Nalp3 inflammasome pathway, which processes IL-1β and IL-18 (Imaeda et al., 2009). Again, the assumption was that the reduced cytokine formation attenuates liver injury through reduced inflammatory cell activation and recruitment. However, using the same pretreatment regimen as described by Imaeda et al. (2009) with the same or higher doses of aspirin, no significant effect on APAP-induced liver injury was observed when compared to APAP-treated mice in our study. Aspirin treatment showed a trend to attenuate the number of neutrophils in the liver but the overall effect was statistically not significant and indeed, the quantification of serum biomarkers of toxicity (ALT, HMGB1, K-18) was not different between groups. These data further support the concept that aspirin pretreatment has no significant effect on the overall extent of experimental APAP hepatotoxicity. APAP metabolism was shown to be unaffected by aspirin pretreatment in our investigation, but this was not assessed in the previous study (Imaeda et al., 2009). Thus, in our hands aspirin was not effective in preventing APAP hepatotoxicity. Even if aspirin would reduce hepatic neutrophil recruitment, the fact that many other interventions against neutrophils were ineffective in this model (Bauer et al., 2000; Cover et al., 2006; James et al., 2003; Lawson et al., 2000; Welty et al., 1993; Williams et al., 2010a), are consistent with the lack of protection by aspirin.
DAMPs and APAP hepatotoxicity
The sterile inflammatory response after APAP overdose requires the release of DAMPs. Previous studies reported measurement of DAMPs including HMGB1, heat shock proteins, and DNA fragments in plasma of APAP-treated animals (Antoine et al., 2009, 2010; Jahr et al., 2001; Martin-Murphy et al., 2010; Scaffidi et al., 2002). In the case of HMGB1, it was shown that a hypo-acetylated form is passively released by necrotic cells and can be used similar to keratin-18 as a biomarker of necrosis (Antoine et al., 2009). In addition, a hyper-acetylated form of HMGB1 can be actively secreted by macrophages or monocytes (Bonaldi et al., 2003) and thus can represent a potential biomarker of inflammation (Antoine et al., 2009). Consistent with these previous observations, we found a substantial increase in nuclear DNA fragments, in plasma keratin-18 and in total HMGB1 levels after APAP treatment. However, the increase in these plasma biomarkers of hepatic necrosis was neither affected by aspirin treatment nor by deficiency of inflammasome genes. Furthermore, the levels of hypo-acetylated HMGB1 (necrosis) and hyper-acetylated HMGB1 (inflammation) when determined by mass spectrometry were not modulated in the knockout mice or affected by aspirin treatment. These data are consistent with both the histology data and other markers (ALT) and demonstrate that the overall APAP-induced cell necrosis in these livers is the same in all groups. Moreover, the similar release of DAMPs in all groups is consistent with the similar overall induction of pro-inflammatory cytokine and chemokine genes and recruitment of neutrophils into the livers. Thus, despite the minor effect of IL-1β processing caused by the activation of the Nalp3 inflammasome (Imaeda et al., 2009; Williams et al., 2010b), the overall inflammatory response after APAP overdose is not dependent on the Nalp3 inflammasome and does not impact on the overall extent of observed toxicity.
The data presented in this manuscript are fully consistent with a previous study (Williams et al., 2010b) showing that IL-1β formation and processing by the inflammasome has no relevant impact on the degree of APAP hepatotoxicity in mice. However, this is not consistent with the results and conclusions presented by Imaeda et al. (2009) and in part by Chen et al. (2007). The conclusions presented herein are based on data from three independent research groups, that the activation of the Nalp3 inflammasome does not play a significant role in the pathogenesis of experimental APAP hepatotoxicity. We have extended the original investigations reporting a critical role for the Nalp3 inflammasome in APAP toxicity by inclusion of the characterization of the inflammatory response, a more extended quantification of cell death and associated biomarker release. Each of these factors was not altered between the genetic or pharmacological manipulation of the inflammasome and the overall degree of toxicity. It is important to point out only low levels of IL-1β produced in the APAP-treated mice could be detected in all studies, but the evidence for a functional consequence of such cytokine release was inconsistent. There is no obvious explanation for these discrepancies. However, there are minor differences in the experimental design that need to be considered between our present study and the experiments reported by Imaeda et al. (2009). For the experiments with the knock-out mice, we used our standard dose of 300 mg/kg compared to 500 mg/kg by Imaeda et al., (2009). However, the aspirin study was performed administering the same dose of APAP as used by Imaeda et al. (2009) but still resulted in no protection. In addition, we terminated our experiments at 24 h compared to 12 h in the Imaeda study. It appears unlikely that there could be protection at earlier time points that is lost at later times given the fact that inflammatory liver injury generally occurs at later time points. Lastly, the sources of the mice used in these studies were different. Although it can not be completely ruled out that the different sources of mice may have affected the results, the fact that our results with the various knock-out mice are fully consistent with pharmacological interventions (caspase inhibitors, aspirin) and additional manipulations (injection of pharmacological doses of IL-1β) (Williams et al., 2010b) as well as a battery of studies on the role of neutrophils in the pathogenesis of APAP hepatoxicity in rats and various mouse strains (Bauer et al., 2000; Cover et al., 2006; James et al., 2003; Lawson et al., 2000; Welty et al., 1993; Williams et al., 2010a), gives us confidence in the general applicability of our data and conclusions. Although, an important aspect of the present study was to demonstrate that the pharmacological manipulations used for investigation of the inflammasone did not alter the metabolic activation of APAP to its toxic metabolite. In addition to unrecognized effects on drug metabolism, pharmacological interventions and genetic manipulations can trigger stress responses with expression of protective genes or cause compensatory responses that may affect APAP toxicity (Jaeschke et al., 2011).
