Abstract
Encephalitozoon cuniculi continues to pose a problem for immunocompromised patients. Previous studies from our laboratory have elucidated the importance of the CD8+ T cell subset in the protection against systemic parasite infection. There have been no studies related to the mucosal immunity induced against this orally acquired pathogen. In the present study, the immune response generated in the gut after oral E. cuniculi infection was evaluated. An early and rapid increase of the intraepithelial lymphocyte (IEL) population of orally infected animals was observed. This increase in the IEL population started as early as day 3 and peaked at day 7 postinfection with persistent elevation thereafter. At day 7 postinfection, IELs expressed strong cytokine messages (IFN-γ and IL-10) and were highly cytotoxic for parasite-infected syngeneic macrophages. At an E:T ratio of 80:1, these cells were able to cause >60% Ag-specific target cell lysis. A significant increase in the CD8αα subset of IEL in response to an oral E. cuniculi infection was observed. To the best of our knowledge, such an early expansion of an IEL population exhibiting strong ex vivo cytotoxicity has not been reported with infectious models. These data suggest that IELs act as important barriers for multiplication of this organism leading to the successful resolution of infection. The protective role of IELs may be due both to their inflammatory (IFN-γ production and cytotoxic response) as well as immunoregulatory (IL-10 production) properties.
Microsporidia are obligate intracellular parasites that infect a wide range of hosts including both vertebrates and invertebrates (1). With the onset of the AIDS pandemic, more attention has been paid to several microsporidia species including Encephalitozoon, due to their ability to cause disease in humans (2–6). Because of its ability to grow in tissue culture, most of what is known about the biology of microsporidia is based on Encephalitozoon cuniculi (7). This organism is know to cause encephalitis, hepatitis, and disseminated disease in many mammals including rabbits and mice (8). Several cases of HIV-infected patients suffering from encephalitis due to E. cuniculi infection have been reported in recent years (2, 9, 10). These patients presented with a wide variety of symptoms, including renal failure, pneumonitis, sinusitis, keratopathy, granulomatous liver necrosis, and peritonitis. Microsporidiosis also occurs in other patients with immunosuppression such as those receiving immunosuppressive drugs for transplantation (11–13). In immune competent hosts (e.g., the elderly, travelers to the tropics, and residents of the tropics), microsporidia have been recognized as a cause of diarrheal syndromes (14).
Protective immunity against E. cuniculi infection is primarily dependent on the cellular immune response (15). In the absence of immune T cells, animals are unable to withstand i.p. infection with the parasite (16). Studies from our laboratory have demonstrated that among the T cell subsets, CD8+ T cells are more important for protection, as gene knockout mice lacking CD8+ T cells were highly susceptible to E. cuniculi infection, whereas those lacking CD4+ cells were not (17).
Although infection with microsporidia is thought to be primarily acquired through the gastrointestinal route (18), most studies related to the immune response have been carried out using an i.p. route of infection. Information regarding the immune response against an oral (natural) route of challenge is not available. These studies are important, especially in light of the observations made with other oral pathogens (19–21), where the mucosal immune response plays a critical role in protection of infected hosts.
In the present study, the mucosal immune response against oral E. cuniculi was analyzed. Induction of strong gut immunity in response to E. cuniculi infection, characterized by a significant and rapid increase in the intraepithelial lymphocyte (IEL)3 population, was observed. The immune IELs exhibited significant Ag-specific ex vivo cytotoxicity and elevated cytokine messages during infection. The IELs appear to be the first line of defense against the parasite, and to play an important role in preventing the dissemination of organisms to peripheral organs.
Materials and Methods
Mice
Six- to 7-wk-old female C57BL/6 and SCID mice with the same genetic background were obtained from the National Cancer Institute (Frederick, MD) and The Jackson Laboratory (Bar Harbor, ME), respectively. Animals were housed under approved conditions at the Animal Research Facility at Louisiana State University Health Sciences Center.
Parasites and infection
A rabbit isolate of E. cuniculi (genotype II), kindly provided by E. Didier (Tulane Regional Primate Research Center, Covington, LA), was used throughout the study. The parasites were maintained by continuous passage in rabbit kidney (RK-13) cells, obtained from American Type Culture Collection (Manassas, VA). The RK-13 cells were maintained in RPMI 1640 (Life Technologies, Gaithersburg, MD) containing 10% FCS (HyClone Laboratories, Logan, UT). Organisms were collected from the culture medium, centrifuged at 325 × g for 10 min and washes twice with PBS. The mice were orally infected with 5 × 107 spores/mouse.
Depletion of CD4+ and CD8+ T cells
C57BL/6 mice were depleted of CD4+ and CD8+ T cells using GK1.5 and 2.43 mAbs, respectively. Abs were purified with a protein G Hitrap column (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Mice were orally infected with 5 × 107 spores of E. cuniculi. One hundred micrograms of anti-CD4, anti-CD8, or both were administered at –1, 0, and 1 day postinfection (p.i.). The treatment was continued once a week thereafter until termination of the experiment. The control animals were treated with equal amount of rat IgG. A >95% depletion of CD4 and CD8+ T cells in splenocytes, mesenteric lymph node (MLN) lymphocytes, and IELs was demonstrated by FACS analysis using this Ab protocol.
Histopathology
Tissues from infected, CD4+ and CD8+ T cell-depleted mice as well as parental control animals were fixed in 10% formalin and processed for 5-μm histological sections, which were stained with H&E using standard methods. Sections were also stained with chromotrope 2R (microsporidia) using the Kokoskin et al. (22) modification of the Weber et al. (23) microsporidia stain which uses a 10-min staining time in chromotrope 2R at 50°C (22, 23).
IEL isolation and subset purification
IELs were obtained according to a procedure described by Guy-Grand et al. (24). Briefly, the small intestines were flushed with PBS and the Peyer's patches were removed. Intestines were open longitudinally and cut into 2-cm sections. The mucosa was scraped and dissociated by mechanical disruption on a stirring platform for 15 min in HBSS containing 1 mM DTT (Sigma-Aldrich, St. Louis, MO). Tissues, debris, and cell aggregates were removed by passage over a glass wool column (1.8 g packed in a 20-ml syringe) in HBSS-10% FCS. The lymphocytes were obtained by centrifugation on a Ficoll layer (density = 1.077; Sigma-Aldrich).
