Abstract
Nonylphenol (NP), a by-product of alkylphenol ethoxylates, is a pervasive surfactant that activates the xenosensing nuclear receptor, the pregnane X-receptor (PXR) in transactivation assays in vitro. We are interested in determining if NP activates PXR in vivo, determining if hPXR and mPXR act similarly, and investigating the role of PXR in protecting individuals from NP. Wild-type (WT), PXR-null, and humanized PXR (hPXR) mice were treated with NP at 0, 50 or 75 mg/kg/day for one week, and cytochrome P450 (CYP) induction, liver histopathology, and serum NP concentrations were examined. WT mice treated with NP showed induction of Cyp2b, and male-specific induction of Cyp2c and Cyp3a. CYPs were not induced in PXR-null mice, demonstrating that PXR is necessary for NP-mediated CYP induction. CAR-mediated CYP induction was not observed in the PXR-null mice despite previous data demonstrating NP is also a CAR activator. hPXR mice only showed moderate Cyp induction, suggesting that hPXR is not as sensitive to NP as mPXR in vivo. NP-mediated Cyp3a induction from three human hepatocyte donors was not significant, confirming that hPXR is not very sensitive to NP-mediated CYP induction. Lastly, mice with PXR (mPXR and hPXR) showed lower NP serum concentrations than PXR-null mice treated with NP suggesting that PXR plays a role in decreasing liver toxicity by basally regulating Phase I-III detoxification enzymes that promote the metabolism and elimination of NP. In summary, PXR is required for NP-mediated CYP-induction, and mPXR mediates greater CYP induction than hPXR in vivo, and the presence of PXR, especially mPXR, is associated with altered histopathology and increased clearance of NP.
Keywords: PXR, P450s, Nonylphenol, CAR
Introduction
Nonylphenol (NP) is a biological degradation product of the alkylphenol ethoxylates that are widely used in the United States as intermediates for the production of industrial products such as detergents, lubricants, agrichemicals, rubber manufacturing, and personal care products (Reed, 1978). Commercial NP is a mixture of various isomers with para-substituted branched NP predominating in the mixture (United States Environmental Protection Agency, 2005)(Fig. 1). A large body of research has demonstrated that NP is an environmental estrogen (Soto et al., 1991; White et al., 1994; Lech et al., 1996; Wilson et al., 2004; Isidori et al., 2010) and one of the few anthropogenic environmental estrogens shown to induce mammary cancer incidence in a rodent model (Acevedo et al., 2005).
Figure 1.
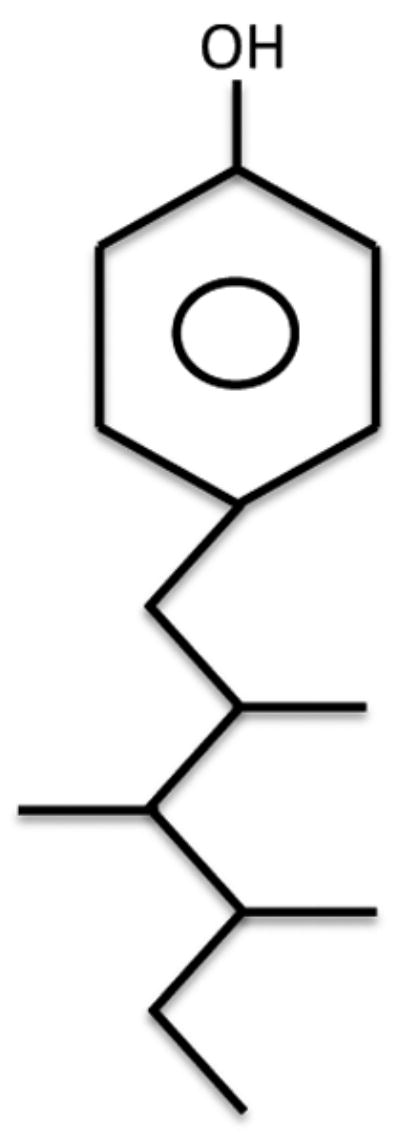
Structure of a para-substituted branched nonylphenol molecule.
Furthermore, NP is one of the most prevalent chemicals in the streams of the United States, and is found in more than 50% of 139 rivers and streams tested in a United States Geological Survey study. When found it is often the chemical present at the highest concentration because of its ability to adsorb strongly to soils and sediments, and its long half-life (Kolpin et al., 2002). Furthermore, NP is quite stable and remains in the sludge even after wastewater treatment (Abad et al., 2005). There is a comprehensive ban of NP and NP ethoxylate surfactants in the European Union in part because of NP's stability in the environment, aquatic toxicity, and endocrine disrupting activity (Renner, 1997; Quednow and Püttmann, 2009).
Most NP is rapidly absorbed, metabolized, and eliminated within 24 hours of exposure in rats (Green et al., 2003). NP is metabolized by several cytochrome P450s in families 1-4; however, the use of recombinant CYP2B6 indicates that this human CYP is the predominant enzyme involved in NPs phase I metabolism (Lee et al., 1998). Phenobarbital-induced rat microsomes and the inhibition of Cyp-mediated activities in mice confirm that Cyp2b > Cyp3a members are the key CYPs involved in NP metabolism (Lee et al., 1998; Acevedo et al., 2005). NP can be glucuronidated directly or after CYP-metabolism by UGT2B enzymes that are primarily by MRP2 (Daidoji et al., 2003).
The Pregnane X Receptor (PXR; NR1I2) is a transcription factor involved in the regulation of several genes crucial in the detoxification of xenobiotics and endobiotics. PXR is activated by a variety of steroids, herbal medicines, pharmaceuticals, and environmental chemicals (Kliewer et al., 1998; Hernandez et al., 2009a), including several environmental estrogens such as DDT, endosulfan, dieldrin and NP (Mikamo et al., 2003; Kretschmer and Baldwin, 2005; Lemaire et al., 2006). PXR's promiscuity is attributed to its flexible ligand binding domain allowing it to accommodate ligands that vary greatly in size, shape, and polarity (Watkins et al., 2001; Xue et al., 2007). Following activation, PXR heterodimerizes with RXRα (NR2B1), binds its response elements, and induces phase I-III enzymes (Hernandez et al., 2009a), including several genes involved in the detoxification of nonylphenol such as MRP2 (Kast et al., 2002), and several CYPs in families 2 and 3 (Waxman, 1999). CYP enzymes induced by mouse PXR include Cyp2b10 and the classical biomarker, Cyp3a11 (Hernandez et al., 2009a).
NP also activates rodent and human PXR in transactivation assays performed in vitro (Masuyama et al., 2000; Hernandez et al., 2007). In addition, NP activates PXR's relative, the constitutive androstane receptor (CAR; NR1I3) as determined by mouse CAR transactivation assays (Hernandez et al., 2007; Baldwin and Roling, 2009). In vivo studies with CAR +/+, and CAR -/- mice demonstrated that Cyp2b10 is induced in a CAR-dependent manner. Furthermore, human hepatocytes and humanized CAR mice treated with NP show Cyp2b10 induction, demonstrating that both mouse and human CAR are activated by NP in vivo (Hernandez et al., 2007). However, similar studies that test whether PXR is required for CYP induction by NP have not been performed in vivo.