It is well recognized that an inflammatory response after an acute tissue injury can have multiple opposing effects (Bulkley and Roberts, 1974). Although inflammation may aggravate tissue injury under certain circumstances, the recruited neutrophils and especially macrophages are critical for removal of necrotic cell debris and promotion of tissue repair. Our data provide further support that in the APAP model, inflammatory cells do not directly cause liver damage. However, there is evidence to indicate that suppressing the late inflammatory response delays liver regeneration (Dambach et al., 2002; Holt et al., 2008). Thus, any intervention that has the potential to affect the inflammatory response caused by tissue damage needs to consider its dual effect.
In summary, our data clearly demonstrated that APAP overdose caused substantial liver injury, release of DAMPs and a sterile inflammatory response. However, mice deficient in components of the Nalp3 inflammasome (ASC-/-, Nalp3-/- and caspase1-/-) or wild type mice treated with aspirin showed very similar responses to APAP as wild type animals treated with APAP alone. These data are consistent with previous pharmacological approaches to inhibit caspase-1, with data obtained in IL-1 receptor-/- mice, and with the lack of effects when pharmacological doses of IL-1β were administered (Williams et al., 2010b). All these data together strongly support the conclusion that the minor endogenous formation of IL-1β and the activation of the Nalp3 inflammasome has no relevant impact on APAP hepatotoxicity and therefore the Nalp3 inflammasome may not be a viable therapeutic target to treat APAP overdose. Caution must therefore be exercised in translating this concept to man with respect to aspirin acting as a hepatoprotective agent against APAP-induced liver injury.
The authors do not have any conflict of interest to disclose.
ACKNOWLEDMENTS
This investigation was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. C.D. Williams was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences. This work was also supported by grants from the National Institutes of Health grant number AR056296 and AI088177, the American Lebanese and Syrian Associated Charities to T-D.K. D.J.A., D.P.W. and B.K.P. would like to acknowledge the financial support of the MRC (Medical Research Council – grant number G0700654) and to the BTS (British Toxicological Society) funded PhD studentship for C.B. awarded to D.P.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
REFERENCES
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol. Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol. Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol. Sci. 2008;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I, Vollmar B, Jaeschke H, Rensing H, Kraemer T, Larsen R, Bauer M. Transcriptional activation of heme oxygenase-1 and its functional significance in acetaminophen-induced hepatitis and hepatocellular injury in the rat. J. Hepatol. 2000;33:395–406. doi: 10.1016/s0168-8278(00)80275-5. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkley BH, Roberts WC. Steroid therapy during acute myocardial infarction. A cause of delayed healing and of ventricular aneurysm. Am. J. Med. 1974;56:244–250. doi: 10.1016/0002-9343(74)90603-2. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Ellebedy AH, Lupfer C, Ghoneim HE, Debeauchamp J, Kanneganti TD, Webby RJ. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc. Natl. Acad. Sci. U.S.A. 2011 Jan 26; doi: 10.1073/pnas.1012455108. in press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R, Van de Veerdonk FL, Netea MG, Joosten LA, Lamkanfi M, Kanneganti TD. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J. Biol. Chem. 2010;285:12454–12462. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2005;1:389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am. J. Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 2011 doi: 10.1016/j.lfs.2011.01.025. in press. DOI : 10.1016/j.lfs.2011.01.025. (published online Feb 4.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic. Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol. Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol. Appl. Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol. Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J. Invest. Surg. 2003;16:141–147. [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi M,E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Welty SE, Smith CV, Benzick AE, Montgomery CA, Hansen TN. Investigation of possible mechanisms of hepatic swelling and necrosis caused by acetaminophen in mice. Biochem. Pharmacol. 1993;45:449–458. doi: 10.1016/0006-2952(93)90082-8. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 2010b;247:169–78. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]