Abstract
Purpose
The purpose of the study was to examine the symptom of itch and its relationship with chronic venous disease, pain, and quality of life.
Design
The study used an exploratory, cross sectional design.
Methods
One hundred sixty one participants completed demographic, health, substance abuse, itch, and pain questionnaires and the SF-12v2 Health Survey to measure health related quality of life.
Results
Participants were mostly men (n = 95, 59%), African American (113, 70.2%) had a mean age of 44.19 years. A history of injection drug use was reported by 91.4%. Using the clinical score of the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) Classification of the worst leg, the most common classification was Class 3, edema without skin changes (45.9%); 18.6% had severe venous disease (Classes 5 and 6). Eighty-eight participants (54.7%) reported itch somewhere on their body with 74 of them (45.9%) reporting itch on the legs/feet. Fourteen participants who reported itch of their legs or feet had wounds on the legs or feet. A positive correlation between magnitude of reported itch and CEAP clinical classification was noted (0.26, p = .025). This relationship was not strictly linear with itch increasing at a faster rate at higher levels of the CEAP classification. Persons with leg or feet itch had poorer health related quality of life, more comorbidities, and higher leg pain than those without itch. Because the SF-12v2 Health Survey is norm based, persons with itch were more than one standard deviation below the mean for the United States for their mental and physical health scores.
Conclusions
Itch on the legs or feet is a clinically relevant problem that is related to the level of venous disease. Persons with lower extremity itch have higher pain ratings and lower quality of life.
Introduction
Itch is a subjective symptom seen with multiple pathologic conditions and with changes to the skin. The term is difficult to define and is often a neglected symptom. More than 340 years ago, Hafenreffer, a German physician, defined itch as an unpleasant sensation provoking the desire to scratch.1,2 This definition has remained largely unchanged over time. Categorization of itch has been difficult because of the multiple dimensions of the phenomenon. Like pain, itch has sensory, cognitive and emotional components. The objective response to itch is scratching or rubbing.3 One example of itch with a pathologic change is venous eczema or stasis dermatitis of chronic venous disease (CVD). Fluid and macromolecules leak into the tissues causing an inflammatory response which includes erythema, scaling and intense itching.4 The trauma produced by the scratching is of great concern because it can result in excoriation, secondary infection, further changes in pigmentation, thickening of the skin, scars, and delayed healing.5 We examined the bodily occurrence of itch and its relationship with CVD, pain, and health related quality of life.
Although generally used interchangeably, some distinctions have been made between itch and pruritus. Waxler and colleagues6 specify that pruritus is a condition in which itch is present without a specific cause. The term pruritus has also been used to indicate itch without visible skin lesions.7 For the purpose of this study, itch and pruritus will be considered synonymous and we will use both terms throughout this article.
Physiology and Classification of Itch
Until 1997, the sensation of itch was thought to follow the same pathways as painful stimuli, but with a less intense stimulus eliciting a symptom of itch rather than pain. In a breakthrough study, Schmelz and coinvestigators identified itch-selective neurons in humans.8 Slow-conducting C-fibers that originate in the skin pass sensory information to the dorsal horn of the spinal cord that travels via the spinothalamic tract to the thalamus in the somatosensory cortex.6,9–11 These C-fibers, sometimes called pruriceptors are a subclass of C-nociceptors for pain; they account for approximately 5% of all afferent C-fibers in human skin and are similar to, but functionally distinct from, pain fibers. They are responsive to histamine and other pruritogens.9 Pruritogens that are hypothesized to be present in open wounds include histamine that is released from granulation tissue and nerve growth factors.5,11,12 The symptom of itch occurs when free nerve endings of the specialized C-fibers are stimulated by pruritogenic substances.
The classification of itch can be determined based on timing and mechanism. For example, acute itch can last from seconds to a week.3 Chronic itch persists for more than three months.1 Twycross and colleagues11 proposed an itch classification system based on underlying mechanism: 1) pruritoceptive, 2) neurogenic, 3) neuropathic, 4) psychogenic, and 5) mixed that has proved most useful in clinical and research settings. Pruritoceptive itch originates in the skin in response to insect bites, eczema, or dry skin. Neurogenic itch originates centrally without evidence of neural pathology and is found with systemic disorders such as chronic liver disease, chronic renal disease, and malignant and hematologic conditions.1,11 Neuropathic itch is associated with diseased neurons along the afferent neuronal pathway’ it is associated with post-herpetic pruritus, multiple sclerosis, and diabetic neuropathy.1 Psychogenic itch is associated with psychological factors such as emotional stress. The origin of itch in the central nervous system is demonstrated with the common event of socially contagious scratching (similar to contagious yawning). With distraction, itch can be forgotten and the symptom can be suppressed with training.11 Itch associated with chronic venous disorders is attributed to mixed mechanisms including cutaneous changes, neuropathic changes, and psychological factors.