There is circumstantial evidence that NP activates PXR in vivo. NP has been shown to induce Cyp3a subfamily members in rat liver (Lee et al., 1996) as well as mouse liver; (Masuyama et al., 2001). Furthermore, while the NP-mediated induction of Cyp2b in WT mice was lost in CAR-null mice; the induction of several other Cyps including Cyp2a4 and Cyp3a11 was observed in CAR-null female mice suggesting NP activation of PXR (Hernandez et al., 2009b). One of the goals of this paper is to prove that NP activates mouse and human PXR in vivo.
Furthermore, we are interested in determining if PXR protects individuals from NP exposure. Even though PXR is crucial in the induction of detoxification enzymes, the presence or activation of PXR has rarely been shown to protect individuals from xenobiotics. In contrast, PXR-null mice show fewer adverse hepatotoxic effects after co-treatment with pregnenolone 16α-carbonitrile (PCN) and acetaminophen (APAP), demonstrating the role of PXR in the bioactivation of acetaminophen to N-acetyl-p-benzoquinone imines (NAPQI) (Guo et al., 2004). However, PXR is important in protecting individuals from bile acids, and PXR-null mice treated with lithocholic acid exhibit significantly higher hepatoxicity than WT mice (Xie et al., 2001). This hepatoxicity is attributed to the inability of PXR-null mice to respond and induce enzymes involved in the detoxification and excretion of this toxic bile acid.
In this study, we investigated PXR's role in the basal regulation of drug metabolizing CYPs as basal regulation may be important in protecting the liver from xenobiotics, especially after initial exposure and before an inductive response can occur (Mota et al., 2010). Then we investigated the role of PXR in NP-mediated CYP induction in WT, PXR-null, and hPXR mice to determine if NP activates mPXR and hPXR in vivo. Cyp3a11 induction was also assessed in primary human hepatocytes to confirm hPXR's role in responding to NP. Lastly, liver histopathology and serum levels of NP were compared between treatment groups to test whether PXR is associated with increased clearance and protection of the liver from NP. Overall, the data indicates that mPXR is necessary for CYP induction, hPXR is not as sensitive as mPXR in vivo, and PXR is associated with lower serum concentrations of NP in mice.
Materials and Methods
Animals
All studies were performed in agreement with NIH guidelines for the humane use of research animals and approved by the Clemson University Animal Care and Use Committee. Mice were provided with food and water ad libitum. Male and female 8-10 week old B6129 (WT), PXR-null (Staudinger et al., 2001) and hPXR (Lichti-Kaiser and Staudinger, 2008) mice were randomly split into groups (n = 4-6). The control mice were fed 100 μl of honey with a syringe. Treated mice were fed 50 or 75 mg/kg/day NP (technical grade with approximately 85% p-isomers; Fluka Chemical Co., Seelze, Germany) dissolved in 100 μl honey for seven consecutive days. The dosages were based on previous studies demonstrating CYP induction (Acevedo et al., 2005; Hernandez et al., 2006; Hernandez et al., 2007). Furthermore, it is estimated that a dose as low as 50 mg/kg/day NP for 7 days can reach metabolic saturation and lead to steady state concentrations in the serum (Green et al., 2003). The positive control, dexamethasone (technical grade 98%; Sigma St. Louis MO) was dissolved in 100 μl of corn oil and injected at 75 mg/kg/day once per day for three days. All mice were anesthetized 6 hours following the last treatment, blood was collected from the mice by heart puncture, and livers excised.
Liver sample preparation
Livers from euthanized mice were excised, weighed, snap frozen, and cut into several pieces for RNA extraction, and microsome preparation. Total RNA was isolated using a modified TRI-reagent protocol of guanidinium thiocyanate-phenol-chloroform extraction following manufacturer's specifications (Sigma, St. Louis, MO). To remove residual DNA from the RNA a DNAse digestion was performed (Promega Corporation, Madison WI). RNA was quantified at 260 nm and 280 nm wavelengths using a spectrophotometer and samples were immediately stored at −80°C. For cDNA preparation reverse transcription was performed using MMLV-RT, dNTP mixture, and random hexamers followed by immediate storage at −20°C. Microsomes were homogenized and isolated by differential centrifugation; aprotonin, leupeptin, and PMSF were used as protease inhibitors. After protein quantification (Bio-Rad, Hercules, CA), microsomes were stored at −80°C.
Quantification of CYP expression
QPCR was performed using previously published protocols and primers (Muller et al., 2002; Wiwi et al., 2004; Hernandez et al., 2009b). All samples were diluted 1:10 and standard curves were performed using a composite of samples diluted from 1:1 to 1:1000. To quantify gene expression amplifications were performed in triplicates using a 96-well iQ5™ multicolor Real-Time PCR Detection System (Bio-Rad) with 0.25× SybrGreen. Data normalization of Q-PCR results was performed using the expression of 18S rRNA as the housekeeping gene. Quantification was done by taking the efficiency curve of the Q-PCR reaction to the power of the threshold cycle (Ct) divided by 18S (Muller et al., 2002).
Immunoblotting was used to quantify CYP protein levels. Quantification of mouse CYP is referred to by subfamily because each antibody may recognize more than one mouse isoform in a subfamily. Antibodies from different sources were used to quantify mouse Cyp2b, 2c, and 3a. Cyp2c and Cyp3a were quantified using a human CYP2C8/9/19 or rat CYP3A1 antibodies respectively from Chemicon, (Billerica, MA). A newly developed polyclonal antibody was used to quantify Cyp2b (Mota et al., 2010). Proteins were separated electrophoretically on a 10% polyacrylamide gel (SDS-PAGE), and transferred to nitrocellulose membrane (Bio-Rad) using 30-50 μg of microsomal protein. After protein transfer the nitrocellulose was blocked in 2.5-5% dried milk for 30 min to 1 hour and washed in Tris Buffer Saline pH=7.4 0.1%Tween 20. Primary antibodies were incubated at room temperature for 2h and overnight at 4°C prior to secondary antibody incubation at room temperature for 2h (Mota et al., 2010). β-Actin (Sigma, St. Louis, MO) was used as a housekeeping gene. Secondary antibodies used were based on the source of the primary antibodies mainly goat anti-mouse or goat anti-rabbit (Bio-Rad; 1:500) Protein detection was performed by chemiluminescence using Immun-Star AP Chemiluminescent Protein Detection Systems and quantified by Chemi Doc XRS HQ using Quantity One 4.6.5 (Bio-Rad, Inc).
Primary human hepatocytes
Human hepatocytes were obtained from Cellz Direct (Pittsboro, NC) and maintained as previously described (Hernandez et al., 2007). The hepatocytes were extracted from three different donors that were Caucasian females with ages of 52, 29 and 77. Cells were treated with DMSO (UT), NP, or phenobarbital (PB) as a positive control (Sigma, St. Louis, MO) for 24h. After treatments, the cells were harvested, RNA extracted, cDNA prepared and Q-PCR performed using CYP3A4 and 18s primers as previously described (Hernandez et al., 2007).
Histology samples
Liver samples from untreated and NP-treated WT, PXR-null, and hPXR mice were fixed in 10% formalin. Samples were trimmed, processed, embedded, sectioned, and stained with hemotoxylin and eosin at Colorado Histo-Prep (Fort Collins, CO) for blind histopathological evaluation. Standard mouse toxicologic pathology criteria and nomenclature were used to evaluate microscopic tissue changes in each of the different treatment groups.