The IEL population was separated into CD8β+ and CD8β– subsets by microbeads (Miltenyi Biotec, Auburn, CA). Briefly, IELs were incubated for 30 min at 4°C with anti-CD8β Ab conjugated to biotin (BD PharM-ingen, San Diego, CA), followed by a 15-min incubation with anti-biotin microbeads with the separation procedure being performed according to the manufacturer's instructions (Miltenyi Biotec). Both CD8β+ and CD8β– IEL populations were recovered with a purity >95% as determined by FACS analysis.
Phenotypic analysis
C57BL/6 mice were infected orally as described above. The animals were sacrificed at different time points postinfection. Following euthanasia, the spleens and MLN were removed and homogenized in a Petri dish. The contaminating RBC from the splenocyte suspension were lysed with RBC lysis buffer (Sigma-Aldrich). Intestinal IEL were separated from the gut according to the above-described procedure. Isolated cells were washed, suspended in 1% PBS-BSA (Sigma-Aldrich), and analyzed by FACS (BD Biosciences, San Jose, CA) for expression of CD8α and CD8β using a direct immunofluorescence assay (BD PharMingen). Cells (1 × 106) were incubated with 1 μg of FITC-labeled mAbs in 1% PBS-BSA. After a 1-h incubation at 4°C, the cells were washed several times in buffer, fixed in 1% formaldehyde, and stored at 4°C for FACS analysis.
Detection of cytokines
Quantitation of cytokine mRNA was performed by PCR. IEL, MLN lymphocytes, and splenocytes from E. cuniculi-infected animals were collected on days 0, 3, 7, 14, 21, 28, and 50 p.i. RNA was isolated using TRIzol (Life Technologies) according to the manufacturer's instructions. Reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase (Life Technologies) and random hexamer primers (Promega, Madison, WI). Expression of mRNA for IFN-γ, IL-10, and IL-4 was performed by quantitative PCR using the PQRS quantitative method (25). The splenocytes from uninfected mice were used to establish a baseline value of 1.0 against which the level of message for cytokine in the test was quantitated.
CTL assay
A CTL assay was performed according to a standard procedure published previously by our laboratory (26). Briefly, mouse peritoneal macrophages were harvested 2 days after i.p. inoculation with 1 ml of thioglycolate. The macrophages were washed three times in PBS and dispensed at a concentration of 5 × 104 cells/well in U-bottom 96-well plates. After overnight incubation, the cells were infected with 2 × 105 spores of E. cuniculi per well for 48 h. The cells were washed extensively with PBS to clear extracellular parasites. Macrophages were labeled with 51Cr (0.5 μCi/well) for 2 h at 37°C. Macrophages were washed five times with PBS and incubated with IEL, MLN, and spleen cells at various E:T ratios in a final volume of 200 μl of culture medium. The microtiter plate was centrifuged at 200 × g for 3 min and incubated at 37°C for 4 h. Samples (100 μl) were removed and assayed for released cpm by scintillation counting. The percentage of lysis was calculated as follow: ((mean cpm of test sample – mean cpm of spontaneous release)/(mean cpm of maximal release – mean cpm of spontaneous release))/100.
IEL adoptive transfer
C57BL/6 donor mice were orally infected with 5 × 107 spores. At day 7 p.i., IEL were purified as already described. SCID mice were adaptively transferred with 107 immune or naive IEL via the i.p. route. The recipient mice were orally challenged with 5 × 107 spores of E. cuniculi at 48 h post-transfer. Mice were monitored for morbidity and mortality on a daily basis until the termination of the experiment. In some of the experiments, SCID animals were injected with IELs from infected Thy1.1 congenic animals. At day 2 post-transfer, the recipients were challenged with E. cuniculi spores as mentioned above. Four days p.i., the recipients were sacrificed and IELs, spleen cells, and MLN lymphocytes recovered. Cells were labeled with biotin-conjugated anti Thy1.1 Ab (BD PharMingen) and FACS analysis was performed.
Statistical analysis
Statistical analysis of the data was performed using a two-sampled Student's t test (27).
Results
Mice depleted of CD4+ and CD8+ T cells are susceptible to oral infection with E. cuniculi
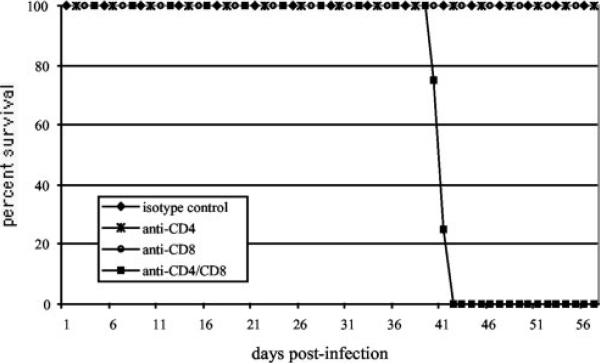
Depletion studies were performed to determine the importance of immune T cells subsets during an oral E. cuniculi infection. Mice were orally infected and T cell depletion was conducted as described in Materials and Methods. As shown in Fig. 1, control mice treated with rat IgG survived E. cuniculi infection and demonstrated no apparent signs of illness. Interestingly, while the depletion of CD8+ T cells during i.p. infection led to the mortality of infected animals (data not shown), orally infected mice did not succumb to infection as a result of anti-CD8 Ab treatment (Fig. 1). Similarly, depletion of CD4+ T cells alone had no effect on the outcome of oral infection. Double depletion of both CD4+ and CD8+ T cells, however, had an adverse effect on the survival of the infected animals and resulted in a 100% mortality by day 42 p.i. (Fig. 1). These animals exhibited severe morbidity (sluggishness, weight loss) before death. The results suggest that unlike i.p. infection, both T cell subsets are involved in protection after an oral E. cuniculi infection.
FIGURE 1.
Depletion of CD4 and CD8+ T cells in E. cuniculi-infected mice. C57BL/6 mice (four mice per group) were infected orally with 5 × 107 spores of E. cuniculi. The infected animals were injected with 100 μg/mouse of either anti-CD4, anti-CD8, or both for 3 consecutive days, starting 1 day before infection. The Ab treatment was continued once weekly thereafter. The controls animals were injected with an equal amount of isotype control Abs. The animals were monitored daily for survival. The study was performed twice with similar results.