In contrast to pain, itching provokes a withdrawal response to remove the offending irritant and protect the skin and integrity of the body.10 The itch-scratch cycle is described as itch eliciting a scratch response.5,13 Scratching causes inflammation and further stimulation of nerve fibers which provokes the sensation of itch. The sensation of itch then prompts further scratching or rubbing and so on. While scratching and rubbing can provide relief, both can also lead to lichenification (scratch marks) and additional trauma to the local skin.13
Chronic Venous Disease and Itch
With CVD, fluid and macromolecules leak into the tissues causing an inflammatory response.4 Soft tissue injury begins in the subcutaneous tissue; it presents as induration and fibrosis and is commonly referred to as lipodermatosclerosis.14 The skin increases in color intensity, often to hues of red, brown, or black, owing to hemosiderin released from the breakdown of red blood cells in the subcutaneous tissue. The skin becomes incessantly dry and flaky due to edema and fibrinogen deposits. This leads to pruritus and scratching with excoriation and eczematous changes referred to as stasis dermatitis.4 Venous ulcers may develop whenever the skin is injured, even when the original injury is minor. In addition, individuals may have other types of wounds or ulcers within areas of CVD that produce itch because of surrounding skin changes.
Duque and cowrkers15 conducted a study among persons with mild to moderate venous insufficiency to estimate the prevalence of itch, pain, and burning sensations. They also examined characteristics of these symptoms and their relation to severity of venous disease, identified factors that aggravated or alleviated these symptoms, and determined their impact on health related quality of life. Eklof and associates16 used The Clinical-Etiology-Anatomy-Pathophysiology (CEAP) Classification to grade venous disease. Sixty-six percent of their subjects (n = 100) had itch at the time of the interview. They reported that itch did not correlate with the severity of venous disease, but they did find a statistically significant negative relationship between itch intensity and health related quality of life (r = .50, p < .001).
Shai and Halevy17 interviewed and reviewed the medical records of 91 persons who had a total of 110 venous ulcers to determine what actually causes ulceration in persons with venous insufficiency. Although the study did not focus specifically on itch, they concluded that 5.4% of the ulcers were triggered by dry skin and subsequent scratching.
Wound Itch
Research related to wound itch is sparse; our literature review revealed 3 studies focusing on itch in acute burns. Mendham18 was interested in determining if itch associated with burns would respond to medications used for neuropathic pain. He observed episodes of itching in 35 children with acute burns and found that a marked reduction in episodes of scratching was noted with gabapentin use. Baker and colleagues12 used a numeric itch scale to assess the effects of histamine antagonists on 32 patients with acute burns; cetirizine with cimetidine provided the best effect. Matheson and colleagues19 looked for a method to reduce itch experienced by persons with burns. They assessed itch ratings of 35 acute burn patients who tried one of two bath oils: one with colloidal oatmeal and one without. Persons using the bath oil with colloidal oatmeal reported a daily mean itch that was half of the magnitude reported by those persons using the product without colloidal oatmeal.
Hareendran and associates20 interviewed 38 persons with venous ulcers to identify their quality of life. Participants reported their ulcers resulted in pain (80.5%), itching (69.4%), altered appearance (66.7%), loss of sleep (66.6%), and functional limitations (58.3%), and disappointment with treatment (50%). Since this study did not focus on itch, its influence on sleep or functional limitations was not reported. In a separate study, Hareendran and cowirkers21 conducted in-depth interviews and focus groups in 36 persons with venous leg ulcers in order to develop and validate a quality of life questionnaire. Symptom severity and bother were assessed. Bother included pain, smell, itching, sleep disturbance, and influence on daily activities. “Ulcer itches” was ranked fourth among ten symptoms causing distress, after ulcer burns and stings, ulcer hurts, and skin irritated.21
Purpose
Itch is a subjective symptom that is difficult to define and categorize. Like pain, itch has sensory, cognitive and emotional components. In addition to skin and wound changes that occur with CVD, itch may cause further skin damage. In order to further elucidate itch in patients with CVD, we asked the following questions: (1) What is the frequency of occurrence of leg or foot itch in persons with CVD? (2) What are the characteristics of itch in persons with CVD? (3) What measures do participants use to minimize or eradicate itch? (4) What is the relationship of itch to pain? (5) What is the relationship of itch to quality of life?
METHODS
Design
This study was part of a larger study described in a previous publication.22 Seven hundred thirteen participants from that study were recruited from 12 methadone treatment clinics located in a large urban Midwestern city between 2005 and 2007. Eligible participants were 25 to 65 years of age, able to understand and speak English, had not undergone lower extremity amputation, able to walk, non-pregnant, and deemed physically and mentally able to participate. The study used a three-group comparative design with stratification. The three groups were determined by site of injection/non-injection drug use: Group 1 consisted of non-injection drug users; Group 2 consisted of participants who injected drugs in their arms and/or upper body only; and Group 3 consisted of participants with a history of injecting in their legs (leg +/− arm). Stratification within each drug group was based on age (25 – 39 years; 40 – 49 years; 50 – 65 years); gender (male, female); and ethnicity (African American; White). We recruited the initial 104 participants from one methadone treatment clinic for the purpose of preliminary psychometrics for test-retest reliability. These results are reported in the Measures section below. Because the itch questionnaire was added late to the study, data analysis is based on responses from 161 participants out of 716 parent study participants (22.6%).
Study Procedures
The study was approved by the Institutional Review Board of the affiliated university. Research assistants obtained informed consent from all participants, administered the questionnaires and completed the leg assessment. Questionnaires were read to participants to facilitate responding and decrease frustration for those who read poorly. Both legs were assessed for clinical manifestations of CVD. Participation comprised 1.5 to 2 hours; participants were compensated $40 for their time.