Nonylphenol Extraction and GC quantification
Nonylphenol was extracted from mice serum using a modified protocol (Danzo et al., 2002). In brief, 150 μl of mouse serum was vigorously vortexed with 1 mL saturated NaCl in glass tubes, afterwards 1 mL of ethyl acetate was added and vortexed. Mixture was allowed to settle for 10 min at room temperature, or until mixture turned biphasic. Supernatant was transferred to new glass tubes, where 1 mL of water was added, vortexed and supernatant was again transferred to a new glass tube. Solvent was evaporated with nitrogen gas, and reconstituted with 400 μl of ethyl acetate.
Standards of NP were prepared at a concentration of 0.01, 0.05, 0.1, 0.5, 1.00 μg/mL in ethyl acetate. Spectra were recorded using Agilent 7890 A gas chromatograph (Agilent Technologies, Inc., Santa Clara, CA) using 70eV electron ionization at an ion source temperature of 230°C. Chromatographic separations were performed using 30m × 0.25mm, 0.25 μm film DB-5ms column (Agilent Technologies, Inc.) GC analyses were done using a splitless injection at 250°C, followed by a column temperature program change of 100 to 300°C over 10°/min. Quantification was done by selected ion monitoring with an ion dwell time of 25 msec. The ions used for NP were, m/z 135, 149, and 220. Quantification of all samples were performed by the external standard technique in which a known amount of a reference standard was analyzed; areas of selected ion monitoring chromatographic peaks were integrated using GC ChemStation© software (Agilent Technologies, Inc.) Areas under the peaks were converted to plasma concentrations using the standard curve of NP. The detection limit was 0.01 ng/μL.
Statistical analysis
One-way ANOVA followed by Dunnett's multiple comparison test was performed on Q-PCR data when comparing the CYP expression between the different mouse genotypes, and to determine statistically significant differences in NP serum concentrations between untreated and NP treated mice of different genotypes. Student's two-tailed t-tests were used to compare differences in CYP3A4 expression in human hepatocytes, and CYP protein expression measured by Western blotting. Fisher's 2×2 was performed to determine a significant difference between untreated and NP treated WT, PXR-null and hPXR mice that exhibited hepatocyte hypertrophy. Values of p < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Comparing basal CYP expression in mPXR, PXR-null, and hPXR mice
Q-PCR was performed to determine basal regulation of CYPs by mouse and human PXR. Different isoforms of CYPs were tested by Q-PCR to compare CYP expression in WT and PXR-null male and female mice, as well as between hPXR and PXR-null mice. The loss of PXR resulted in a significantly lower expression of Cyp3a11 in male and female PXR-null mice compared to WT mice. Interestingly, PXR-null mice showed significantly higher expression of Cyp2b10 and Cyp3a41 than WT mice (Table 1). Negative regulation of Cyp3a41 by PXR has been previously demonstrated (Anakk et al., 2004); however to our knowledge, this is the first time that Cyp2b10 has been shown to be negatively regulated by PXR. Surprisingly, mouse CYPs did not respond the same to human PXR (Table 2). For example, hPXR did not repress Cyp2b10 in the same manner as mPXR, nor was Cyp3a11 repressed in male hPXR mice. Overall, mPXR showed stronger basal regulation of mouse CYPs than hPXR when compared to the PXR-null mice.
Table 1. Comparison of CYP expression between WT and PXR-null mice.
| CYP | WT Female | PXR-null Female | WT Male | PXR-null Male |
|---|---|---|---|---|
| Cyp2b9 | 14.97 ± 4.96 a** | 6.47 ± 1.27 | 1.00 ± 0.81 | 0.69 ± 0.46 |
| Cyp2b10 | 2.42 ± 0.53 | 23.64 ± 5.53 b** | 1.00 ± 0.41 | 6.74 ± 2.28 d** |
| Cyp2c29 | 0.82 ± 0.28 | 1.42 ± 0.85 | 1.00 ± 0.28 | 1.87 ± 0.44 |
| Cyp3a11 | 1.19 ± 0.24 | 0.31 ± 0.06 b* | 1.00 ± 0.24 | 0.26 ± 0.08c* |
| Cyp3a41 | 60.98 ± 15.31 a* | 125.37 ± 23.26 b* | 1.00 ± 0.76 | 0.85 ± 0.31d** |
Data is expressed as relative data ± SEM
P-value < 0.05 and
p-value < 0.01; indicate statistical significance by one-way ANOVA followed by Dunnett's multiple comparison test.
Indicates significant difference between WT female and WT male.
Indicates significant difference between WT female and PXR-null female mice.
Indicates significant difference between WT male and PXR-null male mice.
Indicates significant difference between PXR-null female and PXR-null male mice.
Table 2. Comparison of CYP expression between PXR-null and humanized PXR mice.
| CYP | hPXR Female | PXR-null Female | hPXR Male | PXR-null Male |
|---|---|---|---|---|
| Cyp2b9 | 2.20 ± 0.53 | 4.98 ± 1.02 b* | 1.00 ± 0.64 | 0.05 ± 0.03 d** |
| Cyp2b10 | 0.65 ± 0.14 | 0.79 ± 0.40 | 1.00 ± 0.56 | 0.36 ± 0.11 |
| Cyp2c29 | 0.59 ± 0.27 | 0.63 ± 0.11 | 1.00 ± 0.28 | 1.71 ± 0.62 |
| Cyp3a11 | 0.22 ± 0.05 | 0.25 ± 0.06 | 1.00 ± 0.44 | 0.23 ± 0.06 |
| Cyp3a41 | 9.71 ± 1.75 | 22.78 ± 4.78 b* | 1.00 ± 0.87 | 0.34 ± 0.24 d** |
Data is expressed as relative data ± SEM
P-value < 0.05 and
p-value < 0.01; indicate statistical significance by one-way ANOVA followed by Dunnett's multiple comparison test.
Indicates significant difference between hPXR female and hPXR male.
Indicates significant difference between hPXR female and PXR-null female mice.
Indicates significant difference between hPXR male and PXR-null male mice.
Indicates significant difference between PXR-null female and PXR-null male mice.
CYP induction by NP in vivo is PXR-dependent
Immunoblots were performed to determine NP-mediated induction of CYPs in WT, PXR-null, and hPXR mice. Cyp2b was significantly induced by NP in male and female WT mice (Fig. 2A). Cyp3a and Cyp2c (data not shown) were significantly induced in male mice treated with 50 or 75 mg/kg/day of NP (Fig. 2A). Female WT mice did not demonstrate Cyp3a induction by NP. We have previously observed a lack of Cyp3a induction by NP in WT mice (Acevedo et al., 2005; Hernandez et al., 2009b), presumably because of NP-mediated repression of Cyp3a41, a female specific CYP negatively regulated by PXR (Anakk et al., 2004)(Table 1). WT mice treated with the positive control, dexamethasone, also exhibit significant induction of Cyp2b and Cyp3a (Suppl. Data Fig. 1A). Because NP activates CAR and PXR (Hernandez et al., 2007), Cyp induction may be mediated by either CAR or PXR activation (especially Cyp2b). However, PXR-null mice did not demonstrate CYP induction following NP treatment (Fig. 2B), indicating that NP-mediated CYP induction is PXR-dependent.
Figure 2.