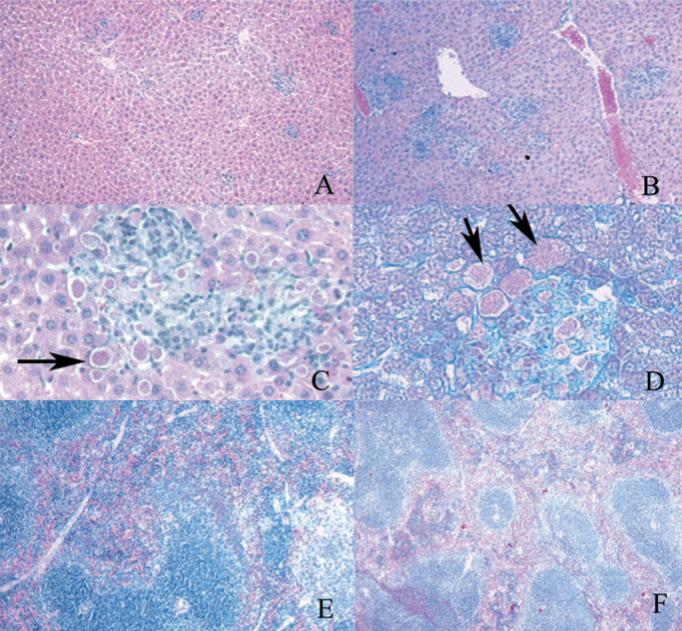
To examine the etiology of mortality in the double (CD8+ CD4+) depleted animals, histopathological analysis of the tissues of these animals and control Ab-treated animals was performed. The liver of control animals demonstrated multiple inflammatory foci with lymphocytes and early granuloma formation; no organisms were seen with either H&E or modified chromotrope 2R (e.g., microsporidia) stain (Fig. 2A). In the CD4+CD8+ T cell-depleted animals, there were increased areas of inflammatory cell infiltration compared with control tissue (Fig. 2B) with no granuloma formation. The majority of these areas contained foci of replicating organisms (Fig. 2, C and D). Such foci were never seen in control animals. The spleen of the infected control animals was 4- to 6-fold larger than that of uninfected mice or infected CD4+CD8+ T cell-depleted mice. There was an expansion of both the white and red pulp with increased hemopoiesis as well as active germinal centers (Fig. 2E). In the CD4+CD8+ T cell-depleted animals, the spleen was at most 2-fold larger than that of uninfected mice. There was also an increase in germinal center size and an increase in hemopoiesis, but not to the degree of uninfected mice (Fig. 2F). There was also some disorganization of the architecture of the spleen with effacement of the germinal centers. As in the liver, multiple foci containing organisms were present in the spleens of these CD4+CD8+ T cell-depleted animals. Intestinal tissue from infected control animals demonstrated an increase in Peyer's patch size compared with uninfected mice or CD4+CD8+ T cell-depleted mice (data not shown). No organisms were visible in the intestinal tissue of either infected control or infected CD4+CD8+ T cell-depleted mice. The intestinal epithelial cell morphology was normal in both infected control and infected CD4+CD8+ T cell-depleted mice.
FIGURE 2.
Photomicrographs of liver and spleen of C57BL/6 infected and depleted of CD4 and CD8+ T cells at day 30 p.i. A, Liver-infected control animals ×20 H&E stain; B, liver-infected CD4+CD8+ T cell-depleted animals ×20 H&E stain; C, liver-infected CD4+CD8+ T cell-depleted animals ×40 H&E stain, microsporidian spores are clearly demonstrated (arrows); D, liver-infected CD4+CD8+ T cell-depleted animals, ×40 chromotrope 2R (microsporidia stain). The microsporidian spores (arrows) stain pink with this stain; E, spleen-infected control animals ×20 H&E stain; F, spleen-infected CD4+CD8+ T cell-depleted animals ×20 H&E stain.
Early increase of IEL following oral E. cuniculi infection
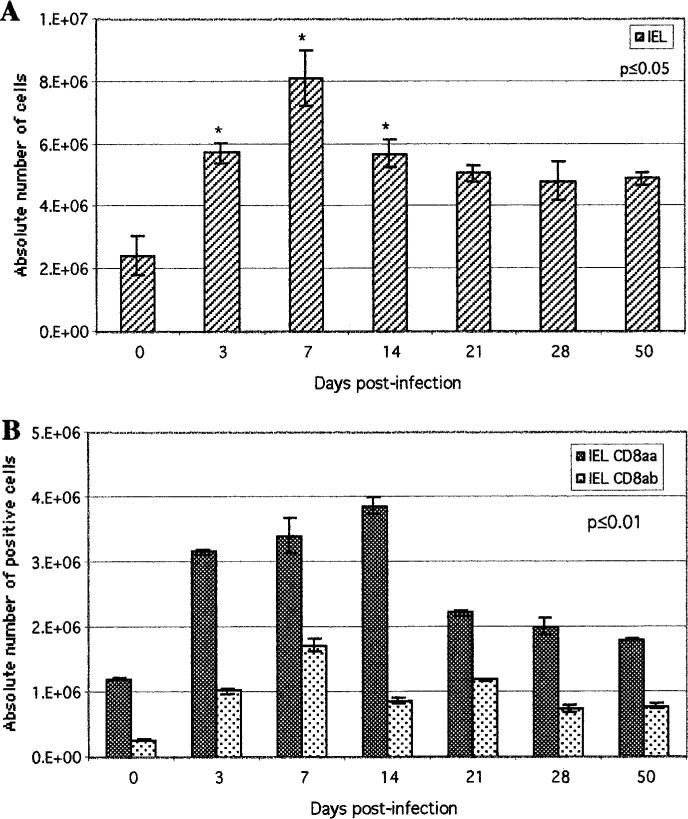
The above observations suggested that protective immunity during oral E. cuniculi infection is mediated by both CD4+ and CD8+ T cells. As IEL are an important component of the gut immune system, we next assayed the response of these cells after per oral infection. A kinetic analysis of IEL induction in the gut at various time points p.i. was performed. The studies demonstrated a significant rise in the IEL population as early as day 3 p.i. (Fig. 3A). The increase in IEL response continued and peaked at day 7 p.i. after which they started to decline. Nevertheless, a significant number of IELs was maintained at least up to day 50 p.i. To further dissect this response, phenotypic analysis within the IEL population was performed by FACS. IELs isolated from infected mice exhibited an increase in both CD8αβ and CD8αα subtypes as early as day 3 p.i. (Fig. 3B). The rise of CD8αβ T cells was maximal on day 7 p.i. (1.7 × 106), while the CD8αα peaked at day 14 p.i. (3.9 × 106). The number of CD8αβ and CD8αα slowly decreased after day 14 p.i. However, a significant increase in both subsets of IELs was observed at all the time points tested (p < 0.01) (Fig. 3B).
