Abstract
Neurogenesis depends on exquisitely regulated interactions between macromolecules on the cell surface and in the extracellular matrix. In particular, interactions between proteoglycans and members of the type IIa subgroup of receptor protein tyrosine phosphatases underlie critical developmental processes such as the formation of synapses at the neuromuscular junction and the migration of axons to their appropriate targets. We report here the crystal structures of the first and second immunoglobulin-like domains of the Drosophila type IIa receptor Dlar and its mouse homologue LAR. These two domains adopt an unusual antiparallel arrangement that has not been previously observed in tandem repeats of immunoglobulin-like domains and that is presumably conserved in all type IIa receptor protein tyrosine phosphatases.
Keywords: cell adhesion, receptor protein tyrosine phosphatase, heparan sulfate proteoglycans, immunoglobulin-like domains, crystal structure
Receptor protein tyrosine phosphatases (RPTPs) are a family of cell surface receptors involved in the growth and development of the nervous system from worms to humans 1, 2. These molecules are typically comprised of intracellular tyrosine phosphatase domains that antagonize tyrosine kinase signaling and large, modular extracellular domains that resemble those found in the ectodomains of cell adhesion molecules. The particular architecture of RPTPs is akin to that of receptor tyrosine kinases, which would suggest that the binding of extracellular ligands could control the intracellular phosphatase activity. However, most RPTPs remain orphan receptors and a unifying mechanism of how ligand binding is transduced into intracellular tyrosine dephosphorylation, if it exists, is still lacking.
Studies carried out in Drosophila were the first to uncover the involvement of RPTPs in neurogenesis 3, 4. In particular, it was demonstrated that the motor axons of flies lacking the leukocyte-antigen related receptor (Dlar) were unable to reach their appropriate targets 3. These pathfinding defects were later linked to aberrant signaling through the tyrosine kinase Abl, which is normally antagonized by the intracellular tyrosine phosphatase activity of Dlar 5. Furthermore, it was shown that the formation of synapses at the neuromuscular junction depended on interactions between ligands expressed at the surface of muscle cells and the N-terminus of Dlar expressed on nerve cells 6. In contrast, in the Drosophila visual system, the appropriate targeting of photoreceptor neuron R7 axons does not depend on the N-terminus of Dlar or its phosphatase activity, but instead requires the presence of domains within the membrane proximal region of the ectodomain of Dlar 7. Consequently, these results indicate that some of the characterized physiological functions of Dlar are mediated by distinct modules in its extracellular region.
Dlar and its three vertebrate homologues LAR/PTPRF, RPTPδ/PTPRD and RPTPσ/PTPRS share a similar modular architecture and belong to the type IIa subgroup of RPTPs. These type I transmembrane proteins are comprised of three immunoglobulin domains (Ig) and eight or nine fibronectin type III (FNIII) repeats followed by a transmembrane region and tandem cytoplasmic tyrosine phosphatase domains 2, 8 (Fig. 1a). Mice lacking either of the vertebrate homologues of Dlar display neurological abnormalities such as learning disabilities (LAR and PTPRD 9, 10), posture/motor defects (PTPRD 10) or spastic movement, tremors and decreased brain size (PTPRS 11, 12). In addition, mice deficient for both PTPRD and PTPRS suffer from severe motoneuron defects and die soon after birth 13. Taken together, these findings suggest that Dlar and its vertebrate homologues LAR, PTPRD and PTPRF play critical roles in neurogenesis across species.
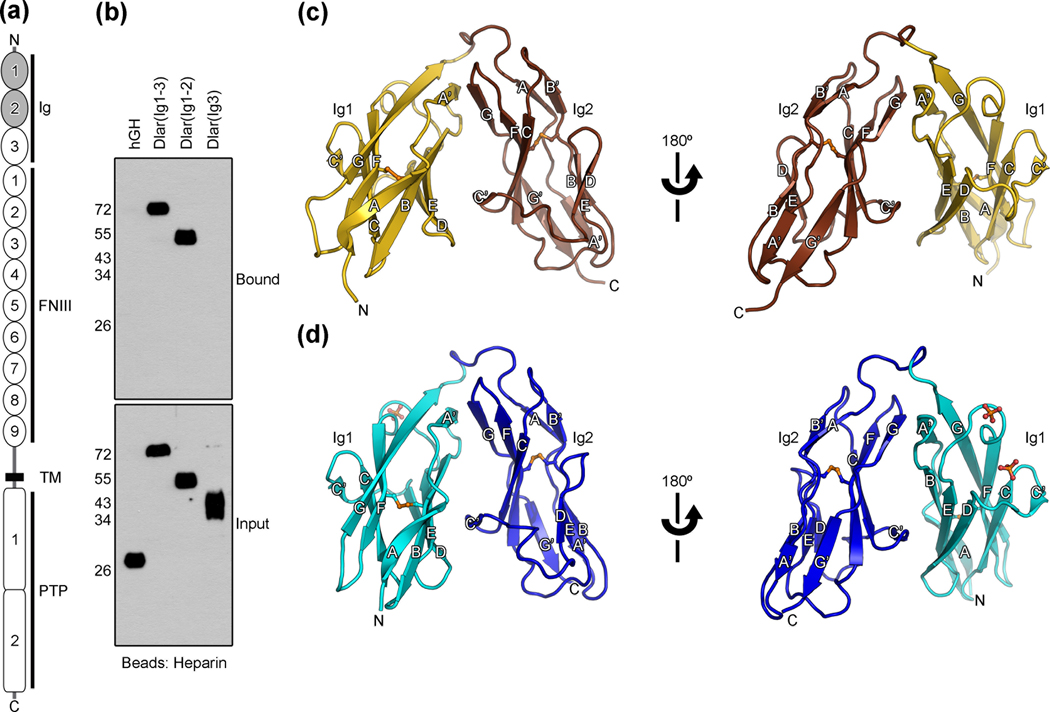
Fig. 1.
Crystal structures of the Ig1–Ig2 domains of Dlar and mouse LAR. (a) Schematic representation of type IIa RPTPs. Ig: immunoglobulin-like domains, FNIII: fibronectin type III domains, TM: transmembrane region, PTP: protein tyrosine phosphatase domains. The Ig domains that were crystallized are shaded. (b) Identification of a heparin-binding region within the Ig domains of Dlar. Fragments of Dlar were fused to human growth hormone (hGH) and expressed transiently in HEK293 cells. Conditioned media were incubated with heparin-sepharose. Bound fusion proteins were visualized by immunoblotting against hGH. (c) Ribbon diagram of Dlar(Ig1-2). The letters N and C indicate the N- and C-termini, respectively. Each β-strand is labeled. Disulfide bonds are shown as orange ball-and-stick models. The first and second Ig domains are colored gold and brown, respectively. (d) Ribbon diagram of mouse LAR(Ig1-2). The first and second Ig domains are colored cyan and blue, respectively. Two bound sulfate ions are shown in ball-and-stick representation. Oxygen and sulfur atoms are colored red and orange, respectively. Structural images were prepared with PYMOL (www.pymol.org).
