Abstract
Antibodies are thought to exert antiviral activities by blocking viral entry into cells and/or accelerating viral clearance from circulation. In particular, antibodies to hepatitis B virus (HBV) surface antigen (HBsAg) confer protection, by binding circulating virus. Here, we used mathematical modeling to gain information about viral dynamics during and after a single or multiple infusions of a combination of two human monoclonal anti-HBs (HepeX-B™) in patients with chronic hepatitis B. The antibody HBV-17 recognizes a conformational epitope, while antibody HBV-19 recognizes a linear epitope on the HBV surface antigen. The kinetic profiles of the decline of serum HBV DNA and HBsAg revealed partial blocking of virion release from infected cells as a new antiviral mechanism, in addition to acceleration of HBV clearance from the circulation. We then replicated this approach in vitro, using cells secreting HBsAg, and compared the prediction of the mathematical modeling obtained from the in vivo kinetics. In-vitro, HepeX-B™ treatment of HBsAg-producing cells showed cellular uptake of antibodies resulting in intracellular accumulation of viral particles. Blocking HBsAg secretion continued also after HepeX-B™ was removed from the cell culture supernatants.
Conclusion
These results identify a novel antiviral mechanism of antibodies to HBsAg involving prolonged blocking of the hepatitis B virus and HBsAg subviral particles release from infected cells. This may have implications in designing new therapies for patients with chronic HBV infection and may also be relevant in other viral infections.
Keywords: Hepatitis B virus, anti-HBs, antiviral antibodies, viral kinetics, immunotherapy
Viruses elicit a range of antiviral antibodies, but only some of these have direct antiviral activity and are referred as neutralizing antibodies, as they render virions non-infectious by blocking viral entry into cells (1). Such antibodies bind to epitopes that interfere with the interaction of the viral surface protein and its receptor by steric hindrance (2), by targeting directly the receptor-binding site on virus (3), or by inducing conformational changes that abrogate the functionality of the viral surface protein (4). In addition, the antiviral activities of antibodies against virus particles in the circulation can include clearance via Fc-mediated effector systems, such as complement-dependent virolysis or phagocytosis (5).
In hepatitis B virus (HBV) infection, antibodies directed to a conserved region, a-determinant, of the HBV surface antigen (HBsAg), are known to confer protection by high-affinity binding of HBsAg, the main component of the virus envelope, as well as the 22-nm subviral particles (6). The efficacy of antibodies to HBsAg (anti-HBs) in preventing HBV infection has been established both when given as passive immunoprophylaxis – for example, to prevent mother-to-child HBV transmission or to prevent HBV reinfection of the liver graft following liver transplantation (7), as well as by the success of universal active immunization using recombinant HBsAg, resulting in high anti-HBs titres (8). The mechanisms of anti-HBs protection are not understood, although the common belief is that these are based on binding HBV particles in circulation, thus preventing the infection of liver cells. According to this paradigm, cells that have already been infected will not be affected by anti-HBs. Importantly, the protection achieved by anti-HBs, at least in the liver transplant setting, is not sterile as HBV DNA is detectable in the new liver even in cases with effective prophylaxis (9).
Recently, in vitro experiments have shown that HBs-specific IgG is internalized into hepatocyte-derived cell lines and inhibits the secretion of HBsAg and virions from these cells (10). The HBsAg and anti-HBs were co-localized within the cells, and the specificity of intracellular HBsAg-anti-HBs interaction was further demonstrated by abrogating the anti-HBs inhibitory effect in cell transfected with HBV genomes expressing antibody-escape mutant HBsAg (10). To investigate further the phenomenon of intracellular blocking of HBV release by antibodies and its potential for therapeutic application, we analyzed both in vivo and in vitro the effect of two human monoclonal antibodies to HBsAg - HBV-Ab17 and HBV-Ab19, that have been shown to have high neutralizing activity against HBV (11, 12). We used mathematical modeling of serum HBV DNA and HBsAg levels to gain information about viral dynamics during a single or multiple infusions of a combination of the two monoclonal anti-HBs (HepeX-B™) in patients with chronic hepatitis B. We then replicated this approach in vitro, using cells secreting HBsAg, and compared the prediction of the mathematical modeling obtained from the in vivo kinetics.
Materials and Methods
Antibodies
Human monoclonal antibodies to HBsAg (HBV-Ab17 and HBV-Ab19) were generated, as described previously (11). The antibodies bind different epitopes on HBsAg - HBV-Ab17 recognizes a conformational epitope, while HBV-Ab19 recognizes a linear epitope between amino acids 140-149. The specific activities of HBV-Ab17 and HBV-Ab19 are 554 IU/mg and 2090 IU/mg, and their affinity constants (Kd) are 7.6×10-10 M and 5×10-10 M, respectively (12). HepeX-B™ is a 3:1 (mg:mg) mixture of HBV-Ab17 and HBV-Ab19. The serum half-lives of HepeX-B™ following a single 10 mg or 40 mg infusion in healthy volunteers were 22.3±5.5 and 24.2±4.4 days, respectively (unpublished data). For the in vitro experiments, a human monoclonal antibody (IgG1) against the envelope protein (E2) of hepatitis C virus (HCV-Ab68) was used as an isotype control.