FIGURE 3.
Induction of IEL response following oral E. cuniculi infection. C57BL/6 mice were infected orally, IEL were isolated at various time points p.i. and pooled (n = 4 mice per group). A, Absolute numbers of total IELs recovered per mouse were determined for each time point. B, Isolated IELs were assayed for CD8αβ and CD8αα expression by FACS analysis. Data represent the mean ± SD of two individual sets of experiments. Statistical significance between each time point and the uninfected control was determined using the Student t test.
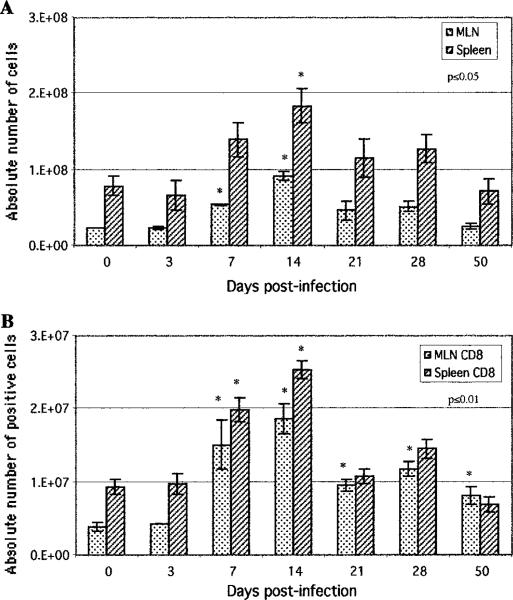
As data demonstrated a strong and early IEL induction during E. cuniculi infection, we were interested to determine whether the response in other immune compartments, like spleen and MLN, was evoked to a similar extent. In comparison to IELs, the splenocytes and MLN cells did not increase significantly until day 7 p.i. (Fig. 4A). The total number of cells in both of these tissues peaked at day 14 p.i. and then started to regress. The analysis of the CD8+ T cells in these organs demonstrated a significant increase at day 7 p.i. onward, with a peak at day 14 p.i. (Fig. 4B). Similar to CD8+ T cells, numbers of CD4+ T cells peaked at day 14 p.i., both in the spleen and the MLN of the infected animal (data not shown).
FIGURE 4.
Induction of MLN and splenic response following oral E. cuniculi infection. C57BL/6 mice were infected orally, MLN lymphocytes, and splenocytes were isolated at various time points postinfection and pooled (n = 4 mice per group). A, Absolute numbers of total MLN and spleen cells recovered per mouse were determined for each time point. B, Isolated cells were assayed for CD8 expression by FACS analysis. Data represent the mean ± SD of two individual sets of experiments. Statistical significance between each time point and the uninfected control was determined using the Student t test.
Early cytokine response of the IEL population after oral infection
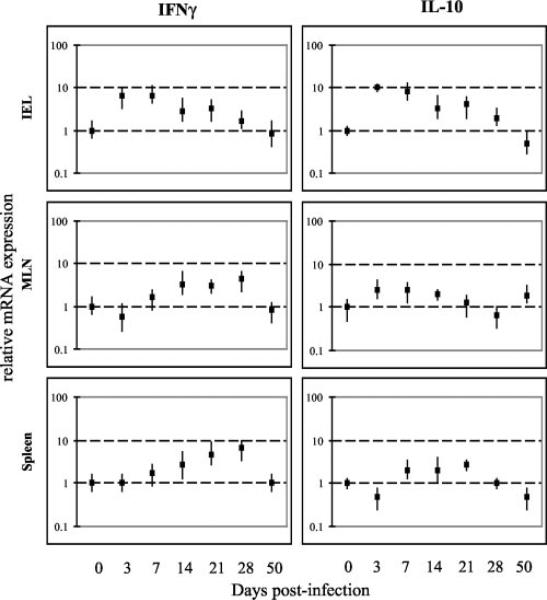
The kinetics of cytokine production by IELs in response to E. cuniculi infection was performed by quantitative PCR. Cells were assayed for IFN-γ and IL-10 message as these cytokines have been reported to be elevated during i.p. E. cuniculi infection (28). Moreover, IL-10 has been reported to play an important immunoregulatory role in other infectious disease models (26, 29). As shown in Fig. 5, an almost 10-fold increase in both IFN-γ and IL-10 message in the IEL population was observed as early as day 3 p.i. The message levels for these cytokines remained elevated until day 21 p.i. In comparison, significant increases in IFN-γ mRNA of MLN and spleen were not observed until day 14 p.i. (Fig. 5). Surprisingly, IL-10 message was not significantly up-regulated in the splenocytes and MLN at all the time points tested. Induction of IL-4 message could not be detected at any of the time points tested for any of the tissues (data not shown). These findings suggest that in addition to producing the Th1 signature cytokine (IFN-γ), IEL due to their ability to produce IL-10, may also play an immunoregulatory role during E. cuniculi infection.
FIGURE 5.
Cytokine mRNA expression of IEL, MLN lymphocytes, and splenocytes from mice infected orally with E. cuniculi. IEL, MLN lymphocytes, and splenocytes from C57BL/6 mice (four mice per group) were isolated at different time points p.i. (days 0, 3, 7, 14, 21, 28, 50). mRNA expression of IFN-γ and IL-10 was assayed by RT-PCR. The differences in the transcriptional levels for the genes are expressed relative to day 0 (assigned as 1). The cDNA concentration examined at each time point was standardized to the hypoxanthine phosphoribosyltransferase mRNA level (data not shown). The experiment was performed twice with similar results, and the data is representative of one experiment.