In addition to their similar architecture and their roles in neural development, type IIa RPTPs interact with related extracellular ligands. In Drosophila, Dlar interacts in vivo with the heparan-sulfate proteoglycans (HSPGs) syndecan and dally-like protein during the formation of synapses at the Drosophila neuromuscular junction 6, 14. HSPGs are composed of a protein core and are decorated with heparan sulfate (HS) chains, which are polymers of negatively-charged disaccharide units. The interactions between Dlar and the HSPGs syndecan and dally-like protein have dissociation constants within the nanomolar range and are sensitive to treatment with the HS-degrading enzyme heparinase, indicating that the HS chains present on these molecules play a crucial role in mediating these interactions 6. In addition, a form of Dlar lacking the first three Ig domains was unable to bind to HSPGs and could not rescue the synaptic defects observed in dlar−/− flies, suggesting that the HSPG-binding site localizes to this region and that these protein-carbohydrate binding events are critical for fly motoneuron development. In vertebrates, the avian PTPRS also binds with high affinity to the HSPGs agrin and collagen XVIII 15. Dissociation constants in the nanomolar range were measured and were nearly indistinguishable to those determined for the binding of the artificial ligand heparin-albumin to PTPRS. These combined studies have highlighted the importance of the interactions between proteoglycans and the extracellular regions of type IIa RPTPs during the growth and development of the nervous system. As a first step towards providing a molecular basis for these protein-carbohydrate interactions, we undertook structural analyses of the first two Ig domains of Dlar and its mouse homologue LAR.
Crystal structures of Ig1–Ig2 repeats of Dlar and mouse LAR
We initially pursued the crystallization of the entire Ig region of Dlar for subsequent structural analyses. The protein was purified from the conditioned medium of transiently transfected N-acetylglucosaminyltransferase I-negative HEK293S cells, followed by removal of the N-linked oligosaccharides using endoglycosidase H 16. However, attempts to grow crystals of Dlar(Ig1-3) were unsuccessful, mostly because of the instability of this protein fragment. Incubation of the protein for several days at 4°C resulted in a reduction of molecular weight of about ~10 kDa as judged by SDS-PAGE. Subsequent analysis by mass spectrometry indicated that the stable form of Dlar included residues 32–237, which span Ig domains 1 and 2. These serendipitous findings resulted in the identification of a discrete form of Dlar that was more amenable to crystallographic analysis because of its improved stability. Mutational analyses of PTPRS previously demonstrated that a cluster of basic residues in domain Ig1 are crucial for binding to HSPGs 15, therefore we predicted that the absence of Ig3 would not impair the ability of Dlar to bind to HS chains. To test this hypothesis, we incubated secreted fragments of Dlar fused to human growth hormone (hGH) expressed in HEK293 cells with heparin-sepharose resin (Fig. 1b). In this assay, the third Ig domain of Dlar does not appear to have any intrinsic heparin-binding property and its absence does not impair the ability of Dlar to associate with heparin. Hence we focused our efforts on determining the crystal structure of the first two Ig repeats of Dlar.
Instead of producing Dlar(Ig1-2) in HEK293S cells, we opted for a more expeditious approach and expressed this fragment in Escherichia coli strain Origami2(DE3) to promote the formation of disulfide bonds within each Ig domain. A similar strategy was employed to produce the Ig1–Ig2 fragment of mouse LAR. Both bacterially-expressed Dlar(Ig1-2) and mouse LAR(Ig1-2) bind to heparin-agarose in affinity isolation assay (supplementary Fig. 1) indicating that these fragments retain the heparin-binding activity observed for Dlar expressed in mammalian cells. Small plate-like crystals of Dlar(Ig1-2) appeared after two rounds of microseeding, whereas crystals of LAR(Ig1-2) grew readily. First, the crystal structure of LAR(Ig1-2) was determined by molecular replacement and refined to 2.0 Å resolution (Rwork / Rfree = 22.0 % / 24.0 %). The structures of Ig1 and Ig2 of LAR were then used as independent search models to calculate initial phases for Dlar(Ig1-2) and the final model was refined to 2.3 Å resolution (Rwork / Rfree = 22.3 % / 26.1 %).
Overall, domains Ig1 and Ig2 of Dlar adopt an I set topology 17 and superimpose with a rmsd of 1.6 Å over 92 residues. Ig1 is most similar to the A168 Ig domain of titin (rmsd of 1.2 Å over 95 residues, 29% identity) whereas the closest structural neighbor of Ig2 is the 2nd Ig domain of muscle-specific kinase (rmsd of 2.4 Å over 97 residues, 38% identity). Strikingly, Ig1 and Ig2 fold back onto one another to adopt a constrained conformation in which the individual Ig domains are arranged in an antiparallel manner (Fig. 1c and d). This horseshoe-like conformation is in contrast to the extended arrangement observed for titin and muscle-specific kinase or in other tandem Ig domains that harbor glycosaminoglycan-binding activity such as Robo and fibroblast growth factor receptors 18–20.
In essence, Dlar(Ig1-2) resembles a triangle of dimensions of ~ 45 × 41 × 49 Å as measured from the N-terminus to the middle of the linker region, from the middle of the linker region to the C-terminus and from the C-terminus to the N-terminus. The four molecules in the asymmetric unit of Dlar(Ig1-2) all adopt this conformation and superimpose with rmsd values of 0.4–0.5 Å for 187–199 equivalent Cα positions indicating that this arrangement of Ig1 and Ig2 in Dlar is rigid. Mouse LAR adopts a very similar, albeit more compact conformation with dimensions of ~ 44 × 41 × 43 Å. The Ig1-2 regions of Dlar and LAR superimpose with a rmsd of 2.3 Å for 193 equivalent Cα positions (49% identity) whereas Ig1 and Ig2 superimpose with rmsd values of 1.5 Å (95 Cα positions) and 1.3 Å (89 Cα positions), respectively, indicating that much of the difference between the two molecules resides in the relative orientations of the individual Ig domains.