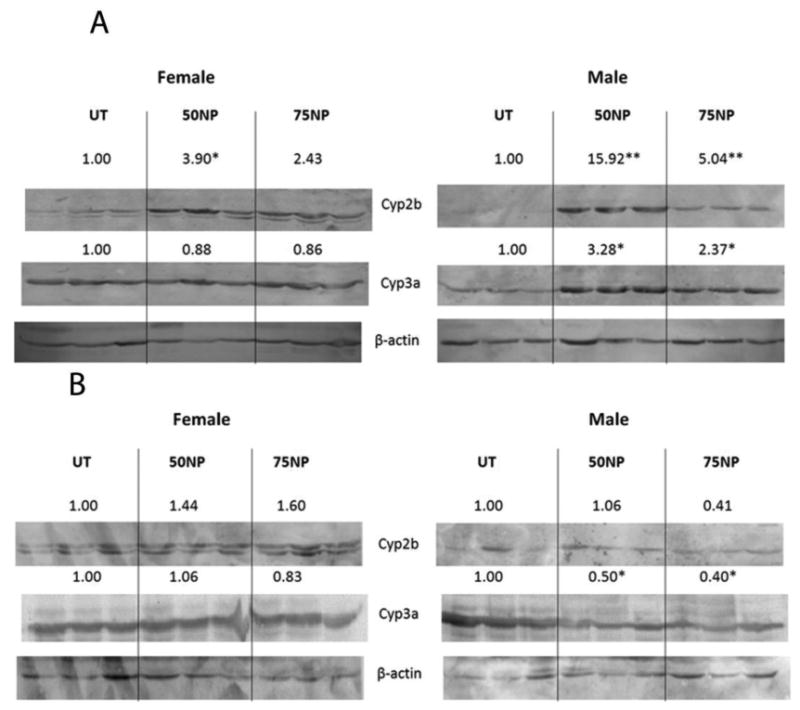
Nonylphenol activates mouse PXR. Western blots of Cyp2b, Cyp3a, and β-actin from hepatic microsomes of untreated, 50 or 75 mg/kg/day nonylphenol treated as described in the Materials and Methods. Actin was used as a housekeeping gene. A) Wild-type male and female untreated and NP-treated mice. B) PXR-null male and female untreated and NP-treated mice. Blots were quantified densitometrically and the relative mean differential expression as compared to the controls is reported above the blots. An asterisk indicates a statistically significant difference compared to the untreated group using Student's t-test (p < 0.05).
hPXR activation by NP
Humanized PXR mice lack mouse PXR and instead contain human PXR. They are used to reduce uncertainty when extrapolating data from rodents to humans, and demonstrate the key role of hPXR in mediating chemical-mediated CYP induction (Lichti-Kaiser and Staudinger, 2008). The hPXR mice did not appear to respond to NP treatment as robustly as mPXR (WT) mice. Female hPXR mice had no significant CYP induction from either of the NP treatments (50 or 75 mg/kg/day) (Fig. 3A). Male hPXR mice treated with NP showed induction of Cyp2b and Cyp3a (Fig. 3B), albeit weaker induction than the WT mice. In comparison, hPXR mice treated with dexamethasone, as a positive control, showed Cyp2b induction in both male and female mice, but Cyp3a induction was not significant (Suppl Data Fig. 1B). Overall, CYP induction was more robust in mPXR mice than hPXR mice suggesting that NP is a weaker hPXR activator than mPXR activator in vivo.
Figure 3.
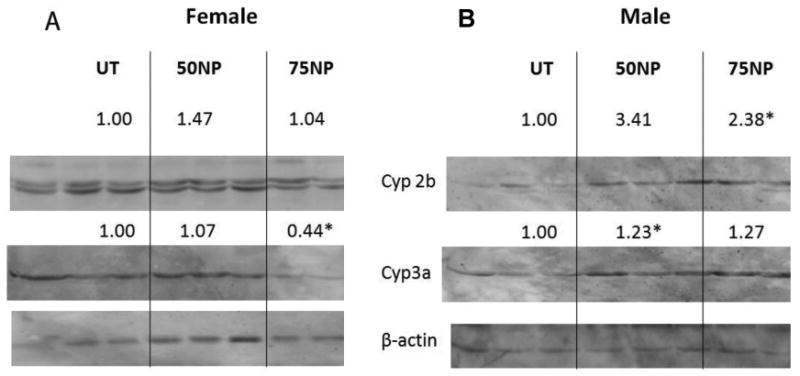
Nonylphenol is a weak activator of human PXR in vivo. Western blots of hepatic microsomes were performed for Cyp2b, Cyp3a, and β-actin from hepatic microsomes of male and female humanized mice treated with 0, 50, or 75 mg/kg/day NP. Actin was used as a housekeeping gene. A) Female untreated and NP-treated hPXR mice. B) Male untreated and NP-treated hPXR mice. Blots were quantified densitometrically and the relative mean differential expression as compared to the controls is reported above the blots. An asterisk indicates a statistically significant difference compared to the untreated group using Student's t-test (p < 0.05).
CYP3A4 expression in human hepatocytes
Weak induction of CYPs in hPXR mice could be caused by poor interactions between the necessary murine coactivators and human PXR. Therefore, we treated fresh human hepatocytes from three donors with NP and phenobarbital (known to activate human CAR and PXR) (Moore et al., 2000) for 24 hours and then measured CYP3A4 expression. While most patients showed weak CYP3A4 induction, there was no statistically significant difference in CYP3A4 mRNA expression between UT and NP treated hepatocytes in any of the three donors (Fig. 4A-4C). A statistically significant higher expression of CYP3A4 was observed in all of the three patients treated with phenobarbital (Fig. 4D-4F). Thus, NP does not significantly increase CYP3A4 expression in human hepatocytes. This result coincides with the hPXR result and indicates that NP is a weaker hPXR activator than mPXR activator.
Figure 4.
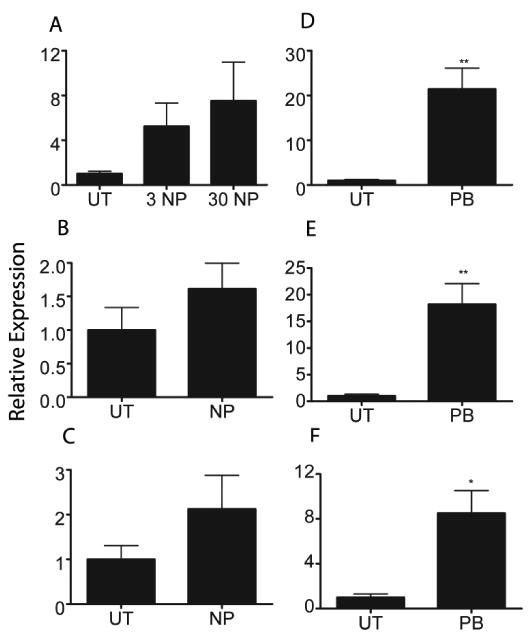
CYP3A4 expression in human hepatocytes treated with nonylphenol. Q-PCR was performed with human hepatocytes from three different donors treated with NP or PB. A) Donor 1 NP treated hepatocytes. B) Donor 2 NP treated hepatocytes C) Donor 3 NP treated hepatocytes D) Donor 1 PB treated hepatocytes E) Donor 2 PB treated hepatocytes F) Donor 3 PB treated hepatocytes. An asterisk indicates a statistically significant difference compared to the untreated group using Student's t-test (p < 0.05).