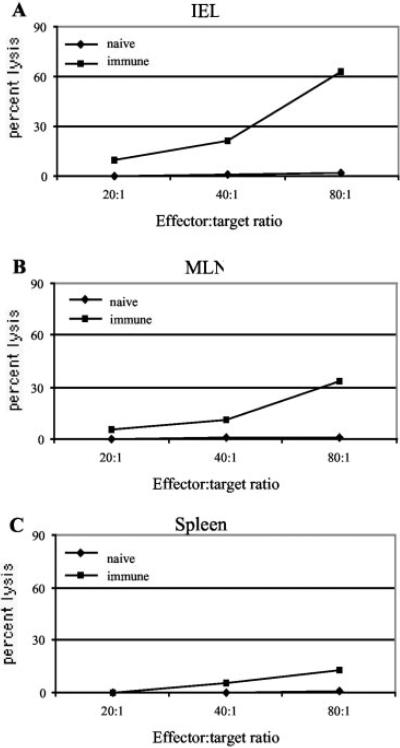
IEL are able to lyse E. cuniculi-infected macrophages
Previous studies have described a limited cytotoxic response of IELs against certain pathogens (19, 30). To determine whether the IEL generated during E. cuniculi infection are able to exhibit an Ag-specific cytotoxicity, a 51Cr release assay was performed using syngeneic target cells infected with parasites. Immune IELs expressed high amounts of direct ex vivo cytotoxic activity (63% at 80:1) at day 7 p.i. (Fig. 6). Conversely, IELs from uninfected controls exhibited background a cytolytic effect against infected targets. Comparatively, MLN lymphocytes and splenocytes showed a much lower cytotoxic response against a parasite-infected target (Fig. 6). Lysis of uninfected targets by immune IELs, MLN lymphocytes, or splenocytes was undetectable (data not shown).
FIGURE 6.
Cytotoxic activity of IEL, MLN lymphocytes, and splenocytes against E. cuniculi-infected macrophages. C57BL/6 mice (six mice per group) were orally infected with 5 × 107 spores of E. cuniculi. At day 7 p.i., the mice were sacrificed and IEL, MLN lymphocytes, and splenocytes were isolated and incubated with 51Cr-labeled uninfected or infected macrophages at various E:T ratios. After 4 h of incubation, the cytolytic activity was determined by radioisotope release into culture supernatant. Data are representative of two separate experiments.
To further assess antigenic specificity of the cytotoxic response described above, IELs isolated at day 7 p.i. were incubated with radiolabeled targets infected with Toxoplasma gondii. Although, as observed earlier, immune IELs were able to lyse E. cuniculi-infected target cells (35% killing at an E:T ratio of 40:1), they failed to show any cytotoxic activity against T. gondii-infected cells (data not shown). These observations confirmed that IEL response generated during E. cuniculi infection is specifically directed against the parasite.
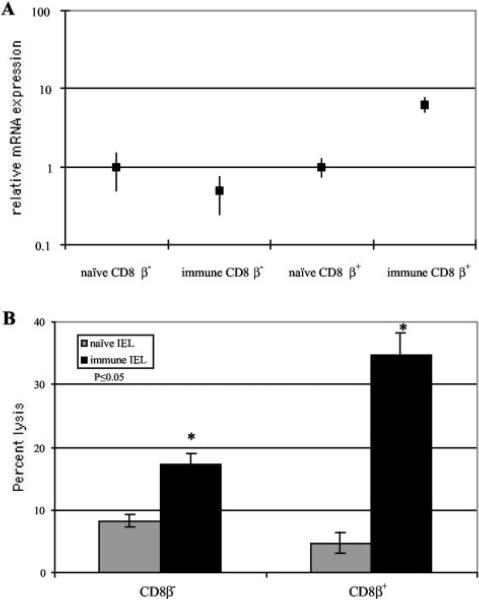
CD8β+ subset of IELs exhibit cytotoxic response and IFN-γ production during E. cuniculi infection
As both CD8αα as well as CD8αβ+ IELs are increased during E. cuniculi infection, we were interested to determine whether the cytotoxic response and IFN-γ production are the property of both or any of these two subsets of IELs. For this purpose, IELs from infected mice were separated into CD8β+ and CD8β– fractions. As shown in Fig. 7A, IFN-γ message was undetectable in CD8β– fraction of IELs. Conversely, at the same time point, strong IFN-γ message (almost 10-fold above the controls) was observed in CD8b+ IELs (Fig. 7A). CD8β+ populations exhibited significantly stronger ( p < 0.05) Ag-specific cytotoxic activity (>30%) compared with the CD8β– fraction of the IELs (Fig. 7B).
FIGURE 7.
Immune response of IEL subsets from mice infected with E. cuniculi. IELs from C57BL/6 mice (four mice per group) were isolated at day 7 p.i., CD8β+ and CD8β– T cell population was purified. A, Cytokine mRNA expression of CD8β+ and CD8β– IELs. mRNA expression of IFN-γ was assayed by RT-PCR as already described. B, Cytotoxic activity of CD8β+ and CD8β– against E. cuniculi-infected macrophages. IEL subsets were incubated with 51Cr-labeled uninfected and infected macrophages at an E:T ratio of 40:1. After a 4-h incubation, the cytolytic activity was determined by radioisotope release into the culture supernatant. The experiments were performed twice with similar results and the data are representative of one experiment.
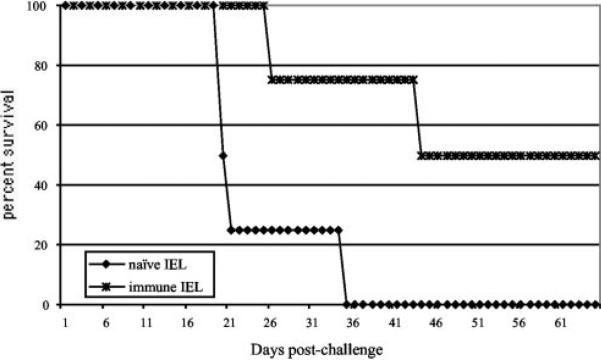
Adoptive transfer of primed IEL protects SCID mice against a lethal challenge with E. cuniculi
The ability of immune IELs to protect immunocompromised hosts against E. cuniculi infection was then analyzed by adoptive transfer studies. C57BL/6 mice were infected orally with E. cuniculi spores and the IELs were isolated at day 7 p.i., as a maximal level of IFN-γ message, as well as a strong Ag-specific cytotoxic response, was observed at this time point. Purified IELs (1 × 107/mouse) were injected i.p. into SCID animals of the same genetic background. The control animals received an equal number of nonimmune IELs. Two days after the transfer of IELs, the recipients were challenged orally with 5 × 107 spores/mouse. As shown in Fig. 8, nonimmune IELs were unable to confer protection to SCID recipients and all the animals were dead by day 36 p.i. In comparison, the SCID mice injected with immune IELs lived longer and 50% of the recipients survived until the termination of the experiment.
FIGURE 8.