Instruments
A demographic questionnaire provided information about sex, race, and age of participants. The Health History Questionnaire was a self-report of 23 medical diagnoses. Participants were measured and weighed on a standard scale to determine body mass index (BMI). In the overall study, the test-retest reliability values for the demographic and health history questionnaires were 0.99 and 0.86 respectively.23
A drug history questionnaire was used to obtain a detailed history about illicit drugs consumed.23 Participants were asked to report age at first use, age at last use, and number of years between first and last use of all substances. Additional questions addressed the number of years spent injecting in the upper body (i.e., hands, arms, and above the waist) and number of injection years in the lower body (i.e., groin, legs, and feet). The scoring of the drug history questionnaire provided the information for the drug use group classification (nominal data) (non-injection drug use, arm/upper body injection only, or legs +/− upper body injection), years of use per substance, and years of injecting in the upper and lower body sites. For this study, the drug use questionnaire had a median kappa value of .79.23
The clinical portion of the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) Classification was used to determine the clinical severity of CVD (Table 1).16 In our previous work, the inter-rater reliability of the CEAP was kappa = 1.0.24 Using 25 of the 104 participants in the test-retest sample, we determined clinical CEAP inter-rater reliability was .97 for the right leg and .94 for the left leg.22
Table 1.
CAEP Classifications16
| Class | Description |
|---|---|
| Class 0 | No visible or palpable signs of venous disease |
| Class 1 | Telangiectasias or reticular veins |
| Class 2 | Varicose veins, distinguished from reticular veins by diameter of 3 mm or more; |
| Class 3 | Edema |
| Class 4a | Pigmentation or eczema |
| Class 4b | Lipodermatosclerosis or atrophie blanche |
| Class 5 | Healed venous ulcers |
| Class 6 | Active venous ulcer |
The Itch Questionnaire was developed for this study and was based on the itch literature.25. Participants initially rated the amount of bodily itch on a 0 (none) to 10 (unbearable) scale and identified its location (head, arms, hands, chest, back, legs, feet, and other). From a list of 19 items (cold pack, lotion, air, etc.), participants selected what decreased or relieved bodily itch. From 5 items (heat, cold, eating certain foods, worry, other), they identified factors that made itch worse. If participants had a wound, they rated the amount of itch on and surrounding the wound (0, none to 10, unbearable), the length of time they had wound itch, and when during the day it was most bothersome. Reliability cannot be calculated for the instrument because it is a survey versus a singular summative rating scale.
The Pain Severity subscore of the Brief Pain Inventory (BPI) Short Form26 was modified to measure leg pain. Severity is a primary factor that determines the impact of pain for the person.27 Participants rated their leg pain for its worst, average, and current level. Each item was scored on an 11-point scale ranging from 0 (no pain) to 10 (worst possible pain). The three items were combined for a total pain score. The pain severity items have a reported Cronbach’s alpha of .85.28 The Cronbach’s alpha for the three pain severity items for this study was .89.
Health Related Quality of Life
The SF-12v2 Health Survey was used to measure health related quality of life. The SF-12v2 consists of 12 questions selected from the SF-36 Health Survey.29 The 12 items measure physical and mental health with questions categorized as: Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. Participants responded to questions in terms of the past 4 weeks. Responses range from all of the time to none of the time. Items were tabulated to obtain the physical and mental health scores. SF-12v2 scores have been norm-based for the general United States (U.S.) population.29 For the general U.S. sample, the reliability coefficients of each of the eight SF12v2 scales range from .73 to .87; the reliability estimates of the physical and mental health scores are .89 and .86, respectively.29
Data Analysis
We first performed simple frequencies to describe the participants. The relationship between itch and other variables was examined with correlations or inferential statistics.
RESULTS
Subjects
The 161 study participants included 95 men (59.01%) and 66 (40.99%) women. A majority (70.2%) were African American. Their mean age was 44.19 ± 9.48 years (Mean ± SD, range 25 to 63 years). Participants reported a mean of 2.32 ± 1.88 co-morbid conditions. Thirty participants (18.63%) reported no history of injection drug use; 36 (22.36%) had a history of injecting drugs in the upper extremities only. Ninety-five participants had a history of injecting drugs in the lower extremities, either with (93.7%) or without upper extremity injection.
Based CEAP classification of the worst leg, 3 subjects (1.9%) did not exhibit clinical changes of CVD; 60.9% percent had mild CVD (Classes 1 – 3), 18.6% had moderate disease (Classes 4a and 4b), and 18.6% had severe CVD disease (Classes 5 and 6). The most common classification was Class 3, edema without skin changes (45.9%).
Eighty eight participants (54.7%) reported itch somewhere on their body, including 74 (45.9%) who reported itch on the legs and/or feet. Fourteen participants with leg/feet itch had lower extremity wounds (Table 2). Among the 74 participants who had itch on their legs and/or feet, their mean itch score was 5.66 ± 2.73. The most common ways to relieve itch were scratching (n = 36), lotion (n = 28), and rubbing (n = 17) (Table 3). Four factors were reported to exacerbate itch; heat and worry were most commonly cited (Table 4).