Histopathology
Given that NP activates PXR and PXR regulates several detoxification genes both basally and when activated, we wanted to test whether PXR protects the liver from NP-mediated damage. Therefore, liver histopathology was assessed in formalin fixed H&E stained slides from the different mouse genotypes, and NP treatments at 0 and 50 mg/kg/day. Untreated WT mice exhibited normal liver tissue (Fig. 5A); whereas NP-treated WT mice (Fig. 5B) showed significant hepatocyte hypertrophy in the periportal region (Table 3). Increased eosin staining was also observed in NP-treated livers from WT mice, which may be a sign of increased protein synthesis. In the PXR-null (Fig. 5-4D) and hPXR (not shown) mice the liver showed minimal hepatocyte hypertrophy in the periportal regions regardless of the treatment (untreated or NP-treated) (Table 3), and increased eosin staining was observed in both PXR-null and hPXR mice. Therefore, there was no statistically significant difference in the hypertrophy scoring between UT and NP-treated PXR-null mice or hPXR mice (Table 3). However, some of the NP-treated PXR-null mice did show a subjectively larger increase in eosin staining (Fig.5), but no significant differences in hypertrophy. The histopathology data for the PXR-null and hPXR mice is difficult to interpret because of minimal hypertrophy in the untreated mice. Overall, NP appears to cause some damage or an acute response as observed through increased eosin staining and hypertrophy in the WT mice, which may be mediated through PXR and was more responsive in mPXR (WT) mice than hPXR mice.
Figure 5.
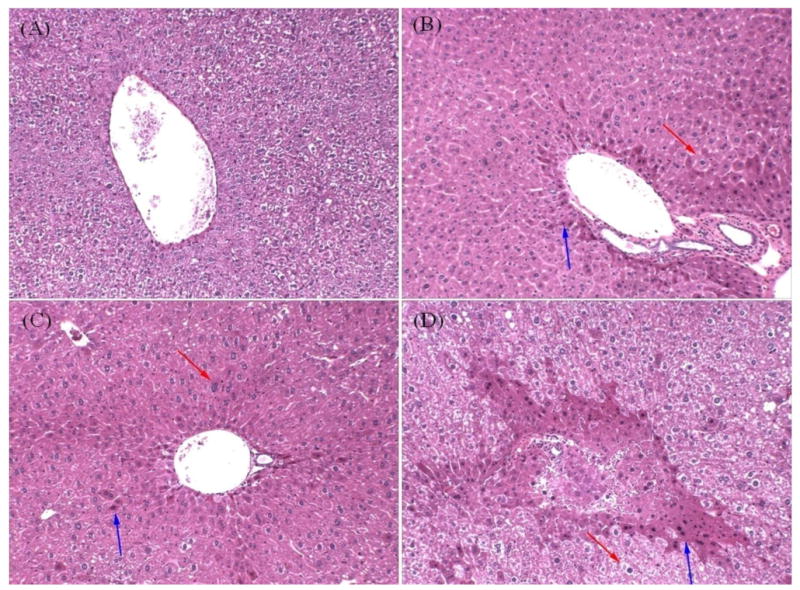
Liver histopathology in untreated and 50 mg/kg/day NP treated mice. Mouse liver fragments were stained using hematoxylin and eosin and examined as described in the Materials and Methods. A) Untreated wild-type mouse liver. B) NP treated wild-type mouse liver. C) Untreated PXR-null mouse liver. D) NP treated PXR-null mouse liver.
Table 3.
Association of PXR status with hepatocyte hypertrophy in untreated and NP treated mice.
| WTa | PXR-nulla | hPXRa | |||
|---|---|---|---|---|---|
| UT | NP | UT | NP | UT | NP |
| 0/6 | 4/6* | 6/8 | 6/8 | 5/8 | 5/8 |
Data shown represents the number of mice that exhibited hepatocyte hypertrophy/total number of mice.
There were no differences between male and females, thus data was combined for analysis.
An asterisk indicates a significant difference between untreated and nonylphenol treated WT mice (p < 0.05) by a one-tailed Fisher's 2×2 test.
Nonylphenol serum concentrations
Because increased damage was observed in the NP-treated WT mice and increased eosin staining was observed in the periportal regions of some PXR-null mice, GC-MS was used to quantify serum NP concentrations and assess whether PXR-status effected the ability of WT, PXR-null, and hPXR mice to clear NP. In general, there was a trend indicating greater NP in the serum of treated mice than untreated mice, especially at 50 mg/kg/day NP. Differences in serum NP concentrations between treatment groups were not significantly different in WT and hPXR mice (Table 4A,B). There was no trend in NP serum concentrations in female hPXR mice (Table 4) because the female untreated hPXR mice showed high concentrations of NP. Male and female PXR-null mice treated with 50 mg/kg/day NP have greater amounts of NP in their serum than untreated PXR-null mice (Table 4A, B), suggesting that the lack of PXR caused perturbed clearance of NP.
Table 4.
Nonylphenol serum concentrations, as measured by GC-MS in mice treated with 0, 50, or 75 mg/kg/day NP.
| A. Female | |||
|---|---|---|---|
| NP | WT# | PXR-null# | hPXR# |
| 0 | 0.0285 + 0.0179 | 0.0221 ± 0.0122b | 0.1182 ± 0.0250 |
| 50 | 0.0926 ± 0.0322 | 0.1493 ± 0.0223b | 0.0950 ± 0.0133 |
| 75 | 0.0791 ± 0.0100 | 0.0574 ± 0.0279 | 0.0796 ± 0.0060 |
| B. Male | |||
| NP | WT | PXR-null | hPXR |
| 0 | 0.0103 ± 0.0042 | 0.0335 ± 0.0255b | 0.0343 ± 0.0038 |
| 50 | 0.0783 ± 0.0468 | 0.1432 ± 0.0118b | 0.1250 ± 0.0343 |
| 75 | 0.0024 ± 0.0011 | 0.0292 ± 0.0144 | 0.0585 ± 0.0121d |
| C. Male and Female | |||
| NP | WT | PXR-null | hPXR |
| 0 | 0.0194 ± 0.0092a | 0.0283 ± 0.0099c | 0.0902 ± 0.0237 |
| 50 | 0.0856 ± 0.0264a | 0.1459 ± 0.0076c | 0.1086 ± 0.0169 |
| 75 | 0.0364 ± 0.0141 | 0.0433 ± 0.0108 | 0.0690 ± 0.0077 |
Data is expressed as mean (μg/ml) ± SEM (n = 4-6 or n = 8-12 for combined male and female data). Detection limit was of 0.01μg/ml. Values calculated below detection limit but higher than 0 were assigned a value of ½ detection limit.
Indicates significant difference between untreated and NP-treated mice (p-value < 0.05)
Indicates significant difference between untreated and NP-treated mice (p-value < 0.01)
Indicates significant difference between untreated and NP-treated mice (p-value < 0.0001)
Indicates significant difference between WT and hPXR mice (p-value < 0.05)
Statistical significance determined by ANOVA followed by Dunnett's multiple comparison test.
Data from male and female mice was combined because there were no significant differences in serum concentration between males and females (Table 4C). NP-treated WT mice showed a significant increase in serum NP concentrations (Table 4C) when the data was combined because of the increased statistical power. The p-values comparing the untreated and 50 mg/kg/day groups from PXR-null mice also increased from 0.01 to 0.001. A trend was observed in the male, female, and combined data where 50 mg/kg/day treated WT mice had lower NP serum concentrations than 50 mg/kg/day treated PXR-null mice, although the data was not statistically significant (Table 4). However, when the data from WT and hPXR mice (all PXR-positive mice) was combined and compared to PXR-null mice, there was significantly less NP in the serum of PXR-positive mice than PXR-null mice (p = 0.045 by Student's t-test). Taken together, this suggests that the presence of PXR is protective from NP potentially by regulating enzymes and transporters important in metabolizing or clearing NP.