Clinical Design
Serum HBV-DNA and HBsAg levels were determined in patients with chronic hepatitis B, who participated in Phase IA and IB clinical trials for evaluation of HepeX-B™ (13). Phase IA was an open-label, single dose study with a total of 15 patients, each receiving a single dose of HepeX-B™ (ranging from 0.26 mg to 40 mg) by an intravenous infusion over 2 to 8 hours. Serum samples were taken at 0, 0.5, 1, 2, 4, 8, 12, 24, 48 and 96 hours post infusion. Phase IB was an open-label study with ascending multiple doses of HepeX-B™ (13). Four sequential cohorts of 3 patients each were given 10, 20, 40, or 80 mg as 4 identical doses of HepeX-B™ at weekly intervals. Serum samples were taken at 0, 4, 12 and 24 hours after each infusion. Serum HBV-DNA levels were quantitated by Amplicor HBV Monitor assay with a limit of detection 200 copies/ml. (Roche Diagnostics, Branchburg, NJ). Serum HBsAg levels were determined by an automated immunoassay (IMX system; Abbott GmbH Diagnostika, Wiesbaden-Delkenhaim, Germany), using a purified HBsAg preparation as standard. The limit of detection of this assay is 0.125 ng/ml.
Design of in vitro experiments
The PLC/PRF/5 cell line was established from hepatocellular carcinoma (14). These cells contain integrated HBV DNA fragments and produce 22-nm non-infectious HBsAg particles (15-17). The HBsAg production was shown to be constant on a per cell basis during culture (18, 19). In the present study, PCL/PRF/5 cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, Paisley, UK), supplemented with 10% fetal calf serum (FCS, Invitrogen), 500 U/ml Penicillin, 500 μg/ml streptomycin and 2mM L-glutamine. The cells were seeded in 24-well plates at 50,000 per well. After 48 hours, the cells were confluent, which was the starting time point (T0) of the experimental conditions outlined below.
i) Internalisation of anti-HBs and effect on intracellular HBsAg
At T0 the supernatants were removed and replaced with medium only (DMEM/5% FCS, control); medium plus HBV-Ab17 at two concentrations (0.2 and 0.5 mg/ml); medium plus HBV-Ab19 (0.2 and 0.5 mg/ml); HepeX-B™ (0.5 mg/ml) or medium plus isotype IgG (0.2 and 0.5 mg/ml) as a further control. After 48 hours (T48), the supernatants and the cells were collected separately. The cell lysates were tested for cellular IgG and HBsAg by Western blot. The HBsAg secreted in the supernatants was quantitated by an enzyme immunoassay (ELISA).
ii) Kinetic of HBsAg secretion in the presence of anti-HBs
In this set of experiments, at T0 the supernatants of PLC/PRF/5 cells were replaced with: i) medium only; ii) isotype IgG control (0.5 mg/ml); iii) human AB serum, as another source of non-immune IgG (0.5 mg/ml); iv) HBV-Ab17 (0.5 mg/ml); v) HBV-Ab19 (0.5 mg/ml); vi) HepeX-B™ (0.5 mg/ml). The cells were cultured continuously for 48 hours, during which period an aliquot of the supernatants was taken (without adding new medium) at 3; 6; 12; 24; 36 and 48 hours and the HBsAg levels were determined by ELISA.
iii) Kinetic of HBsAg secretion from cells pre-treated with anti-HBs
The supernatants at T0 were replaced with the same controls or antibodies, as outlined above. During the first 24 hours an aliquot of the supernatants was collected at 3; 6; 12 and 24 hours. After 24 hours (T24) the supernatants in all wells were replaced with fresh medium, without non-immune IgG or anti-HBs, and the cells were kept in culture for further 24 hours. During the second 24-hour period, aliquots were collected at the same time points as during the first period, i.e. at 27; 30; 36 and 48 hours, and the HBsAg levels were quantitated by ELISA.
Immunoblot analysis
After 48 hours, the cells were trypsinized and washed two times in PBS and resuspended in lysis buffer. The presence of human IgG and HBsAg in PLC/PRF/5 cells was analysed by Western blot in cells cultured in the presence or absence of monoclonal anti-HBs IgG, as described previously (10).