Table 2.
Demographic Data
| Variable | Total Sample (N = 161) |
Leg/Feet Itch (n = 74) |
Wound Itch (n = 14) |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (range, 25 to 63) | 44.19 | 9.48 | 46.09 | 8.86 | 48.86 | 7.88 |
| Comorbidities (range, 0 to 8) | 2.32 | 1.88 | 2.95 | 2.01 | 3.21 | 2.04 |
| Itch general (range, 0 to 10) | 2.99 | 3.41 | 5.66 | 2.73 | 6.14 | 2.69 |
| Wound Itch (range, 0 to 10) | 5.71 | 3.27 | ||||
| Periwound Itch (range, 0 to 10) | 6.36 | 2.81 | ||||
| Leg Pain (range, 0 to 10) | 3.73 | 3.01 | 4.79 | 2.73 | 5.90 | 2.61 |
| CEAP clinical score (range, 0 to 6) | 3.27 | 1.34 | 3.69 | 1.35 | 4.71 | 1.27 |
| Physical SF-12 (range, 0 to 100) | 42.31 | 8.85 | 39.66 | 8.49 | 34.79 | 5.92 |
| Mental SF-12 (range, 0 to 100) | 40.48 | 12.08 | 39.03 | 11.40 | 37.45 | 11.56 |
| Sex (% Male) | 59.01 | 62.16 | 78.57 | |||
| Race (% African American) | 70.19 | 75.58 | 71.43 | |||
| Drug Use (% Injecting) | 81.37 | 85.14 | 85.70 | |||
Table 3.
Methods Used to Decrease Leg/Feet Itch (n = 74) and Wound Itch (n = 12)
| Methods Used to Decrease Itch | Leg/Feet Itch |
Wound Itch |
||
|---|---|---|---|---|
| n | % of cases |
n | % of cases |
|
| Scratching | 36 | 48.6 | 3 | 25 |
| Lotion | 28 | 37.8 | 3 | 25 |
| Rubbing the area | 17 | 23.0 | ||
| Petrolatum | 13 | 17.6 | 4 | 33.3 |
| Diphenhydramine/hydroxyzine | 8 | 10.8 | 1 | 8.3 |
| Hot/warm water | 5 | 6.8 | 1 | 8.3 |
| Shower or tub bath | 6 | 8.1 | 1 | 8.3 |
| Steroid ointment | 3 | 4.1 | ||
| Antibiotic ointment | 3 | 4.1 | 5 | 41.7 |
| Cold pack/ice | 3 | 4.1 | ||
| Epsom salt (2) or menthol cream (2) or blowing air on area (1) | 5 | 6.8 |
Note: More than one item could be selected. Percent of cases is presented because it gives the percent of persons who selected that option.
Table 4.
Factors That Exacerbated Leg/Feet Itch (n = 70)
| Factor | n | % of cases |
|---|---|---|
| Heat | 19 | 27.1 |
| Worry | 18 | 25.7 |
| Cold | 6 | 8.6 |
| Certain foods | 3 | 4.3 |
Note: More than one item could be selected. Percent of cases is presented because it gives the percent of persons who selected that option.
Fourteen participants had wounds on their legs or feet; 7 had one or more wounds that were classified as venous ulcers and the other 7 had diabetic or trauma ulcers. Wound itch had a mean rating of 5.71 ± 3.27; itch around the wound had a mean rating of 6.36 ± 2.82. Itch within and around the wound was associated (r = .76, p = .001). Wound itch was most commonly relieved by antibiotic ointment (n = 5), petrolatum (n = 4), scratching and lotion (n = 3 for each) (Table 3).
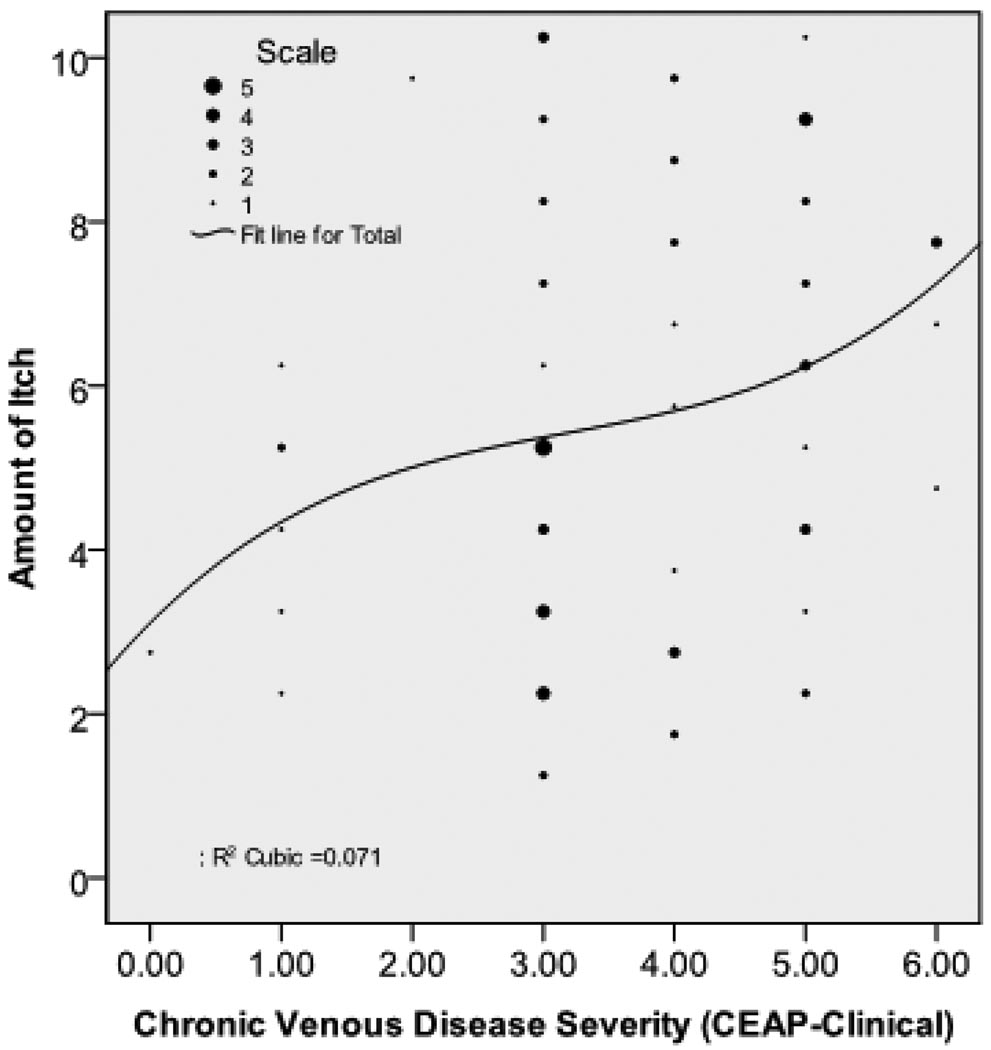