Adoptive transfer of immune IEL protects SCID mice against a lethal challenge with E. cuniculi. IEL from naive and infected C57BL/6 (10 mice per group) were isolated at day 7 p.i. A total of 107 purified IELs were injected i.p. into SCID mice (four mice per group). Control animals received an equal amount of cells from uninfected mice. After 48 h, the mice were challenged orally with 5 × 107 spores of E. cuniculi and the survival of the animals was monitored until the end of the experiment.
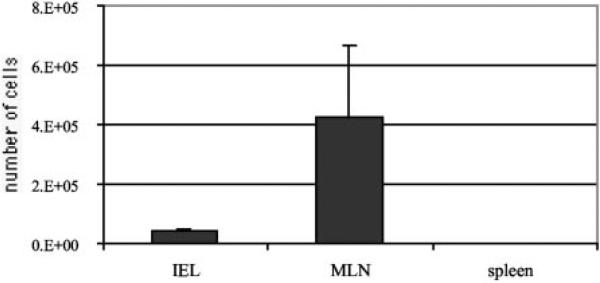
To determine that donor IELs were migrating back to the gut of the recipient mice, IELs from congenic Thy1.1 mice on the C57BL/6 background were isolated and adoptively transferred to naive SCID animals as described above. Two days post-transfer, recipient mice were challenged with E. cuniculi spores and sacrificed at day 4 p.i. IELs, spleen cells, and MLN lymphocytes from the recipients were recovered and subsequently stained and analyzed for the presence of a Thy1.1 marker by FACS. The majority of the transferred Thy1.1 IELs were detected in the MLN compartment (4.23 × 105 ± 2.44 × 105), while a comparatively smaller number of donor Thy1.1 cells (4 × 104 ± 7.8 × 103) were also recovered from the IEL compartment (Fig. 9). Donor Thy1.1 cells were undetectable in the spleen of the recipient SCID animals (Fig. 9).
FIGURE 9.
Trafficking of immune IELs in SCID recipients. Thy1.1 C57BL/6 (10 mice) were infected orally with 5 × 107 E. cuniculi spores. Animals were sacrificed at day 7 p.i. and IELs recovered as described in Materials and Methods. Purified IELs (1 × 107) were adoptively transferred to naive SCID animals (three per mice) as described earlier. Same number of control SCID animals received an equal volume of sterile PBS alone. Recipients were challenged orally with 5 × 107 E. cuniculi spores 2 days post-transfer. At day 4 p.i., the recipients were sacrificed and IELs, spleen cells, and MLN lymphocytes were analyzed for Thy1.1 marker by FACS. Experiment was performed twice and the data are representative of one experiment.
Discussion
To date, host immunity against E. cuniculi has been conducted using the i.p. route of infection. Studies related to the gut immune response against E. cuniculi infection have not been performed. Observations presented in this manuscript demonstrate, for the first time, that oral E. cuniculi infection induces a strong immune response in the gut which is manifested by an early and rapid increase in the IEL population. In response to oral infection, IELs were elevated as early as day 3 p.i., while MLN and spleen cells increased only after day 7 p.i. The immune IELs showed increase in cytokine message and exhibited strong ex vivo Ag-specific cytotoxic response. Moreover, purified IELs from the infected animals were also able to transfer protective immunity to SCID animals. The majority of the donor IELs home to gut-associated lymphoid tissue associated compartments (the MLN compartment of the gut and the intestinal epithelium), while none of them home to peripheral lymphoid organs consistent with the idea that mucosal immune cells respond to E. cuniculi following an oral infection and that the effect of IELs in preventing disease is related to the immune response occurring in the gut-associated lymphoid tissue.
Although IELs have been identified as being T cells, with a primarily cytolytic phenotype, the critical benefits of these cells to the host are not well characterized (31, 32). Their location suggests they may be important in the regulation of local immune responses and the protection of mucosal surfaces against pathogenic infection. Intestinal IEL are predominantly CD8+ T cells with expression of either a homodimeric CD8αα or heterodimeric CD8αβ molecules, whereas, at other peripheral sites, CD8+ T cells form a minority of T cells and the heterodimeric form of the CD8 molecule is normally expressed (33, 34). Despite the fact that IELs still remain enigmatic in several aspects, recent studies have shown that these cells could play an important role in protective immunity against mucosal pathogens (35–37).
Oral T. gondii infection causes an increase in IEL population between days 9 and 13 p.i. (19). IFN-γ message of the IEL population from T.gondii-infected animals was increased by day 11 p.i. (35). Although the immune IEL population protected naive animals against lethal parasitic infection, this IEL population exhibited a poor Ag-specific cytotoxic response. Cytotoxic activity of IELs, assayed at day 13 post-Toxoplasma infection, was 23% at an E:T ratio of 60:1. The protective role of IELs during infection with the protozoan parasite Cryptosporidium muris has been demonstrated (38). Although a kinetic analysis of IEL induction was not performed in this study, protection was attributed to the minority CD4+ T cells present within the IEL population. Induction of an IEL response during systemic infection with lymphocytic choriomeningitis virus has been analyzed by Muller et al. (21). In these studies, an increase in the IEL population was observed at day 8 postviral infection. A purified CD8αβ subset of IEL exhibited maximum cytotoxic activity of 45% lysis at an E:T ratio of 5:1. Comparatively, SIV-specific IEL could display 60% lytic activity only when the E:T ratio was raised to 200:1 (30).
The significance of these findings is that none of the pathogens mentioned above evoke such an early and rapid IEL response as demonstrated during E. cuniculi infection. The only similar example available in the literature is that of Eimeria vermiformis (20), which induces high levels of γδ+ IELs at day 3 p.i. However, in this Eimeria model, the IEL population is depleted at day 7 p.i. and increases again at day 12 p.i. In contrast, during E. cuniculi infection IEL response rises significantly at day 3 postinfection and continues to increase until day 14 p.i. when the response is at the peak. Although a slight decrease in the IEL population is noted beyond this time point, IELs remain elevated at least until day 50 p.i. Isolated IELs from E. cuniculi-infected animals exhibited an elevated IFN-γ message and a strong ex vivo cytotoxic response against parasite-infected targets. At an E:T ratio of 80:1, 63% target cell lysis was observed. Compared with viral infections, parasites in general do not induce a strong CTL response in the host (19, 21). Our findings are unique in the sense that it is the first report in which strong Ag-specific ex vivo cytotoxic activity by any lymphocyte population during a parasitic infection has been demonstrated.