Discussion
PXR is important in the regulation of detoxification enzymes that are required to metabolize and eliminate compounds that may have deleterious effects such as NP (Waxman, 1999; Xie et al., 2001; Kretschmer and Baldwin, 2005; Hernandez et al., 2009a). Past studies have shown NP-mediated induction of CYP3A (Lee et al., 1996), and NP activation of PXR has been demonstrated in transactivation assays using mouse (Masuyama et al., 2000), rat, and human PXR (Hernandez et al., 2007). It is presumed that PXR is activated by NP; however, activation of PXR in vivo has not been demonstrated using knockout models. Instead only activation of CAR by NP has been definitively demonstrated in vitro and in vivo (Hernandez et al., 2007; Hernandez et al., 2009b). The data from this study showed that NP-mediated induction of Cyp2b and Cyp3a in male and female mice is PXR-dependent as induction was completely lost in PXR-null mice.
This is somewhat surprising because NP also activates CAR and we hypothesized that some CYP induction, such as the induction of Cyp2b, would be preserved in PXR-null mice due to CAR activation. Research has shown weak CYP induction by Q-PCR in CAR-null mice (Hernandez et al., 2009b) presumably due to activation of PXR; however, in this study there was no CYP induction observed via immunoblotting in NP-treated PXR-null mice. Overall, this suggest that CAR and PXR work together to regulate NP-mediated CYP induction. Most studies indicate that activated nuclear receptors often compete for resources (Yan et al., 1998; Miao et al., 2006; Lee et al., 2008); however, in this case CAR and PXR appear to work together to increase NP-mediated CYP induction.
Male mice showed larger NP-mediated CYP induction than females. Cyp2b and Cyp3a induction in males was observed at 50 and 75mg/kg/day NP with the 50mg/kg/day NP treatment showing greater CYP induction than the 75mg/kg/day NP-treatment. Female mice only showed a significant induction of Cyp2b by the 50mg/kg/day NP treatment, and not the 75 mg/kg/day NP treatment (Fig. 2). WT female mice did not show induction of Cyp3a protein levels following NP-treatment, which is common and even NP-mediated down-regulation of Cyp3a protein expression has been observed (Laurenzana et al., 2002; Acevedo et al., 2005; Hernandez et al., 2006; Hernandez et al., 2009b), presumably due to repression of Cyp3a41 and potentially Cyp3a44 (Anakk et al., 2004; Hernandez et al., 2006; Hernandez et al., 2009b).
Male and female mice treated with 75 mg/kg/day consistently showed less CYP-induction than mice treated with 50 mg/kg/day NP (Fig. 2). Mice treated with 75 mg/kg/day were harder to feed and showed less inclination for the NP-tainted honey. It is possible that the dose they ultimately received was less in the 75 mg/kg/day group than the 50 mg/kg/day group because they did not fully ingest their share. Corroborating evidence was provided from the serum concentrations of NP as the 75 mg/kg/day group showed consistently lower NP concentrations than the 50 mg/kg/day group (Table 4). Therefore, it is our opinion that the reduced induction of CYPs and reduced serum concentrations at 75 mg/kg/day is indicative of the poor feeding rate of the NP-treated mice at 75 mg/kg/day.
CYP induction was also tested in hPXR mice, a model that is more relevant to human health (Xie et al., 2000; Lichti-Kaiser and Staudinger, 2008). Interestingly, NP is a weak activator of hPXR relative to mPXR in vivo. For example, hPXR mice treated with NP showed no significant induction of CYPs in females and much weaker induction of Cyp2b and Cyp3a in males than WT (mPXR) mice (Fig. 3). hPXR may not work as well as mPXR because of poor interactions with mouse co-activators in the hPXR mouse, or NP is not as strong of an hPXR activator as mPXR activator in vivo.
Therefore, we obtained hepatocytes from human donors to determine NP's ability to induce CYP3A4 expression in another model directly relevant to human health. NP-treated human hepatocytes have hPXR and the necessary human co-activators for proper CYP induction. Q-PCR results showed that NP did not induce CYP3A4 significantly, but the positive control phenobarbital showed significant induction (Fig. 4). A previous study in our laboratory demonstrated CYP2B6 induction by NP in hepatocytes, and attributed the induction to CAR, but could not rule out PXR (Hernandez et al., 2007). CYP2B6 induction in the human hepatocytes and hCAR mice was also weaker than WT mice and PB-mediated induction. This indicates the importance of in vivo results as transactivation assays suggested similar activation of rodent and human PXR by NP (Hernandez et al., 2007). Overall, the results indicate that NP is a weaker human PXR activator than mouse PXR activator and this is in part the reason for the weak CYP induction in hPXR mice and human hepatocytes.
PXR was also found to be important in the basal regulation of some CYPs. For example, male and female mice showed significantly lower expression of Cyp3a11 in PXR-null mice than WT mice, indicative of a direct role of PXR in Cyp3a11 basal regulation when a chemical activator is not present. Cyp2b10 and Cyp3a41 were found to be negatively regulated by PXR, as higher expression of these genes was observed in PXR-null mice (Table 1). Our results confirm earlier work that Cyp3a41 is negatively regulated by PXR (Anakk et al., 2004). To our knowledge this is the first time that Cyp2b10 has been shown to be negatively regulated by PXR. hPXR did not always demonstrate similar basal regulation as mPXR (Table 2). For example, the mouse Cyp2b members, Cyp2b9 and Cyp2b10 did not respond to human PXR regulation in the same manner as mPXR. This suggests either differential binding of hPXR to co-regulators than mPXR, different function of hPXR than mPXR, or this result could be an artificial function of the hPXR mouse. hPXR is under the control of the transthyretin promoter in the hPXR mouse (Lichti-Kaiser and Staudinger, 2008), not the typical hPXR or mPXR promoter and therefore may be overexpressed or expressed at different times than PXR would typically be expressed leading to altered expression of CYPs.
Histopathology demonstrated that NP caused hypertrophy in the periportal regions of WT mice. A previous study with rats also demonstrated increased hypertrophy following oral NP-treatment (Woo et al., 2007). The hepatocyte hypertrophy coincides with CYP induction and increased eosin staining around periportal regions, which is thought to be attributed to increased protein synthesis. Therefore, this data is indicative of an acute compensatory response to NP exposure in WT mice.
However, it is difficult to establish whether PXR-null mice are more sensitive to the toxic or hypertrophic effects of NP on the liver than WT mice. A significant difference in hypertrophy was not measured between treated and untreated PXR-null mice. This is because PXR-null and hPXR mice show hypertrophy regardless of treatment (Table 3). The observed hypertrophy in treatments of PXR-null and hPXR mice (Fig. 5C-5D; hPXR data is not shown) indicates that mouse PXR is important for the normal physiology of the liver, such as the constitutively regulating the expression of some CYPs (Table 1,2) There have been other studies that have observed hepatocyte hypertrophy in knockout mouse models, including those that lack peroxisomes and HNF4α-null mice (Hayhurst et al., 2001; Dirkx et al., 2005). Interestingly, there is more eosin staining around periportal regions in some of the NP-treated PXR-null mice than in untreated PXR-null mice (Fig. 5C-5D), which suggests increased protein synthesis. Whether this is indicative of minor cellular damage caused by NP is not known, but suggests some sensitivity in treated PXR-null mice relative to untreated PXR-null mice.