ELISA for quantitation of secreted HBsAg
Aliquots of cell culture supernatants were added to wells coated with P2D3 monoclonal anti-HBs (20), and the detection was with peroxidase-conjugated monoclonal anti-HBs (D2H5) (21). The HBsAg in supernatants was quantitated in nanograms per milliliter, based on a parallel testing of 8 standard amounts of HBsAg between 5 and 2,000 ng, which were included in the same run of the assay. Details of the Western blot and ELISA methodology are given in the Supplementary Material.
Mathematical Modeling
In-vivo and in-vitro models
Mathematical modeling of the anti-viral effect of the antibodies on viral kinetics in patients was performed by extension of the standard model by Neumann et al. (22) to include the dynamics of HBsAg particles in circulation, in addition to the hepatitis B virions in the serum, as measured by HBV DNA, and a number of possible anti-viral effects. In addition, we developed a model in order to simulate the in-vitro kinetics of supernatant HBsAg produced by PLC/PRF/5 cells in culture and the possible effects of antibodies on that process. Both models, their parameters’ estimates and fitting procedures are explained in detail in the Supplementary Material.
Results
Viral dynamics after a single anti-HBs infusion
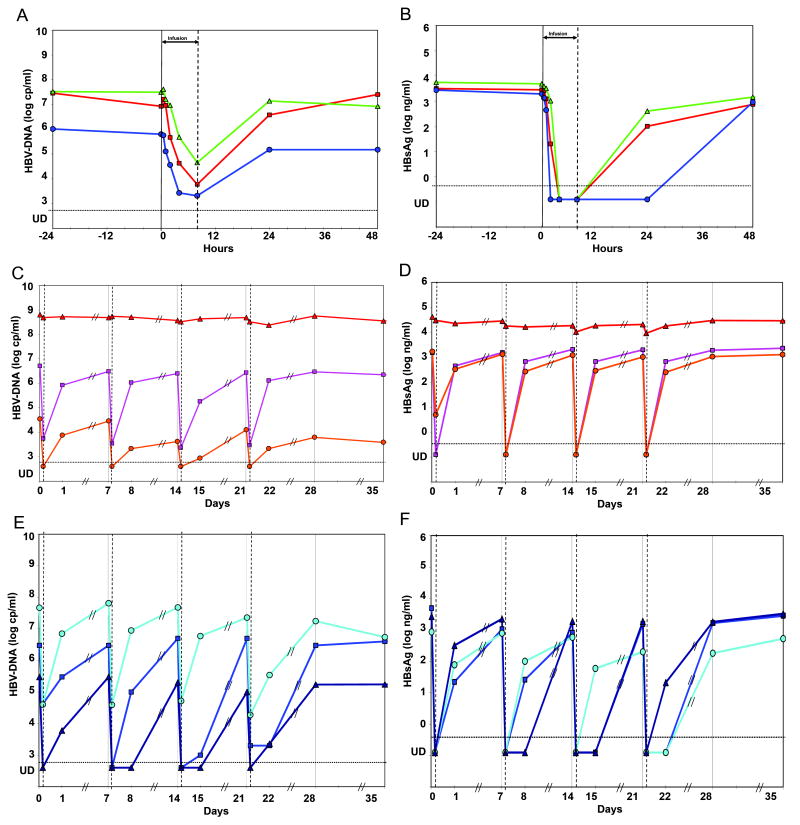
Analysis of viral kinetics after a single infusion of 40 mg HepeX-B™ showed a rapid HBV-DNA decline starting 0.5 hours after initiation of infusion and continuing throughout the 8-hour infusion period, reaching 2.5-3.3 log10copies/ml reduction from baseline with a half-life of 0.33-0.53 hours (Figure 1 and Table 1). A parallel HBsAg decline to undetectable levels (<0.125 ng/ml) was observed in all 3 patients with a half-life of 0.09-0.19 hours and maximal decline of 4.3-4.6 log10cp/ml relative to baseline.
Figure 1. HBV-DNA and HBsAg kinetics in-vivo during and following HepeX-B™ infusion.
Plasma HBV-DNA and HBsAg were measured frequently during a single infusion of 40 mg Hepex-B™ (A and B, 3 patients respectively), and following multiple infusions of 40 mg (C and D) or 80 mg (E and F). An immediate rapid decline after the beginning of infusion is observed for HBV-DNA and even more pronounced for HBsAg, with the exception of 1 non-responding patient (triangles in C and D). After the end of the infusion, a rebound in viraemia is observed, although it is delayed in some of the patients.