Table 5 shows the relationship between leg or foot itch CEAP classification. Itch occurred with the skin changes of venous disease (CEAP clinical classifications 4 and 5) (n = 33, 44.6%), but not necessarily with the presence of an ulcer (n = 5, 6.8%). The correlation between amount of itch and CEAP classification was .26, p = .025. This relationship was not strictly linear; itch increased at a faster rate with higher CEAP classification categories (Figure 1).
Table 5.
Cross Tabulation of CEAP Clinical Classification with Itch in Feet/Legs
| Itch in Feet/Legs | |||||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| CEAP classification | Class 0 | Count | 2 | 1 | 3 |
| % within category | 66.7% | 33.3% | 100% | ||
| Class 1 | Count | 15 | 6 | 21 | |
| % within category | 71.4% | 28.6% | 100.0% | ||
| Class 2 | Count | 2 | 1 | 3 | |
| % within category | 66.7% | 33.3% | 100.0% | ||
| Class 3 | Count | 46 | 28 | 74 | |
| % within category | 62.2% | 37.8% | 100.0% | ||
| Class 4a and b | Count | 16 | 14 | 30 | |
| % within category | 53.3% | 46.7% | 100.0% | ||
| Class 5 | Count | 4 | 19 | 23 | |
| % within category | 17.4% | 82.6% | 100.0% | ||
| Class 6 | Count | 2 | 5 | 7 | |
| % within category | 28.6% | 71.4% | 100.0% | ||
| Total | Count | 87 | 74 | 161 | |
| % within category | 54.0% | 46.0% | 100.0% | ||
Figure 1.
The figure shows the relationship between leg/feet itch (n = 74) and chronic venous disease severity. The vertical axis is the leg/feet itch score (0 to 10 scale). The horizontal axis is the clinical CEAP score (Class 0 to Class 6). This relationship was not strictly linear with itch increasing at a faster rate at higher levels of clinical CEAP
Analysis of variance was used to examine the relationships between itch with pain, comorbid conditions, and health related quality of life (Table 6). Persons with leg or feet itch had poorer physical quality of life [F(1, 159) = 13.18, p < .001], more comorbid conditions [F(1, 129) = 12.79, p < .001], and higher leg pain [F(1, 159) = 18.90, p < .001]. The two groups did not differ significantly on the mental health quality of life score. Because the SF-12 has norm based statistics/scoring, the T scores for the mental health and physical health scores for the United States population are 50 with a standard deviation of 10.29 When leg/feet itch or wound itch was present, participants’ mean physical and mental health scores were more than one standard deviation below the national mean for these scores [leg/feet itch (Mean Physical = 39.66; Mean Mental = 39.03) and wound itch (Mean Physical = 34.79; Mean Mental = 37.45)] (Table 2). These scores indicate the negative effect of itch on quality of life.
Table 6.