The antiparallel arrangement of Ig1 and Ig2
The most striking feature of the structures of Dlar(Ig1-2) and LAR(Ig1-2) is the presence of the horseshoe-like conformation of the two Ig domains. This antiparallel arrangement is made possible because of the flexible 7-aa linker region between the domains (EGDKTPA in Dlar and EEDQLPSG in LAR) and the extensive contacts between Ig1 and Ig2 (Fig. 2a and b). Overall, the interface buries 1,199 Å2 with a shape complementarity coefficient of 0.56 in Dlar(Ig1-2) and 1,295 Å2 with a shape complementarity coefficient of 0.69 in LAR(Ig1-2). These values are consistent, albeit slightly inferior in the case of Dlar(Ig1-2), to these found in known biological interfaces 21, 22. The contacts between Ig1 and Ig2 include mostly van der Waals interactions and are localized to a minor and a major site (Fig. 2a and b). The minor contact site is located at the linker region between the two Ig domains. It includes R48, Y130, P136, F139 and V141 in Dlar(Ig1-2) and L45, L125, L130 and P131 in LAR(Ig1-2). The major contact site involves residues in the ABED face of the β-sheet in Ig1 and the GFCC’ face of the β-sheet in Ig2 (Fig. 1c and d). It encompasses amino acid residues Q45, V52, Y56, L85, R96, Q177, K179, E208, T217, E218 and H219 in Dlar and S51, I81, V90, R92, F169, F172, L173, P174, E206 and Y217 in LAR. There is little conservation in the residues involved in Ig1–Ig2 contacts in Dlar and mouse LAR (Fig. 2c): a single salt bridge (R96/E208 in Dlar(Ig1-2) and R92/E206 in LAR(Ig1-2)) is the sole interaction that is strictly conserved between the two proteins.
Fig. 2.
The Ig1–Ig2 interface in Dlar and mouse LAR. (a) Stereo view of the interface between Ig domains 1 and 2 in Dlar(Ig1-2). Residues at the interface between the two domains are shown as ball-and-sticks and colored gold (Ig1) or brown (Ig2). This view is in the same orientation as the right view in Fig. 1c. Transparent gray spheres and dashed lines denote residues involved in van der Waals contacts and potential hydrogen bonds and salt bridges, respectively. (b) Stereo view of the interface between Ig domains 1 and 2 in LAR(Ig1-2). Residues at the interface between the two domains are shown as ball-and-sticks and colored cyan (Ig1) or blue (Ig2). This view is in the same orientation as the right view in Fig. 1d. (c) Alignment of amino acid sequences of Ig domains 1–2 of Dlar and mouse type IIa RPTPs. Strictly conserved residues are shaded in black and similar residues are colored gray. The numbering refers to Dlar. Cysteine residues involved in disulfide bridges are numbered in green below the sequences. Gold and brown triangles indicate the residues involved in interactions between Ig domains 1 and 2 of Dlar that are unique to Dlar. Cyan and blue triangles indicate the residues involved in interactions between Ig domains 1 and 2 of mouse LAR that are unique to LAR. Red stars indicate the positions of residues involved in interactions between Ig1 and Ig2 of both Dlar and mouse LAR. (d) Analysis of interference-free SAXS curve for LAR(Ig1-2). The experimental scattering profile (black) for LAR(Ig1-2) and the theoretical scattering (red line, χ2=1.2) calculated from the LAR(Ig1-2) crystal structure are shown on the left panel. The right panel shows the Guinier plots with linear fit (red line). (e) Crystal structure of LAR(Ig1-2) used to calculate the theoretical scattering profile in which the red regions indicate disordered residues at the N- and C-termini that have been added in extended form and optimized by BILBOMD 41. These residues are GPGSSRG at the N-terminus and VRRVAPRFS at the C-terminus. SAXS data were collected at the ALS beamline 12.3.1 LBNL Berkeley, California 42. Tunable wavelength λ 1.0–1.5 Å and the sample-to-detector distances were set to 1.5 m resulting in scattering vectors, q, ranging from 0.01 Å−1 to 0.32 Å−1. The scattering vector is defined as q = 4π sinθ/λ, where 2θ is the scattering angle. All experiments were performed at 20 °C and data was processed as described 42. Data acquired at short and long time exposure (0.5 and 5 s) were merged for calculations using the entire scattering profile. The experimental SAXS data for different protein concentrations were investigated for aggregation using Guinier plots 43. The radius of gyration RG is 20.5 ± 0.1 Å and was derived by the Guinier approximation I(q) = I(0) exp(−q2RG 2/3) with the limits qRG < 1.6. The theoretical SAXS profile and the corresponding fit to the experimental data were calculated using the program FoXS 44. (f) Fusion proteins of hGH with wild-type Dlar(Ig1-2) (Dlar(Ig1-2), WT) and a mutant form of Dlar(Ig1-2) with cysteine residues at positions 52 and 219 (Dlar(Ig1-2), Cys)) were analyzed by SDS-PAGE (9% gel) under reducing and non-reducing conditions followed by immunoblotting against hGH. (g) Schematic drawing of the Drosophila larval central nervous system, illustrating the positions of the two brain hemispheres (BH) and the ventral nerve cord (VNC). (h) Confocal micrographs of the VNC region shown in (g) (dotted box). Fc fusion proteins of histidine-tagged wild-type and mutant Dlar(Ig1-2) were expressed in HEK293 cells and purified from conditioned media by cobalt-affinity chromatography. The brain/VNC complex was dissected from second instar larvae in phosphate-buffered saline (PBS) and incubated with the indicated fusion proteins for 30 minutes. After fixation for 30 minutes in PBS/formaldehyde, the larval VNC was washed with PBS with 0.05% (v/v) Triton X-100 and incubated with protein G-Alexa Fluor 488. Both wild-type Dlar(Ig1-2) (left panel) and its cysteine mutant (middle panel) showed a staining pattern consistent with ventral nerve cord staining that was not observed in mock (cobalt-affinity chromatography eluate of conditioned media from untransfected HEK293 cells, right panel).
The fact that the Ig1–Ig2 fragments from two orthologous receptors adopt a similar antiparallel arrangement while crystallizing in distinct lattice environments strongly indicates that this conformation is not a consequence of crystallization, but rather likely represents the biologically active form of these proteins. To test this hypothesis directly, we analyzed the conformation of mouse LAR(Ig1-2) produced in E. coli in solution by small angle X-ray scattering (SAXS) (Fig. 2d and e). Since this technique is sensitive to the low-resolution shape of a molecule in solution 23, it can be employed to discern to which extent LAR(Ig1-2) adopts an extended or compact conformation independently of the crystal lattice constraints. Data sets were acquired at different concentrations and exposures and merged to obtain an experimental SAXS profile for LAR(Ig1-2). This profile was then compared to the theoretical profile of LAR(Ig1-2), which was calculated by using the crystal structure in which it adopts a horseshoe-like shape (Fig. 2d and e). The experimental and theoretical scattering profiles match closely, thus indicating that the antiparallel conformation found in LAR crystals reflects its conformation in solution.