PXR appears to provide some minor protection from NP as PXR-null mice treated with NP showed increased eosin staining relative to untreated PXR-null mice. Furthermore, only PXR-null mice showed significantly higher NP serum concentrations (Table 4A,B) as mice with PXR (mPXR or hPXR) had lower serum concentrations of NP after treatment with 50 mg/kg/day than PXR-null mice treated with 50 mg/kg/day NP, especially the mPXR mice. Because CYP induction occurred in PXR-positive mice, but not PXR-null mice; the data suggests that PXR positive mice respond to NP and induce detoxification enzymes that are crucial in helping eliminate a toxic insult. In addition, the role that PXR plays in the basal regulation of CYPs and potentially other enzymes and transporters cannot be discounted when considering PXR's protective role (Table 1-2).
However, PXR-status was not associated with increased clearance in female hPXR because of contamination of the serum with NP (Table 4A) for unknown reasons. NP contamination has been observed in previous studies (Soto et al., 1991; Danzo et al., 2002) where measurable concentrations of NP were found in untreated samples. These NP concentrations have been attributed to NP leaching from the plastic tubes during storage and centrifugation steps. Another possible source of contamination, in this study, might be from the plastic water bottles, as well from the honey fed to the mice that was also stored in plastic bottle. One study showed significant concentrations of NP found in bottled water in all commercial bottles tested (Li et al., 2010). Why the contamination occurred in several untreated hPXR female mice to a greater degree than other treatments is unknown. If the contamination occurred prior to treatment of the mice then this may explain the poor induction in female hPXR mice relative to mPXR mice. However, this does not explain why male hPXR mice also showed much less CYP induction than mPXR mice. Furthermore, we did not observe significant induction in the control hPXR mice, but weak induction of CYPs in the treated hPXR mice. Therefore, most likely the contamination of the samples occurred during serum storage and NP extraction.
PXR has been shown to induce several detoxification enzymes in response to many different chemicals (Hernandez et al., 2009a). PXR also protects individuals from bile acids (Staudinger et al., 2001; Xie et al., 2001). However, the presence or activation of PXR has rarely been shown to protect individuals from anthropogenic xenobiotics. This study indicates that the presence of PXR reduces serum NP concentrations in mice treated with 50 mg/kg/day NP compared to mice that lack PXR. Further, the reduced NP serum concentrations in PXR-positive mice are associated with PXR-dependent CYP induction, suggesting some level of protection is provided by PXR. The reduced CYP-induction in hPXR mice and human hepatocytes suggests less protection by hPXR than mPXR, but also may leave humans less susceptible to PXR-mediated adverse drug reactions following NP exposure.
Human NP exposure is thought to vary greatly depending on occupation and fish consumption. Exposure to NP from consumer products is about 0.6 μg/kg/day; exposure from regional environmental sources of which fish intake is 70-80% of a typical consumers NP consumption, is probably less than 6 μg/kg/day (Commission and Center, 2002). Therefore, the average person is exposed to 10,000× less than per day than these experimental murine dosages However, a textile laborer may be exposed to as much as 4.42 mg/kg/day of NP (Commission and Center, 2002), or approximately only 10-fold less than what was provided to the mice per day during this acute exposure. How individuals may respond after chronic treatment was not examined. Metabolic saturation does occur after approximately 7-days of treatment in doses of NP above 50 mg/kg/day (Green et al., 2003). It is hypothesized that the metabolic saturation leads to enhanced bioaccumulation and ultimately biological effects such as endocrine disruption (Green et al., 2003). Whether PXR activation or CYP induction would be different after longer periods of time is unknown, but probably would not significantly differ. Other parameters such as increased hyperplasia are probably more sensitive to the exposure time, and therefore may increase following longer exposure regimens.
In summary, results suggest that the presence of PXR is important in the clearance of NP from mice as PXR-positive mice (especially mPXR mice) treated with 50 mg/kg/day NP have lower serum NP concentrations than PXR-null mice. This may be due to PXR-dependent induction of drug metabolizing enzymes such as CYPs, or may be because of PXR's role in the basal regulation of CYPs and presumably other drug metabolizing enzymes. However, whether PXR provides actual protection from hepatotoxicity by NP is unresolved. NP-induced liver pathology in PXR-null mice was only subjectively increased over untreated PXR-null mice. In addition, hPXR appears to be less sensitive to the activating effects of NP than mPXR thus the protective role of PXR may be less in humans than mice. Given the role of PXR in basal and inducible regulation of CYPs, and the lower level of serum NP in PXR-positive mice, PXR probably provides some level of protection from NP in humans. Susceptible populations that have lower expression (newborns) or activity of PXR (polymorphsims) (Lim et al., 2005; Vyhlidal et al., 2006) may exhibit greater sensitivity to plasticizers such as NP due to their limited detoxification and elimination capacity.
Supplementary Material
Supplemental Data; Figure 1. Dexamethasone activates PXR. Western blots of Cyp2b and Cyp3a were performed using hepatic microsomes from untreated, and 75 mg/kg/day Dexamethasone treated male and female mice as described in the Materials and Methods. (A) Cyp induction in WT (mPXR) mice treated with Dexamethasone. (B) Cyp induction in hPXR mice treated with Dexamethasone. An asterisk indicates statistically significant difference compared to the untreated group performed by Student's t-test (p < 0.05).