Analysis of Variance of Itch on Legs/Feet with Quality of Life, Comorbidities, and Leg Pain
| Sum of Squares |
df | Mean Square |
F | Sig. | |||
|---|---|---|---|---|---|---|---|
| PHYSICAL HEALTH - SF12 | Between Groups | (Combined) | 959.63 | 1 | 959.63 | 13.18 | p <.001 |
| Within Groups | 11575.68 | 159 | 72.80 | ||||
| Total | 12535.31 | 160 | |||||
| MENTAL HEALTH T - SF12 | Between Groups | (Combined) | 285.84 | 1 | 285.84 | 1.97 | ns |
| Within Groups | 23052.55 | 159 | 144.99 | ||||
| Total | 23338.39 | 160 | |||||
| Comorbidities | Between Groups | (Combined) | 33.10 | 1 | 33.10 | 12.79 | p <.001 |
| Within Groups | 333.85 | 129 | 2.59 | ||||
| Total | 366.95 | 130 | |||||
| Leg Pain | Between Groups | (Combined) | 154.33 | 1 | 154.33 | 18.90 | p <.001 |
| Within Groups | 1298.09 | 159 | 8.16 | ||||
| Total | 1452.42 | 160 |
DISCUSSION
We examined body, leg/feet and wound itch in a group of patients with CVD undergoing treatment in a methadone treatment program. A majority of participants (55%) experienced body itch; 19% of them rated its magnitude as unbearable. We found that itch negatively affected physical components of health related quality of life, and associated with more intense leg pain. The most common locations for itch were the legs and feet. Scratching and rubbing were used to relieve itch; worry and heat made the symptom worse.
Itch is an unpleasant sensation that induces a desire to scratch; it is associated with multiple disorders or of psychogenic origin.30 When interviewing physicians about impact of psoriasis, the first symptom mentioned was itch.31 Blome and colleagues32 reported the first expressed need of patients with pruritus is to get rid of itching (97%); this was followed in importance by finding a diagnosis of the itch (94%) and having confidence in the treatment (92%). Verhoeven and colleagues33 questioned 492 persons with various skin diseases about itch, pain and fatigue. Itch (53.5%) and fatigue (52.4%) were more frequently reported than pain (23%). In contrast, Maida and colleagues34 studied 67 cancer patients at the time of referral for palliative care. The prevalence of pruritus was 6%, as compared to 31.3% for pain and 11.9% for odor. Pruritus was reported within the wound itself as well as in the periwound area. Because Maida’s study was done at a random point in time, not when participants were seeing their practitioners, the findings may underestimate the presence and severity of symptoms.
Dawn and colleagues35 asked 304 persons with atopic dermatitis to complete a web-based Characteristics of Itch Questionnaire. A correlation (p < .001) between itch descriptors and itch intensity was found. Strong adjectives, such as annoying and bothersome, were selected by participants to depict the intense degree of suffering and unpleasantness they experienced.
CVD affects about 2.5 million persons in the U.S.36 With CVD, inflammatory mechanisms are up-regulated in the skin.37 Our findings are similar with other researchers who report that that itch is associated with advanced CVD20,21 The effect of itch is negative for someone with CVD since scratching can further damage the skin and result in ulceration.
Our findings are also consistent who report that pruritus of any origin leads to psychosocial burden and impairs quality of life.14, 25, 30,31 For example Globe and colleagues31 reported psoriatic itch symptoms influenced a person’s concentration, regular physical activity, and sleep quality. Similarly, Yosipovitch and coworkers25 reported that 60% of respondents on dialysis indicated that pruritus was associated with nervousness (36%) and depression (8%). However, itch is not typically included in studies evaluating the influence of CVD on health related quality of life.14
Research reveals physiologic associations between pain and itch processing, leading to the Selectivity Theory of Itch and the Pattern Theory of Itch.38 This theory is based on evidence that a subset of afferent nociceptors which respond to pruritogens exist that have separate central connections and neurons. They theory also postulates that an inhibitory process occurs so that pain and itch are not sensed simultaneously. Nevertheless, pain and itch are difficult phenomena to separate. No single “itch center” in the brain has been identified.39 Testing of itch theories has been difficult, as there are no good animal models for itch.40, 41
Implications for WOC Nursing
WOC nurses care for many patients with CVD and lower extremity wounds. Our findings add support to the findings of others indicating that recognition of itch as a problem, assessment a patient’s itch, and inclusion of itch management in treatment is a crucial component of this care. Often the cause of itch cannot be identified and causal therapy is not possible.30 Lawton42 noted managing dry and itching skin conditions is a skill required in all nursing specialties. Dry and itchy skin conditions require emollient therapy to restore the skin barrier.42 Our participants reported frequent use of petrolatum and various lotions to treat itch. Antihistamines also may be used to relieve itch symptoms. Globe and colleagues31 observed that no oral agent has been developed or approved by the United States Food and Drug Administration for managing itch. First generation antihistamines such as diphenhydramine and hydroxyzine may be used, but they are associated with sedation that may affect day time performance. WOC nurse counseling should include information about managing adverse side effects including administration of these agents in the evening. Topical corticosteroids may provide relief from itch, but they should be used with caution in patients with DVD since they may cause local atrophy. Additional treatment strategies include psychoeducational interventions, stress training, and relaxation techniques.30
Limitations
The itch questionnaire was added late in the study, resulting in a smaller sample size. We did not explore a participant’s history of skin conditions that may have influenced itch such as psoriasis or associated conditions. The number of participants with wounds was small limiting our ability to evaluate wound itch. In addition, we did not collect data about the influence of treatments on leg/feet or wound itch. Future studies need larger samples and specific questions to specify itch by various body locations. Longitudinal data about what is effective in treating itch of advanced CVD are needed.