Because of the homology between LAR and Dlar, the results from our SAXS analysis strongly suggest that Dlar also adopts an antiparallel arrangement in solution. We engineered a mutant form of Dlar(Ig1-2) to test this hypothesis directly. In the crystal structure of Dlar(Ig1-2), residues V52 in Ig1 and H219 in Ig2 form van der Waals interactions across the interdomain interface (Fig. 2a). Based on our structural analysis, substitution of these residues for cysteine would result in the formation of an interdomain disulfide bond, which would constrain the protein in its horseshoe-like conformation. This mutant protein was expressed as a fusion protein with hGH in HEK293 cells. It migrates slightly faster than wild-type Dlar(Ig1-2) as judged by SDS-PAGE performed under non-reducing conditions, indicating that the disulfide bridge is indeed formed. Furthermore, introduction of the interdomain disulfide bond had no effect on the binding activity of Dlar(Ig1-2) as Fc fusions of wild-type and the cysteine mutant of Dlar(Ig1-2) were able to stain cells in the ventral nerve cord of Drosophila larvae to a similar extent 14, 24 (Fig. 2g and h). Taken together, these findings demonstrate that the biologically active forms of Dlar(Ig1-2) and LAR(Ig1-2) are the compact conformations identified in their crystal structures. Furthermore, all the residues involved in the interdomain contact in LAR are strictly conserved in its homologues PTPRD and PTPRS (Fig. 2c), indicating that these receptors are likely to contain a similar antiparallel arrangement. Overall, this analysis strongly suggests that the horseshoe-like conformation identified in Dlar(Ig1-2) and LAR(Ig1-2) is a hallmark of all type IIa RPTPs.
Comparison with other horseshoe-like conformations
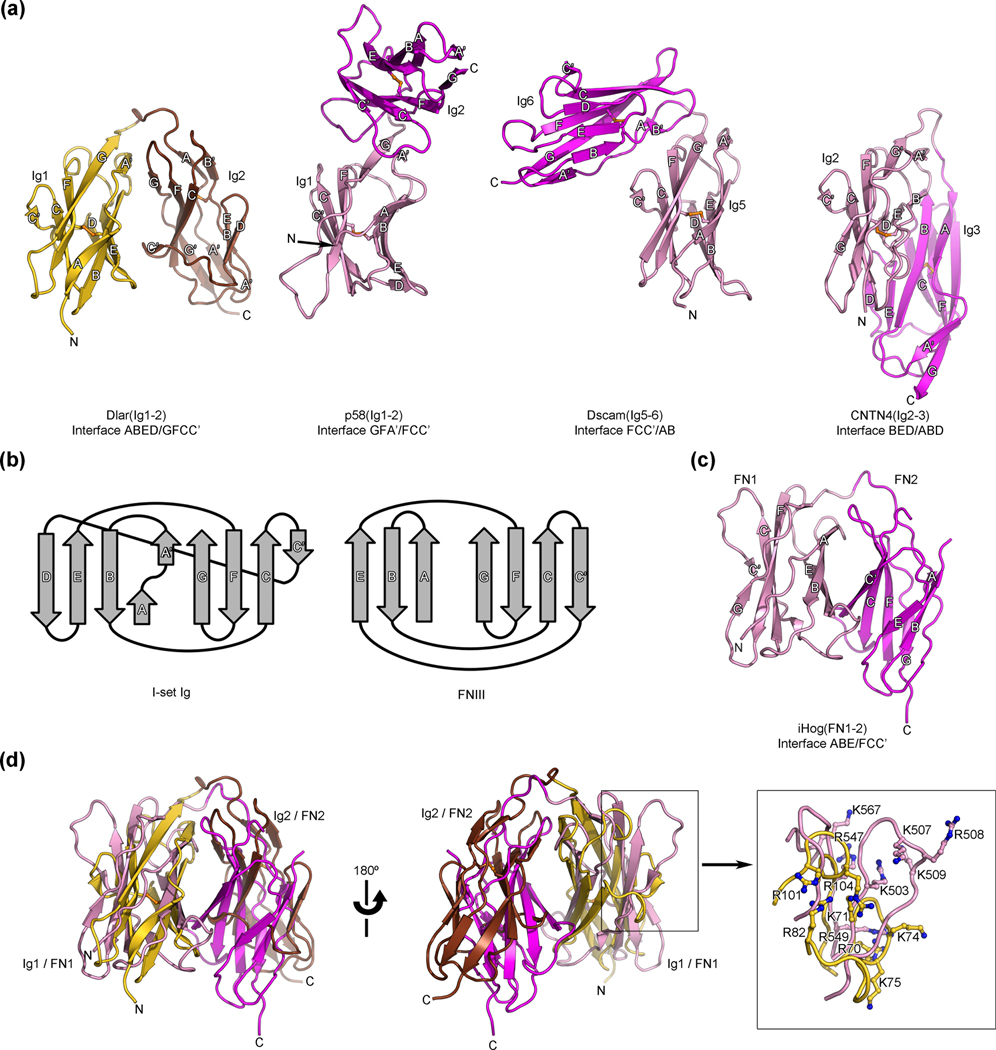
Horseshoe-like arrangements of Ig domains have been reported for several Ig-containing proteins. These cell surface receptors include the natural killer cell receptor p58 25 and more recently Ig superfamily proteins such as the insect hemolymph protein hemolin, the neuronal receptors axonin/TAG1/contactin-2 (CNTN2), CNTN4 and isoforms of the Down syndrome cell adhesion molecule (Dscam) 16, 26–30. In these latter structures, the four N-terminal Ig domains adopt closely-related horseshoe-like conformations mediated by extensive contacts between Ig1 and Ig4 on one hand and Ig2 and Ig3 on the other hand. In addition, Ig5 and Ig6 in Dscam also adopt an antiparallel arrangement distinct from the one adopted by Ig2 and Ig3 29. Hence, it was of interest to compare the structure of Dlar(Ig1-2) with these known structures containing horseshoe-like motifs. To this end, we superimposed Ig1 of Dlar with Ig1 of p58 (rmsd value of 2.7 Å over 81 equivalent Cα positions), with Ig2 of CNTN4 (4.5 Å rmsd value over 93 equivalent Cα positions) or with Ig5 of Dscam (3.5 Å rmsd over 96 equivalent Cα positions) to assess to which extent the C-terminal domains of the tandem overlapped (Fig. 3a). Proteins were superimposed using the DaliLite server 31 and the program SUPERPOSE as implemented in CCP4 32. CNTN4 was chosen in this case because its 2nd Ig domain shares the most sequence identity (26%) with Ig1 of Dlar among hemolin, CNTN2, CNTN4 or Dscam. This analysis shows that the Ig1–Ig2 conformation observed in Dlar is distinct from the ones reported for p58, CNTN4 and Dscam. The differences in these structures can be rationalized when comparing the interfaces between the Ig domains. In Dlar, residues at the ABED face of the β-sheet in Ig1 are in contact with residues in the GFCC’ face of the β-sheet in Ig2. In contrast, in p58, the contacting faces involve strands G, F and A’ in Ig1 and strands F, C and C’ in Ig2 whereas in CNTN4, the contacting faces involve strands B, E and D in Ig2 and strands A, B and D in Ig3. The comparison with Ig5-Ig6 of Dscam shows a striking change of direction, with, essentially, the backbone “turning left” in Dscam and “turning right” in Dlar. Likewise, the contacting faces in Ig5 and Ig6 are distinct from the ones in Ig1 and Ig2 of Dlar as they involve residues in strands F, C and C’ in Ig5 and strands A and B’ in Ig6. These comparisons with other horseshoe-like conformations of Ig domains indicate that the arrangement of Ig1 and Ig2 in type IIa RPTPs is so far unique among tandem Ig repeats.