Another noticeable feature of our current observations is simultaneous expansion of both CD8αα and CD8αβ subsets of the IEL population, observed during very early stages of infection. CD8αα cells are not readily evoked during viral infections and expansion of these cells is supposed to be partially dependent on microbial flora and the food Ags in the gut (39). However, as our current studies demonstrate an early and significant increase in the CD8αα subset, the possibility of their involvement in the regulation of immune response during E. cuniculi infection is very likely and will need to be evaluated in future studies.
As CD8+ T cells are critical during i.p. infection with E. cuniculi (17) and a majority of the IEL population bears the CD8+ phenotype, it could be predicted that depletion of CD8+ T cells would result in the mortality of E. cuniculi-infected animals. However, our data demonstrates that treatment with both anti-CD4 and CD8 Ab is required for animals to succumb to oral challenge. One explanation for this observation is that, similar to C. muris (40), CD4+ IELs also play a protective role during E. cuniculi infection. Against this theory is the observation that no increase in this subtype was observed at any time postparasite infection (data not shown). A more likely explanation is that CD4+ T cells from other immune compartments of the gut like lamina propria or MLN are responsible for protection in the CD8-depleted animals. This mechanism of CD4+ T cell-mediated protection is probably dependent on IFN-γ, as depletion of IFN-γ has been shown to cause mortality in the animals infected with E. cuniculi via the i.p. route (28). Based on our current data, we postulate that CD8+ IELs are a primary source of immune defense against oral E. cuniculi infection. However, in the absence of CD8+ T cells, the CD4+ T cell population from other immune compartments of the gut can provide a second line of defense against the parasite. This may be the reason why CD8+ T cell depletion in E. cuniculi-infected mice did not result in a lethal outcome. These data raise an important question about the primary site of CD4+ T cell induction during oral E. cuniculi infection and the mechanism by which they provide protection to the infected host. The role of gut-associated CD4+ T cells in immunity against E. cuniculi is an important focus of active research in our laboratory.
Although IELs apparently may not be the sole components of protective immunity against oral E. cuniculi infection, they are strongly induced and are able to protect immunocompromised hosts against a lethal challenge. These findings have significant implications in HIV-infected individuals suffering from complications due to E. cuniculi infection or the elderly non-HIV-infected population who recently have been reported to acquire symptomatic microsporidial infection as a result of drinking contaminated water (14, 18). As therapeutic strategies against microsporidiosis (such as E. cuniculi) are still poorly developed, a plan for targeting the IEL population for improved therapeutic intervention in these individuals could be devised.
Our hypothesis, based on the observations made in the current study, for the immune response to oral microsporidia infection is as follows: infection of mice with E. cuniculi evokes a strong proliferation of IELs. Most of the proliferating IELs belong to the CD8αβ and CD8αα subset. Due to their ability to produce IFN-γ and exhibit strong cytolytic property, these cells are able to impede parasite multiplication. IFN-γ production and most of the Ag-specific cytotoxic response is dependent on the CD8αβ+ subset of IEL. To counterbalance the potential harmful effect of overproduction of IFN-γ, IELs play an immunoregulatory role, especially through the expression of IL-10. It is noteworthy to mention that in our studies, expression of IL-10 message was detected only within the IEL population.
The data in this manuscript raise several important additional questions: 1) do CD8αβ and CD8αα have a defined role during E. cuniculi infection? 2) Can the immune IELs generate a long-term memory response against E. cuniculi infection? 3) Can the role of IELs be extended to other microsporidian infections like Encephalitozoon intestinalis and Enterocytozoon bieneusii which are more prevalent in HIV-infected subjects and are well known to cause chronic diarrhea (41–44)? Studies aimed at addressing these questions are currently under investigation in our laboratory.
Acknowledgments
We thank Joseph Chaiban for his help with the phenotypic studies.
Footnotes
This work was supported by National Institutes of Health Grants AI43693 (to I.A.K.) and AI31788 (to L.M.W.).
Abbreviations used in this paper: IEL, intraepithelial lymphocyte; p.i., postinfection; MLN, mesenteric lymph node.
References
- 1.Canning E, Lom J. The Microsporidia of Vertebrates. Academic Press; New York: 1986. [Google Scholar]
- 2.Fournier S, Ligory O, Safarti C, David-Ouaknine F, Derouin F, Decazes JM, Molina JM. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 2000;1:155. doi: 10.1046/j.1468-1293.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 3.Weitzel T, Wolff M, Dabanch J, Levy I, Schmetz C, Visvesvara GS. Dual microsporidial infection with Encephalitozoon cuniculi and Enterocytozoon bieneusi in an HIV-positive patient. Infection. 2001;29:237. doi: 10.1007/s15010-001-1164-0. [DOI] [PubMed] [Google Scholar]
- 4.Botterel F, Minozzi C, Vittecoq D, Bouree P. Pulmonary localization of Enterocytozoon bieneusi in an AIDS patient: case report and review. J. Clin. Microbiol. 2002;40:4800. doi: 10.1128/JCM.40.12.4800-4801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scaglia M, Sacchi L, Croppo GP, da Silva A, Gatti S, Corona S, Orani A, Bernuzzi AM, Pieniazek NJ, Slemenda SB, et al. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J. Infect. 1997;34:119. doi: 10.1016/s0163-4453(97)92414-2. [DOI] [PubMed] [Google Scholar]
- 6.Svedhem V, Lebbad M, Hedkvist B, Del Aguila C, Hedman P, Larsson R, Navajas R, Aust-Kettis A. Disseminated infection with Encephalitozoon intestinalis in AIDS patients: report of 2 cases. Scand. J. Infect. Dis. 2002;34:703. doi: 10.1080/00365540210147598. [DOI] [PubMed] [Google Scholar]
- 7.Zender HO, Arrigoni E, Eckert J, Kapanci Y. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am. J. Clin. Pathol. 1989;92:352. doi: 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]
- 8.Keeble E. Encephalitozoon cuniculi in rabbits. Vet. Rec. 2001;149:688. [PubMed] [Google Scholar]
- 9.Weber R, Deplazes P, Fleep M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N. Engl. J. Med. 1997;336:474. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 10.Mertens RB, Didier ES, Fishbein MC, Bertucci DC, Rogers LB, Orenstein JM. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod. Pathol. 1997;10:68. [PubMed] [Google Scholar]