Acknowledgments
The authors would like to thank Dr. Jeff Staudinger for donating his PXR-null and hPXR mouse models, and Dr. Melissa Riley for help with the GC-MS analysis. This work was supported by NIH grant R15-ES017321 and Clemson University start-up funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abad E, Martínez K, Planas C, Palacios O, Caixach J, Rivera J. Priority organic pollutant assessment of sludges for agricultural purposes. Chemosphere. 2005;61:1358–1369. doi: 10.1016/j.chemosphere.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Acevedo R, Parnell PG, Villanueva H, Chapman LM, Gimenez T, Gray SL, Baldwin WS. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J Appl Toxicol. 2005;25:339–353. doi: 10.1002/jat.1078. [DOI] [PubMed] [Google Scholar]
- Anakk S, Kalsotra A, Kikuta Y, Huang W, Zhang J, Staudinger JL, Moore DD, Strobel HW. CAR/PXR provide directives for Cyp3a41 gene regulation differently from Cyp3a11. Pharmacogenomics J. 2004;4:91–101. doi: 10.1038/sj.tpj.6500222. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol Sci. 2009;107:93–105. doi: 10.1093/toxsci/kfn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission E, Center JR. In: 4-Nonylphenol (branched) and nonylphenol: Summary risk assessment report. I. f. H. a. C. Protection, editor. European Communities; 2002. p. 26. [Google Scholar]
- Daidoji T, Inoue H, Kato S, Yokota H. Glucuronidation and excretion of nonylphenol in perfused rat liver. Drug Metab Dispos. 2003;31:993–998. doi: 10.1124/dmd.31.8.993. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Shappell HW, Banerjee A, Hachey DL. Effects of nonylphenol, 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p’-DDE), and pentachlorophenol on the adult female guinea pig reproductive tract. Reprod Toxicol. 2002;16:29–43. doi: 10.1016/s0890-6238(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Dirkx R, Vanhorebeek I, Martens K, Schad A, Grabenbauer M, Fahimi D, Declercq P, Van Veldhoven PP, Baes M. Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology. 2005;41:868–878. doi: 10.1002/hep.20628. [DOI] [PubMed] [Google Scholar]
- Green T, Swain C, Van Miller JP, Joiner RL. Absorption, bioavailability, and metabolism of para-nonylphenol in the rat. Regul Toxicol Pharmacol. 2003;38:43–51. doi: 10.1016/s0273-2300(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Guo GL, Moffit JS, Nicol CS, Ward JM, Aleksunes LA, Slitt AL, Kliewer SA, Manautou JE, Gonzalez FJ. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS. Gender-specific induction of cytochrome P450s in nonylphenol treated FVB/NJ mice. Toxicol Appl Pharmacol. 2006;216:186–196. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Huang W, Chapman LM, Chua S, Moore DD, Baldwin WS. The environmental estrogen, nonylphenol, activates the constitutive androstane receptor. Toxicol Sci. 2007;98:416–426. doi: 10.1093/toxsci/kfm107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Current Pharmacog Person Med. 2009a;7:81–105. doi: 10.2174/187569209788654005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS. Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR) Toxicology. 2009b;256:53–64. doi: 10.1016/j.tox.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori M, Cangiano M, Palermo FA, Parrella A. E-screen and vitellogenin assay for the detection of the estrogenic activity of alkylphenols and trace elements. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152:51–56. doi: 10.1016/j.cbpc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Laurenzana EM, Weis CC, Bryant CW, Newbold R, Delclos KB. Effect of dietary administration of genistein, nonylphenol or ethinyl estradiol on hepatic testosterone metabolism, cytochrome P-450 enzymes, and estrogen receptor alpha expression. Food Chem Toxicol. 2002;40:53–63. doi: 10.1016/s0278-6915(01)00095-3. [DOI] [PubMed] [Google Scholar]
- Lech JJ, Lewis SK, Ren L. In vivo estrogenic activity of nonylphenol in rainbow trout. Fundam Appl Toxicol. 1996;30:229–232. [PubMed] [Google Scholar]
- Lee HJ, Hwang M, Chattopadhyay S, Choi HS, Lee K. Hepatocyte nuclear factor-3 alpha (HNF-3alpha) negatively regulates androgen receptor transactivation in prostate cancer cells. Biochem Biophys Res Commun. 2008;367:481–486. doi: 10.1016/j.bbrc.2007.12.162. [DOI] [PubMed] [Google Scholar]
- Lee P, Marquardt M, Lech JJ. Metabolism of nonylphenol by rat and human microsomes. Toxicol Lett. 1998;99:117–126. doi: 10.1016/s0378-4274(98)00153-2. [DOI] [PubMed] [Google Scholar]
- Lee PC, Patra SC, Struve M. Modulation of rat hepatic CYP3A by nonylphenol. Xenobiotica. 1996;26:831–838. doi: 10.3109/00498259609046753. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Mnif W, Pascussi JM, Pillon A, Rabenoelina F, Fenet H, Gomez E, Casellas C, Nicolas JC, Cavaillès V, Duchesne MJ, Balaguer P. Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci. 2006;91:501–509. doi: 10.1093/toxsci/kfj173. [DOI] [PubMed] [Google Scholar]
- Li X, Ying GG, Su HC, Yang XB, Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int. 2010;36:557–562. doi: 10.1016/j.envint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Staudinger JL. The traditional Chinese herbal remedy tian xian activates pregnane X receptor and induces CYP3A gene expression in hepatocytes. Drug Metab Dispos. 2008;36:1538–1545. doi: 10.1124/dmd.108.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YP, Liu CH, Shyu LJ, Huang JD. Functional characterization of a novel polymorphism of pregnane X receptor, Q158K, in Chinese subjects. Pharmacogenet Genomics. 2005;15:337–341. doi: 10.1097/01213011-200505000-00009. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, Kunitomi M, Kudo T, MacDonald PN. Endocrine disrupting chemicals phthalic acid and nonylphenol, activate Pregnane X Receptor mediated transcription. Mol Endocrinol. 2000;14:421–428. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, Mizutani Y, Inoshita H, Kudo T. The expression of pregnane X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell Endocrinol. 2001;172:47–56. doi: 10.1016/s0303-7207(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem. 2006;281:14537–14546. doi: 10.1074/jbc.M510713200. [DOI] [PubMed] [Google Scholar]
- Mikamo E, Harada S, Nishikawa J, Nishihara T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl Pharmacol. 2003;193:66–72. doi: 10.1016/j.taap.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Mota LC, Hernandez JP, Baldwin WS. CAR-null mice are sensitive to the toxic effects of parathion: Association with reduced CYP-mediated parathion metabolism in CAR-null mice. Drug Metab Dispos. 2010;38:1582–1588. doi: 10.1124/dmd.110.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Quednow K, Püttmann W. Temporal concentration changes of DEET, TCEP, terbutryn, and nonylphenols in freshwater streams of Hesse, Germany: possible influence of mandatory regulations and voluntary environmental agreements. Environ Sci Pollut Res Int. 2009;16:630–640. doi: 10.1007/s11356-009-0169-6. [DOI] [PubMed] [Google Scholar]
- Reed HWB. Kirk-Othmer: Encyclopedia of chemical technology. John Wiley and Sons; New York: 1978. Alkylphenols. [Google Scholar]
- Renner R. European bans on surfactant trigger transatlantic debate. Environ Sci Technol. 1997;31:316A–319A. doi: 10.1021/es972366q. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency Aquatic Life Ambient Water Quality Criteria-Nonylphenol 2005 [Google Scholar]
- Vyhlidal CA, Gaedigk R, Leeder JS. Nuclear receptor expression in fetal and pediatric liver: correlation with CYP3A expression. Drug Metab Dispos. 2006;34:131–138. doi: 10.1124/dmd.105.005967. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- Waxman DJ. Minireview P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptor CAR, PXR, and PPAR. Arch Biochem and Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135:175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LEJ. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4a-deficient mice. Mol Endocrinol. 2004;18:1975–1987. doi: 10.1210/me.2004-0129. [DOI] [PubMed] [Google Scholar]
- Woo GH, Shibutani M, Ichiki T, Hamamura M, Lee KY, Inoue K, Hirose M. A repeated 28-day oral dose toxicity study of nonylphenol in rats, based on the ‘Enhanced OECD Test Guideline 407′ for screening of endocrine-disrupting chemicals. Arch Toxicol. 2007;81:77–88. doi: 10.1007/s00204-006-0129-6. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, NeuschwanderTetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Moore LB, Orans J, Peng L, Bencharit S, Kliewer SA, Redinbo MR. Crystal structure of the pregnane X receptor-estradiol complex provides insights into endobiotic recognition. Mol Endocrinol. 2007;21:1028–1038. doi: 10.1210/me.2006-0323. [DOI] [PubMed] [Google Scholar]
- Yan ZH, Karam WG, Staudinger JL, Medvedev A, Ghanayem BI, Jetten AM. Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4. J Biol Chem. 1998;273:10948–10957. doi: 10.1074/jbc.273.18.10948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data; Figure 1. Dexamethasone activates PXR. Western blots of Cyp2b and Cyp3a were performed using hepatic microsomes from untreated, and 75 mg/kg/day Dexamethasone treated male and female mice as described in the Materials and Methods. (A) Cyp induction in WT (mPXR) mice treated with Dexamethasone. (B) Cyp induction in hPXR mice treated with Dexamethasone. An asterisk indicates statistically significant difference compared to the untreated group performed by Student's t-test (p < 0.05).