Fig. 3.
Structural comparisons of the antiparallel arrangement of Ig domains 1 and 2 of Dlar with other horseshoe-like structures. (a) Comparison of the Dlar(Ig1-2) structure (left, colored gold and brown) with three structures of tandem Ig repeats that adopt a horseshoe-like conformation. The distinct conformations of horseshoe-like arrangements of these tandem β-sandwiches repeats arise from interactions between residues found in distinct strands, which are listed below each structure. In each of the compared structures, the N-terminal and C-terminal Ig repeats are colored pink and magenta, respectively. Disulfide bonds are shown as orange ball-and-stick models. The letters N and C indicate the N- and C-termini, respectively. These views of Dlar(Ig1-2), p58(Ig1-2), CNTN4(Ig2-3) and Dscam(Ig5-6) were obtained by superimposing their N-terminal Ig repeats in an effort to highlight the distinct positions of the C-terminal Ig repeats that result from changes in the contacting faces in each of the horseshoe-like structures. (b) Topology diagrams for I set Ig domains and FNIII domains highlighting the similarities between the two folds 45. (c) Ribbon diagram depicting the horseshoe-like arrangement observed for the tandem FNIII repeats of Drosophila iHog. The first and second FNIII repeat of iHog are colored pink and magenta, respectively. This view was obtained by superimposing the 1st FNIII iHog and the 1st Ig repeat of Dlar using secondary structure matching. (d) Overlay of the Ig1–Ig2 fragment of Dlar with the first (pink) and second (magenta) FNIII domains of Drosophila iHog. The HSPG-binding site for iHog and the presumed glycosaminoglycan-binding site for Dlar partially overlap and are shown in the boxed view.
In contrast, the structures of Dlar(Ig1-2) and LAR(Ig1-2) are reminiscent of the arrangement adopted by the FNIII repeats 1 and 2 of iHog, a Drosophila Ig superfamily protein that functions as a coreceptor for the morphogen hedgehog (Fig. 3c) 33. Using secondary structure alignment 34, Ig1 of Dlar can be superimposed onto FN1 of iHog with a rmsd of 3.2 Å over 60 equivalent Cα positions and, overall, the Ig1–Ig2 pair of Dlar can be superimposed onto the FN1–FN2 pair of iHog with a rmsd of 4.0 Å over 143 equivalent Cα positions even though the sequence identity is only 6% (Fig. 3d). Similarly, the Ig1–Ig2 fragment of mouse LAR can be superimposed onto the FN1–FN2 pair of iHog with a rmsd of 3.6 Å over 147 residues. Furthermore, the interdomain interface in iHog encompasses residues located in the ABE face of the β-sheet in FN1 and strands FCC’ in FN2 33, which is similar to the contacting faces in Ig1 and Ig2 of Dlar (Fig. 3a and c). Overall, these structural findings suggest that horseshoe-like conformations are recurring motifs in Ig superfamily proteins involved in cell adhesion and signaling.
Binding to heparin
A noteworthy feature of the Dlar(Ig1-2) and LAR(Ig1-2) structures is the presence of a shallow groove bordered by positively-charged residues that localizes to the first Ig domain in each structure and could function as a glycosaminoglycan-binding site. In Dlar, this site encompasses residues R70, K71, K74, K75 and R82 located at the C-terminal end of the C strand and on strand C’ as well as residues R101 and R104 found in the loop between strands E and F (Fig. 1c and 4a). Similarly, in mouse LAR, the surface is composed of the equivalent residues K68, K69, K71, K72, R77, R97 and R100 (Fig. 1d and 4b). In addition, two sulfate ions are bound to this positively-charged region in the vicinity of R97 and R100. Interestingly, the location of this putative glycosaminoglycan-binding site in the region between strands C and D corresponds to the heparin-binding sites found in the 14th FNIII repeat of fibronectin 35 and in the 1st FNIII repeat of iHog 33 (Fig. 3d), further emphasizing the resemblance between Dlar and iHog. Furthermore, the basic residues in LAR are conserved in PTPRD and PTPRS and introduction of pairs of alanine residues in avian PTPRS in place of the residues equivalent to positions K68/K69, K71/K72, and R97/R100 in LAR impaired binding to HSPGs 15 (Fig. 4b). Taken together, these observations indicate that this positively-charged region is the likely binding site for glycosaminoglycans and that it is a conserved surface feature of type IIa RPTPs.
Fig. 4.