- 11.Mohindra A, Lee MW, Vivesvara G, Moura H, Parasuraman R, Leitch GJ, Xiao L, Yee J, Del Busto R. Disseminated microsporidiosis in a renal transplant recipient. Transpl. Infect. Dis. 2002;4:102. doi: 10.1034/j.1399-3062.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 12.Gumbo T, Hobbs RE, Carlyn C, Hall G, Isada CM. Microsporidia infection in transplant patient. Transplantation. 1999;67:482. doi: 10.1097/00007890-199902150-00024. [DOI] [PubMed] [Google Scholar]
- 13.Latib MA, Pascoe MD, Duffield MS, Kahn D. Microsporidiosis in the graft of a renal transplant recipient. Transpl. Int. 2001;14:274. doi: 10.1007/s001470100332. [DOI] [PubMed] [Google Scholar]
- 14.Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidisis in mammals. Microbes Infect. 2000;2:709. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt EC, Schadduck JA. mechanism of resistance of the intracellular protozoan Encephalitozoon cuniculi in mice. J. Immunol. 1984;133:2712. [PubMed] [Google Scholar]
- 16.Schmidt EC, Schadduck JA. Murine encephalitozoonosis model for studying the host-parasite relationship of a chronic infection. Infect. Immun. 1983;40:936. doi: 10.1128/iai.40.3.936-942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan IA, Schwartzman JD, Kasper LH, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J. Immunol. 1999;162:6086. [PubMed] [Google Scholar]
- 18.Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens MA, Trepo C. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J. Infect. Dis. 1999;25:2003. doi: 10.1086/315112. [DOI] [PubMed] [Google Scholar]
- 19.Chardes T, Buzoni-gatel D, Lepage A, Berard F, Bout DT. Toxoplasma gondii oral infection induces specific CD8αβ+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite infected enterocytes. J. Immunol. 1994;153:4596. [PubMed] [Google Scholar]
- 20.Findly RC, Roberts J, Hayday AC. Dynamic response of murine gut intraepithelial T cells after infection by coccidian parasite Eimeria. Eur. J. Immunol. 1993;23:2557. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 21.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J. Immunol. 2000;164:1986. doi: 10.4049/jimmunol.164.4.1986. [DOI] [PubMed] [Google Scholar]
- 22.Kokoskin E, Gyorkos TW, Canus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of microsporidia. J. Clin. Microbiol. 1994;32:1974. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates: The Enteric Opportunistic Infection Working Group. N. Engl. J. Med. 1992;326:161. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 24.Guy-Grand D, Griscelli C, Vassali P. The mouse gut T lymphocyte, a novel type of T cell: nature, origin, and traffic in mice in normal and graft-versus-host conditions. J. Exp. Med. 1978;148:1661. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner SL, Zheng S, Corry DB, Locksley RM. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods. 1993;165:37. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 26.Khan IA, Matsuura T, Kasper LH. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 27.Neter J, Wasserman W, Kuter MH. Applied Linear Statistical Models. Irwin; Homewood: 1985. [Google Scholar]
- 28.Khan IA, Moretto M. Role of γ interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect. Immun. 1999;67:1887. doi: 10.1128/iai.67.4.1887-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Wysocka M, Hieny S, Scarton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependant on CD4+ T cells and accompanied by overproduction of IL-2, IFN-γ and TNF-α. J. Immunol. 1996;157:798. [PubMed] [Google Scholar]
- 30.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet JG, Venet A. Direct ex-vivo simian immunodeficiency virus (SIV)-specific cytotoxicity activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J. Virol. 1997;71:1052. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies MD, Parrott DM. The early appearance of specific cytotoxic T cells in murine gut mucosa. Clin. Exp. Immunol. 1980;42:273. [PMC free article] [PubMed] [Google Scholar]
- 32.Tagliabue A, Befus A, Clark D, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J. Exp. Med. 1982;155:1785. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarry A, Cerf-Bensussan N, Brousse N, Seiz F, Guy-Grand D. Subsets of CD3+ and CD3– lymphocytes isolated from normal human gut epithelium display phenotypic features different from their counterparts in peripheral blood. Eur. J. Immunol. 1990;20:1097. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- 34.Nagler-Anderson C. Man the barrier: strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001;1:59. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 35.Buzoni-Gatel D, Lepage AC, Dimier-Poisson IH, Bout DT, Kasper L. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J. Immunol. 1997;158:5883. [PubMed] [Google Scholar]
- 36.Adjei AA, Shrestha AK, Castro M, Enriquez FJ. Adoptive transfer of immunity with intraepithelial lymphocytes in Cryptosporidium parvum-infected severe combined immunodeficient mice. Am. J. Med. Sci. 2000;320:304. doi: 10.1097/00000441-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Dharakul T, Rott L, Greenberg HB. Recovery from chronic rotavirus infection with mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8 T lymphocytes. J. Virol. 1990;64:4375. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald V, Robinson HA, Kelly JP, Bancroft GC. Immunity to Cryptosporidium muris infection in mice is expressed through gut CD4+ intraepithelial lymphocytes. Infect. Immun. 1996;64:2556. doi: 10.1128/iai.64.7.2556-2562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2001;2:997. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 40.Culshaw RJ, Bancroft GJ, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving γ interferon production. Infect. Immun. 1997;65:3074. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Buckholt MA, Tzipori S. Enterocytozoon bieneusi among children with diarrhea attending Mulago hospital in Uganda. Am. J. Trop. Med. Hyg. 2002;67:299. doi: 10.4269/ajtmh.2002.67.299. [DOI] [PubMed] [Google Scholar]
- 42.Conteas CN, Berlin OG, Ash LR, Pruthi JS. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 2000;63:121. doi: 10.4269/ajtmh.2000.63.121. [DOI] [PubMed] [Google Scholar]
- 43.Wanachiwanawin D, Manatsathit S, Lertlaituan P, Thakerngpol K, Suwanagool P. Intestinal microsporidiosis in HIV infected patients with chronic diarrhea in Thailand. Southeast Asian J. Trop. Med. Public Health. 1998;29:767. [PubMed] [Google Scholar]
- 44.Ferreira FM, Bezerra L, Santos MB, Bernardes RM, Avelino I, Silva ML. Intestinal microsporidiosis: a current infection in HIV-sero-positive patients in Portugal. Microbes Infect. 2001;3:1015. doi: 10.1016/s1286-4579(01)01465-4. [DOI] [PubMed] [Google Scholar]