The Itch Questionnaire we used provided descriptive information about the occurrence of itch and its severity, but it further development of a robust instrument for itch is needed. The Eppendorf Itch Questionnaire has been developed and tested that evaluates itch intensity and health related quality of life.43 It is based on the long form of the McGill Pain Questionnaire and provides information about itch and its psychosocial effects.44
Conclusions
Itch of the legs or feet was a common occurrence in patients with advanced CVD irrespective of ulceration. Persons with itch reported higher lower extremity pain and had lower scores on physical components of a validated health related quality of life instrument. These findings indicate the need for further studies of the itch phenomenon, including interventions for its prevention and treatment.
Acknowledgments
Funding: This project was funded by the National Institute of Nursing Research/National Institute of Health (NINR/NIH), Effect of Drug Use on the Legs: Chronic Venous Insufficiency, Mobility and Pain, R01 NR009264.
The authors gratefully acknowledge Terri Gibbons, BS, Valerie Grech, ADN, RN, and Joyce Peck, BSN, RN as research assistants. The authors acknowledge the invaluable contributions of the following methadone treatment centers: Department of Human Services, Building 5 & Gratiot, Detroit; Metropolitan Rehabilitation Clinics, Oak Park; Millennium Treatment Services, Madison Heights & Warren; Nardin Park Recovery Center, Detroit; New Light Recovery Center, Detroit; Parkview Counseling Centers Detroit, Dearborn Heights, & Pontiac; STAR Center Inc., Detroit; University Psychiatric Centers – Jefferson, Detroit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julia Claire Paul, College of Nursing, Wayne State University, Detroit, MI 48202, Phone: 313-577-4057, Fax: 313-577-4188, bb3410@wayne.edu.
Barbara Pieper, College of Nursing, Wayne State University, 5557 Cass Avenue, Detroit, MI 48202, Phone: 313-577-4057, Fax: 313-577-4188, bpieper@wayne.edu.
Thomas N. Templin, Center for Health Research, College of Nursing, Wayne State University, 5557 Cass Avenue, Detroit, MI 48202, Phone: 313-577-7992, Fax: 313-577-5777, t.templin@wayne.edu.
REFERENCES
- 1.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Yosipovitch G, Stander S. Meeting report of the 3rd International Workshop for the Study of Itch. J Invest Dermatol. 2006;126:1928–1930. doi: 10.1038/sj.jid.5700411. [DOI] [PubMed]
- 3.Yosipovitch G, Greaves M. Definitions of itch. In: Yosipovitch G, Greaves MW, Fleischer AB, McGlone F, editors. Itch: Basic Mechanisms and Therapy. New York: Marcel Dekker, Inc.; 2004. 2004. [Google Scholar]
- 4.Burrows C, Miller R, Townsend D, Bellefontaine R, MacKeon G, Orsted HL, Keast DH. Best practice recommendations for the prevention and treatment of venous leg ulcers: Update 2006. Adv Skin Wound Care. 2007;20(11):611–623. doi: 10.1097/01.ASW.0000284937.32707.c4. [DOI] [PubMed] [Google Scholar]
- 5.Stander S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003;139(11):1463–1470. doi: 10.1001/archderm.139.11.1463. [DOI] [PubMed] [Google Scholar]
- 6.Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiol. 2005;103:168–178. doi: 10.1097/00000542-200507000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Bernhard JD, editor. Itch: Mechanisms and Management of Pruritus. New York: McGraw-Hill, Inc.; 1994. [Google Scholar]
- 8.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymann WR. Itch. J Am Acad Dermatol. 2006;54(4):705–706. doi: 10.1016/j.jaad.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116(5):1174–1185. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: Scratching more than the surface. Q J Med. 2003;96(1):7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 12.Baker R, Zeller R, Klein R, Thornton R, Shuber MarshallR, et al. Burn wound itch control using H1 and H2 antagonists. J Burn Care Rehabil. 2001;22(4):263–268. doi: 10.1097/00004630-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Yosipovitch G, Hundley JL. Practical guidelines for relief of itch. Dermatol Nurs. 2004;16(4):325–238. [PubMed] [Google Scholar]
- 14.Bergan JJ, Schmid-Schonbein GW, Coleridge Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 15.Duque MI, Yosipovitch G, Chan YH, Smith R, Levy P. Itch, pain, and burning sensation are common symptoms in mild to moderate chronic venous insufficiency with an impact on quality of life. J Am Acad Dermatol. 2005;53(3):504–508. doi: 10.1016/j.jaad.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 16.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Shai A, Halevy S. Direct ulceration in patients with venous insufficiency. Int J Dermatol. 2005;44:1006–1009. doi: 10.1111/j.1365-4632.2005.02317.x. [DOI] [PubMed] [Google Scholar]