(a) Electrostatic surface representation of Dlar(Ig1-2). This view is related to the left view on panel c by a counterclockwise rotation of 120° along a vertical axis. Regions with negative electrostatic potential are colored red and regions with positive electrostatic potential are colored blue (scale ± 5 e/kT). (b) Electrostatic surface representation of mouse LAR(Ig1-2). Electrostatic potentials were calculated with DELPHI 46, 47. (c) Interactions between mutants of Dlar(Ig1-2) and heparin. Fragments of Dlar fused to hGH were expressed transiently in HEK293 cells. These fragments include wild-type Dlar(Ig1-2) (labeled Dlar(Ig1-2), WT), Dlar(Ig1-2) with cysteine residues at positions 52 and 219 (Dlar(Ig1-2), Cys), with asparagine residues at positions 52 and 218 to introduce N-linked carbohydrates (Dlar(Ig1-2), Asn), Dlar(Ig1) and Dlar(Ig2). The left panel shows wild-type Dlar(Ig1-2) along with its cysteine and asparagine mutants to illustrate the difference in size between the proteins upon introduction of consensus N-linked glycosylation sites in Dlar(Ig1-2). Samples were resolved by SDS-PAGE (9% gel) and fusion proteins were visualized by immunoblotting against hGH. The right panel shows the results of heparin affinity isolation assays. Conditioned media were incubated with heparin-sepharose in PBS with 1 % (v/v) Tween-20 for one hour at room temperature. Resins were washed in the same buffer and samples were resolved by SDS-PAGE (12% gel). Bound fusion proteins were visualized by immunoblotting against hGH.
The positively-charged region identified in the crystal structures of Dlar and LAR localizes to the 1st Ig repeat and no such region can be observed in the 2nd Ig repeat, which would suggest that only the 1st Ig domain is required to bind heparin. Therefore, we used a heparin affinity isolation assay to test this hypothesis and to determine to which extent the horseshoe conformation adopted by Dlar is important for heparin binding. Secreted fragments of Dlar fused to hGH were expressed in HEK293 cells and incubated with heparin-sepharose. As expected from our previous analyses (Fig. 2g and h), a mutant form of Dlar(Ig1-2) locked into a horseshoe conformation by formation of a disulfide bridge across the Ig1–Ig2 interface supports heparin binding. Similar results were obtained for Ig1, whereas repeat Ig2 of Dlar appears unable to bind heparin (Fig. 4c). To determine to which extent disruption of the Ig1–Ig2 interface would impair heparin binding, we introduced asparagine residues at positions V52 and E218 to create N-linked carbohydrate consensus sites N52AS in Ig1 and N218HS in Ig2, respectively. We would expect that these changes would prevent Dlar from adopting a horseshoe conformation because N-linked glycans at these positions would present a steric barrier to the formation of the Ig1–Ig2 interface. This mutant migrates slightly slower than wild-type Dlar(Ig1-2) and the obtained band is broader, indicating that additional N-linked carbohydrates were indeed appended (Fig. 4c, left panel). As expected, our affinity isolation assay shows that this mutant form of Dlar binds to heparin, which is in line with the fact that Ig1 alone supports heparin binding. Overall, these results indicate that the horseshoe conformation is not necessary to bind to heparin and, presumably, the glycosaminoglycans ligands of Dlar and LAR, thus raising the question as to what the exact role of this unusual antiparallel arrangement is.
Conclusion
Dlar and its vertebrate counterparts LAR, PTPRD and PTPRS mediate critical interactions with proteoglycans during neurogenesis. The crystal structures of the Ig1–Ig2 fragments of Dlar and mouse LAR make it possible to identify a group of conserved lysine and arginine residues that form a positively-charged patch in the first Ig domain, which is the likely glycosaminoglycan-binding site in type IIa RPTPs. Interestingly, PTPRS has been shown to bind to two different classes of proteoglycans with dissociation constants in the nanomolar range. Indeed, avian PTPRS associates with the HSPGs agrin and collagen XVIII 15, whereas murine PTPRS is a physiological receptor for chondroitin sulfate proteoglycans (CSPGs) 36. Much like HSPGs, CSPGs are composed of a core protein to which chondroitin sulfate (CS) chains are attached. However, CS chains differ in their carbohydrate composition from HS chains as they include repeats of a disaccharide formed by glucuronic acid and N-acetyl galactosamine instead of repeats of either glucuronic acid or its epimer iduronic acid linked to N-acetyl glucosamine found in HS chains. In addition, HS disaccharide units can be sulfated on up to 4 of the available hydroxyl groups whereas CS chains are usually less sulfated than HS chains. The interactions between proteoglycans and PTPRS were sensitive to treatment with either the HS-degrading enzyme heparinase or the CS-degrading enzyme chondroitinase ABC suggesting that the glycosaminoglycan chains, but not the protein cores, are primarily responsible for these binding events 15, 36. In addition, mutations of a cluster of basic residues in the first Ig domain of avian PTPRS that impaired binding to HSPGs had an identical effect on the binding of CSPGs to mouse PTPRS. Avian and murine PTPRS share more than 80% amino acid sequence identity and are virtually identical in the Ig1–Ig2 region so that differences in ligand-binding specificities are unlikely to reflect species differences. Taken together, these findings indicate that PTPRS in particular and type IIa RPTPs in general may not discriminate between HSPGs and CSPGs, but would rather appear to harbor a more generic glycosaminoglycan-binding site, which would be the positively-charged surface identified in the work presented here.
Conversely, the unexpected resemblance between type IIa RPTPs and the hedgehog coreceptor iHog could suggest a more complex role for the positively-charged surface on Ig1. In particular, iHog also binds to HSPGs and its HS-binding site is located in its FN1 repeat in a loop region that partially overlaps the putative HSPG-binding site in Dlar and LAR (Fig. 3d). Even more interesting is the fact that iHog forms a ternary complex with hedgehog and HSPGs 33. Although this would appear to be in contrast with the idea of a generic glycosaminoglycan-binding site on Ig1, it is tempting to speculate that type IIa RPTPs may also associate with protein ligands in a HSPG-dependent manner. In this case, there may be two distinct classes of ligands for type IIa RPTPs, CSPGs on one hand and HSPGs/HSPG-dependent protein ligands on the other hand, which could in turn elicit distinct biological functions. However, to this point, no HSPG-dependent protein ligand has been identified for type IIa RPTPs. In the future, structural investigations glycosaminogycan-bound forms of type IIa receptors as well as renewed efforts to identify physiological ligands for these proteins could shed light on the protein-carbohydrate interactions that underlie critical cell adhesion and signaling events during the development and growth of the nervous system.
Methods
cDNA fragments encoding Dlar(Ig1-2) (residues 32–237) and mouse LAR(Ig1-2) (residues 29–235) were amplified from a Drosophila cDNA library and a mouse embryonic day 13.5 cDNA library, respectively. These fragments were subcloned into a modified pET32 plasmid (Novagen, La Jolla, CA) that directs the expression of thioredoxin, a hexahistidine tag, a human rhinovirus 3C protease cleavage site and the protein of interest. Proteins were expressed in Escherichia coli strain Origami 2(DE3) and purified by immobilized nickel-affinity chromatography, ion exchange, heparin-affinity and size exclusion chromatography using published protocols 16.
Crystals were grown at 20°C by hanging drop vapor diffusion. Plate-like crystals of Dlar(Ig1-2) were grown in 50 mM imidazole-HCl pH 7.0, 10 mM (NH4)2SO4 and 20% (w/v) PEG 2,000 MME after two consecutive rounds of microseeding. Crystals of LAR(Ig1-2) were grown in 50 mM sodium citrate pH 5.5, 200 mM of Li2SO4 and 25% (w/v) PEG 1,500. For cryoprotection, crystals were transferred to a solution of their respective mother liquor to which glycerol was added to a final concentration of 15% to 20% (v/v).
Diffraction data were collected at beamline 22-ID of the Advanced Photon Source of Argonne National Laboratory and processed with HKL2000 38. The structure of LAR(Ig1-2) was solved by molecular replacement with PHASER as implemented in PHENIX using the structures of telokin (PDB ID IFHG, residues 40–123) and MuSK (PDB ID 2IEP, residues 129–210) as two independent search models for the 1st and 2nd Ig domains of LAR(Ig1-2), respectively. The final model for LAR(Ig1-2) was obtained after manual model building using COOT and refinement in PHENIX and consists of residues 30–226, 85 water molecules and two sulfate ions 39, 40. The Ig domains from LAR(Ig1-2) were then used as two independent search models to obtain a molecular replacement solution for Dlar(Ig1-2). The final model for Dlar(Ig1-2) consists of 4 chains with residues 32–230 in molecule A, residues 32–230 in molecule B, residues 33–76, 81–114, 120–131 and 134–230 in molecule C, residues 33–131 and 134–230 in molecule D and 138 water molecules.
Supplementary Material
The bacterially-expressed form of Dlar(Ig1-2) and mouse LAR(Ig1-2) bind to heparin. Dlar(Ig1-3) was purified from the conditioned media of transiently transfected N-acetylglucosaminyltransferase I-negative HEK293S cells whereas Dlar(Ig1-2) and LAR(Ig1-2) were expressed in Escherichia coli strain Origami2(DE3). For the affinity isolation assay, 10 µg of each purified protein were incubated with heparin-sepharose in phosphate-buffered saline with 1% Tween-20 (v/v) for 1 hour at room temperature. Resins were washed in phosphate-buffered saline with 1% Tween-20 (v/v) and samples were resolved by SDS-PAGE (15% gel). Bound proteins were visualized by Coomassie blue staining. Lysozyme is used as a negative control.
Table 1.
Crystallographic data table and refinement statistics
| Beamline | APS 22-ID | APS 22-ID |
| Crystal | Dlar(Ig1-2) | LAR(Ig1-2) |
| Wavelength (Å) | 0.97242 | 0.97242 |
| Number of unique reflections | 39,287 | 15,225 |
| Resolution (Å) | 50 – 2.3 | 50 – 2.0 |
| Space group | P21 | P3221 |
| Unit cell | ||
| a, b, c (Å) | 72.96, 77.51, 81.73 | 77.45, 77.45, 68.50 |
| α, β, γ (°) | 90.00, 101.08, 90.00 | 90.00, 90.00, 120.00 |
| Rsyma | 0.117 (0.415) | 0.069 (0.364) |
| Completeness (%) | 98.7 (98.2) | 92.6 (65.4) |
| Redundancy | 2.7 (2.0) | 15.4 (8.7) |
| I/σI | 5.7 (1.9) | 28.2 (3.6) |
| Refinement | ||
| RCSB accession number | 3PXJ | 3PXH |
| Molecules in asymmetric unit | 4 | 1 |
| Resolution (Å) | 41.4 – 2.3 | 33.5 – 2.0 |
| Reflections | 37,084 | 14,492 |
| Rwork c / Rfree | 0.223 / 0.261 | 0.220 / 0.240 |
| Number of protein atoms | 5,965 | 1,494 |
| Number of water atoms | 138 | 85 |
| r.m.s. deviation from ideal bonds (Å) | 0.032 | 0.030 |
| r.m.s. deviation from ideal angles (°) | 1.66 | 1.67 |
| Average B factors (Å2) | 58.2 | 53.4 |
| Protein | 58.4 | 53.6 |
| Water | 51.2 | 49.0 |
| Sulfate | - | 69.6 |
| Ramachandran statistics | ||
| Favored (%) | 97.9 | 99.5 |
| Allowed (%) | 2.0 | 0.5 |
| Outlier (%) | 0.1 | - |
Ramachandran statistics were calculated using RAMPAGE 37 as implemented in CCP4.
Rsym = Σh Σi|Ii(h) - <I(h)>| / Σh Σi Ii(h), where Ii(h) is the ith measurement of reflection h and <I(h)> is a weighted mean of all measurements of h.
Values in parentheses apply to the high-resolution shell.
R = Σh|Fobs(h) – Fcalc(h)| / Σh|Fobs|. Rwork and Rfree were calculated from the working and test reflection sets, respectively. The test set constituted 5% of the total reflections not used in refinement.
Acknowledgments
The authors thank Brian Geisbrecht for helpful comments on the manuscript. This work was supported by Award Number R01GM088806 from the National Institute Of General Medical Sciences. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. Data were collected at Southeast Regional Collaborative Access Team beamlines at the Advanced Photon Source, Argonne National Laboratory. A list of supporting member institutions may be found at www.ser-cat.org/members.html. X-ray scattering technologies at the Lawrence Berkeley National Laboratory SIBYLS beamline of the Advanced Light Source are supported in part by the U.S. Department of Energy program Integrated Diffraction Analysis Technologies (IDAT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data accession numbers
The atomic coordinates and structure factors have been deposited in the Protein Data Bank (www.pdb.org) under accession codes 3PXH for mouse LAR(Ig1-2) and 3PXJ for Dlar(Ig1-2).