- 18.Mendham J. Gabapentin for the treatment of itching produced by burns and wound healing in children: a pilot study. Burns. 2004;30(8):851–853. doi: 10.1016/j.burns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Matheson J, Clayton J, Muller M. The reduction of itch during burn wound healing. J Burn Care Rehabil. 2001;22(1):76–81. doi: 10.1097/00004630-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care. 2005;14(2):53–57. doi: 10.12968/jowc.2005.14.2.26732. [DOI] [PubMed] [Google Scholar]
- 21.Hareendran A, Doll H, Wild DJ, Moffat CJ, Musgrove E, Wheatley C, Franks PJ. The venous leg ulcer quality of life (VLU-Qol) questionnaire: Development and psychometric validation. Wound Repair Regen. 2007;15:465–473. doi: 10.1111/j.1524-475X.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 22.Pieper B, Templin TN, Kirsner RS, Birk TJ. Impact of injection drug use on distribution and severity of chronic venous disorders. Wound Rep Reg. 2009;17(4):485–491. doi: 10.1111/j.1524-475X.2009.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieper B, Templin TN, Birk TJ, Kirsner RS. Reliability and clinical validity of a technique to assess lifetime illicit drug use. Ostomy Wound Manage. 2008;54(2):16–34. [PubMed] [Google Scholar]
- 24.Pieper B, Templin T. Chronic venous insufficiency in persons with a history of injection drug use. Res Nurs Health. 2001;24:423–432. doi: 10.1002/nur.1042. [DOI] [PubMed] [Google Scholar]
- 25.Yosipovitch G, Zucker I, Boner G, Gafter U, Shapira Y, David M. A questionnaire for the assessment of pruritus: Validation in uremic patients. Acta Derm Venereol. 2001;81:108–111. doi: 10.1080/00015550152384236. [DOI] [PubMed] [Google Scholar]
- 26.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 27.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate, or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 28.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. 2002 How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 30.Pogatzki-Zahn E, Marziniak M, Schneider G, Luger TA, Stander S. Chronic pruritus: targets, mechanisms and future therapies. Drug News Perspect. 2008;21(10):541–551. doi: 10.1358/dnp.2008.21.10.1314057. [DOI] [PubMed] [Google Scholar]
- 31.Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62. doi: 10.1186/1477-7525-7-62. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blome C, Augustin M, Siepmann D, Phan NQ, Rustenbach SJ, Stander S. Measuring patient-relevant benefits in pruritus treatment: development and validation of a specific outcomes tool. Brit J Dermatol. 2009;161:1143–1148. doi: 10.1111/j.1365-2133.2009.09328.x. [DOI] [PubMed] [Google Scholar]
- 33.Verhoeven EWM, Kraaimaat FW, van der Kerkhof PCM, van Weel C, Duller P, van der Valk PGM, van den Hoogen HJM, Bor JHJ, Schers HJ, Evers AWM. Prevalence of physical symptoms of itch, pain, and fatigue in patients with skin disease in general practice. Br J Dermatol. 2007;156:1346–1349. doi: 10.1111/j.1365-2133.2007.07916.x. [DOI] [PubMed] [Google Scholar]
- 34.Maida V, Ennis M, Kuzienisky C, Trozzolo L. Symptoms associated with malignant wounds: A prospective case study. J Pain Symptom Manage. 2009;37(2):206–211. doi: 10.1016/j.jpainsymman.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Dawn A, Papoiu ADP, Chan YH, Rapp SR, Rassette N, Yosipovitch G. Itch characteristics in atopic dermatitis: Results of a web-based questionnaire. Brit J Dermatol. 2009;160:642–644. doi: 10.1111/j.1365-2133.2008.08941.x. [DOI] [PubMed] [Google Scholar]
- 36.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circ. 2005;111:2398–2409. doi: 10.1161/01.CIR.0000164199.72440.08. [DOI] [PubMed] [Google Scholar]
- 37.Smith PC. The causes of skin damage and leg ulceration in chronic venous disease. Int J Low Extrem Wounds. 2006;5:160–167. doi: 10.1177/1534734606292429. [DOI] [PubMed] [Google Scholar]
- 38.McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- 39.Darsow U, Drzezga A, Ring J. Central nervous system imaging of itch with PET. In: Yosipovitch G, Greaves MW, Fleischer AB Jr, McGlone F, editors. Itch: Basic mechanisms and therapy. New York: Marcel Dekker, Inc.; 2004. pp. 63–70. [Google Scholar]
- 40.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 41.Carsten E, Kuraishi Y. Animal models of itch: Scratching away at the problem. In: Yosipovitch G, Greaves MW, Fleischer AB Jr, McGlone F, editors. Itch: Basic mechanisms and therapy. New York: Marcel Dekker, Inc.; 2004. pp. 35–50. [Google Scholar]
- 42.Lawton S. Practical issues for emollient therapy in dry and itchy skin. Br J Nurs. 2009;18(16):978–984. doi: 10.12968/bjon.2009.18.16.43964. [DOI] [PubMed] [Google Scholar]
- 43.Yosipovitch G. Itch questionnaires as tools for itch evaluation. In: Yosipovitch G, Greaves MW, Fleischer AB, McGlone F, editors. Itch: Basic Mechanisms and Therapy. New York: Marcel Dekker, Inc.; 2004. [Google Scholar]
- 44.Darsow U, Scharein E, Simon D, Walter G, Bromm B, Ring J. New aspects of itch pathophysiology: Component analysis of atopic itch using the ‘Eppendorf Itch Questionnaire.’. Int Arch Allergy Immunol. 2001;124:326–331. doi: 10.1159/000053748. [DOI] [PubMed] [Google Scholar]