References
- 1.Chang C, Yu TW, Bargmann CI, Tessier-Lavigne M. Inhibition of netrin-mediated axon attraction by a receptor protein tyrosine phosphatase. Science. 2004;305:103–106. doi: 10.1126/science.1096983. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 3.Desai CJ, Gindhart JG, Goldstein LS, Zinn K. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell. 1996;84:599–609. doi: 10.1016/s0092-8674(00)81035-1. [DOI] [PubMed] [Google Scholar]
- 4.Krueger NX, Van Vactor D, Wan HI, Gelbart WM, Goodman CS, Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- 5.Wills Z, Bateman J, Korey CA, Comer A, Vactor V. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeyer K, Treisman JE. The receptor protein tyrosine phosphatase LAR promotes R7 photoreceptor axon targeting by a phosphatase-independent signaling mechanism. Proc. Natl. Acad. Sci. USA. 2009;106:19399–19404. doi: 10.1073/pnas.0903961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Møller NP. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolkman MJ, Streijger F, Linkels M, Bloemen M, Heeren DJ, Hendriks, Van der Zee CE. Mice lacking leukocyte common antigen-related (LAR) protein tyrosine phosphatase domains demonstrate spatial learning impairment in the two-trial water maze and hyperactivity in multiple behavioural tests. Behav. Brain Res. 2004;154:171–182. doi: 10.1016/j.bbr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, Yakura H, Asano M, Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elchebly M, Wagner J, Kennedy TE, Lanctôt C, Michaliszyn E, Itié A, Drouin J, Tremblay ML. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat. Genet. 1999;21:330–333. doi: 10.1038/6859. [DOI] [PubMed] [Google Scholar]
- 12.Wallace MJ, Batt J, Fladd CA, Henderson JT, Skarnes W, Rotin D. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat. Genet. 1999;21:334–338. doi: 10.1038/6866. [DOI] [PubMed] [Google Scholar]
- 13.Uetani N, Chagnon MJ, Kennedy TE, Tremblay Iwakura. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J. Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr. Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol. Cell. Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc. Natl. Acad. Sci. USA. 2010;107:2443–2448. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harpaz Y, Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994;238:528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara N, Howitt JA, Hussain S-A, Hohenester E. Structural and Functional Analysis of Slit and Heparin Binding to Immunoglobulin-like Domains 1 and 2 of Drosophila Robo. J. Biol. Chem. 2008;283:16226–16234. doi: 10.1074/jbc.M800688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morlot C, Thielens NM, Ravelli RBG, Hemrika W, Romijn RA, Gros P, Cusack S, McCarthy AA. Structural insights into the Slit-Robo complex. Proc. Natl. Acad. Sci. USA. 2007;104:14923–14928. doi: 10.1073/pnas.0705310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 22.Lo Conte L, Chothia C, Janin J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999;285:2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 23.Putnam CD, Hammel M, Hura GL, Tainer JA. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 24.Tian SS, Tsoulfas P, Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991;67:675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- 25.Fan QR, Mosyak L, Winter CC, Wagtmann N, Long EO, Wiley DC. Structure of the inhibitory receptor for human natural killer cells resembles haematopoietic receptors. Nature. 1997;389:96–100. doi: 10.1038/38028. [DOI] [PubMed] [Google Scholar]
- 26.Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 27.Meijers R, Puettmann-Holgado R, Skiniotis G, Liu JH, Walz T, Wang JH, Schmucker D. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–491. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- 28.Mortl M, Sonderegger P, Diederichs K, Welte W. The crystal structure of the ligand-binding module of human TAG-1 suggests a new mode of homophilic interaction. Protein Sci. 2007;16:2174–2183. doi: 10.1110/ps.072802707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawaya MR, Wojtowicz WM, Andre I, Qian B, Wu W, Baker D, Eisenberg D, Zipursky SL. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;134:1007–1018. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su XD, Gastinel LN, Vaughn DE, Faye I, Poon P, Bjorkman PJ. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science. 1998;281:991–995. doi: 10.1126/science.281.5379.991. [DOI] [PubMed] [Google Scholar]
- 31.Holm L, Kääriäinen S, Rosenström P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collaborative Computational Project, N. 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Cryst. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 33.McLellan JS, Yao S, Zheng X, Geisbrecht BV, Ghirlando R, Beachy PA, Leahy DJ. Structure of a heparin-dependent complex of Hedgehog and Ihog. Proc. Natl. Acad. Sci. USA. 2006;103:17208–17213. doi: 10.1073/pnas.0606738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Askari JA, Humphries MJ, Jones EY, Stuart DI. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468–1479. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPσ Is a Receptor for Chondroitin Sulfate Proteoglycan, an Inhibitor of Neural Regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 38.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 40.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 41.Pelikan M, Hura GL, Hammel M. Structure and flexibility within proteins as identified through small angle X-ray scattering. Gen. Physiol. Biophys. 2009;28:174–189. doi: 10.4149/gpb_2009_02_174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hura GL, Menon AL, Hammel M, Rambo RP, Poole FLn, Tsutakawa SE, Jenney FEJ, Classen S, Frankel KA, Hopkins RC, Yang SJ, Scott JW, Dillard BD, Adams MW, Tainer JA. Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS) Nat. Methods. 2009;6:606–612. doi: 10.1038/nmeth.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guinier A, Fournet F. Small Angle Scattering of X-rays. New York: Wiley Interscience; 1955. [Google Scholar]
- 44.Schneidman-Duhovny D, Hammel M, Sali A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 2010;38:W540–W544. doi: 10.1093/nar/gkq461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leahy DJ, Hendrickson WA, Aukhil I, Erickson HP. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992;258:987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- 46.Rocchia W, Alexov E, Honig B. Extending the applicability of the nonlinear Poisson-Boltzman equation: multiple dielectric constants and multivalent Ions. J. Phys. Chem. 2001;B105:6507–6514. [Google Scholar]
- 47.Rocchia W, Sridharan S, Nicholls A, Alexov E, Chiabrera A, Honig B. Rapid grid-based construction of the molecular surface and the use of induced surface charge to calculate reaction field energies: applications to the molecular systems and geometric objects. J. Comput. Chem. 2002;23:128–137. doi: 10.1002/jcc.1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bacterially-expressed form of Dlar(Ig1-2) and mouse LAR(Ig1-2) bind to heparin. Dlar(Ig1-3) was purified from the conditioned media of transiently transfected N-acetylglucosaminyltransferase I-negative HEK293S cells whereas Dlar(Ig1-2) and LAR(Ig1-2) were expressed in Escherichia coli strain Origami2(DE3). For the affinity isolation assay, 10 µg of each purified protein were incubated with heparin-sepharose in phosphate-buffered saline with 1% Tween-20 (v/v) for 1 hour at room temperature. Resins were washed in phosphate-buffered saline with 1% Tween-20 (v/v) and samples were resolved by SDS-PAGE (15% gel). Bound proteins were visualized by Coomassie blue staining. Lysozyme is used as a negative control.