Table 1.
| a. Results of non-linear fitting in-vivo data
| |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBV-DNA dynamics | HBsAg dynamics | ||||||||||||||||
| HepeX- B™ dose |
Patient number |
Baseline ALT |
Baseline HBV- DNA |
Half- life Decline |
Total Decline |
Clearance Acceleration |
Blocking Effectiveness |
HBeAg status |
Baseline HBsAg |
Half- life Decline |
Total Decline |
Clearance Acceleration |
Blocking Effectiveness |
||||
| (mg) | (U/L) | (log cp/ml) |
(hours) | (log cp/ml) |
low (c=5.5) |
high (c=0.69) |
low (c=0.69) |
high (c=5.5) |
(log ng/ml) |
(hours) | (log ng/ml) |
low (c=0.69) |
high (c=0.02) |
low (c=0.02) |
high (c=0.69) |
||
| 40 | 310 | 46 | 7.1 | 0.33 | 3.3 | 9.1 | 72.7 | 96.1% | 99.5% | NEG | 3.4 | 0.19 | 4.4 | 126 | 4800 | 80.9% | 99.50% |
| 40 | 311* | 102 | 7.6 | 0.53 | 2.9 | 5.7 | 45.5 | 94.0% | 99.3% | NEG | 3.7 | 0.13 | 4.6 | 179 | 6816 | 82.4% | 99.54% |
| 40 | 201 | 60 | 5.8 | 0.35 | 2.5 | 8.6 | 68.6 | 76.2% | 97.0% | NEG | 3.3 | 0.09 | 4.3 | 282 | 10729 | 46.2% | 98.6% |
| b. Analysis of in-vivo dynamics
| |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBV-DNA dynamics | HBsAg Dynamics | ||||||||||||||||
| HepeX-B™ dose |
Patient number |
Baseline ALT |
Baseline HBV-DNA |
Half-life Decline |
Total Decline |
Acceleration of Clearance |
Effectiveness Blocking Release |
HBeAg status |
Baseline HBsAg |
Half-life Decline |
Total Decline |
Clearance Acceleration |
Blocking Effectiveness 3 |
||||
| (mg) | (U/L) | (log cp/ml) |
(hours) | (log cp/ml) |
low (c=5.5) |
high (c=0.69) |
low (c=0.69) |
high (c=5.5) |
(log ng/ml) |
(hours) | (log ng/ml) |
low (c=0.69) |
high (c=0.02) |
low (c=0.02) |
high (c=0.69) |
||
| 40 | 310 | 46 | 7.1 | 0.40 | 2.3 | 7.6 | 60.5 | 69.5% | 96.2% | NEG | 3.4 | 0.24 | 4.4 | 102 | 3859 | 82.8% | 99.55% |
| 40 | 311* | 102 | 7.6 | 0.56 | 1.8 | 5.3 | 42.5 | 34.4% | 91.8% | NEG | 3.7 | 0.23 | 4.6 | 106 | 4024 | 89.6% | 99.73% |
| 40 | 201 | 60 | 5.8 | 0.48 | 2.4 | 6.3 | 50.1 | 78.7% | 97.3% | NEG | 3.3 | 0.25 | 4.2 | 96 | 3644 | 76.3% | 99.38% |
|
| |||||||||||||||||
| 4 × 40 | 203 | 38 | 6.7 | 0.35 | 3.0 | 8.6 | 68.9 | 92.8% | 99.1% | POS | 3.2 | 0.29 | 4.1 | 83 | 3142 | 77.7% | 99.41% |
| 4 × 40 | 105 | 47 | 4.5 | 0.66 | 1.6 | 4.6 | 36.4 | 3.1% | 87.9% | NEG | 3.2 | 0.33 | 3.6 | 72 | 2736 | 33.3% | 98.2% |
| 4 × 40 | 202 | 19 | 8.8 | 13.9 | 0.1 | Non-Resp | Non-Resp | POS | 4.6 | 5.1 | 0.2 | Non-Resp | Non-Resp | ||||
|
| |||||||||||||||||
| 4 × 80 | 301 | 137 | 6.5 | 0.31 | 3.3 | 9.6 | 76.5 | 96.2% | 99.5% | NEG | 3.7 | 0.29 | 4.1 | 82 | 3108 | 75.5% | 99.36% |
| 4 × 80 | 106 | 87 | 7.7 | 0.34 | 3.1 | 8.9 | 71.3 | 94.1% | 99.3% | NEG | 2.9 | 0.33 | 3.6 | 72 | 2746 | 35.0% | 98.3% |
| 4 × 80 | 311* | 120 | 5.5 | 0.38 | 2.7 | 7.8 | 62.7 | 87.8% | 98.5% | NEG | 3.4 | 0.28 | 4.2 | 84 | 3206 | 81.2% | 99.51% |
|
| |||||||||||||||||
| Mean | 6.4 | 0.44 | 2.5 | 7.3 | 58.6 | 69.6% | 96.2% | 3.5 | 0.28 | 4.1 | 87 | 3308 | 68.9% | 99.18% | |||
Patient participated in both phase 1A and phase 1B studies
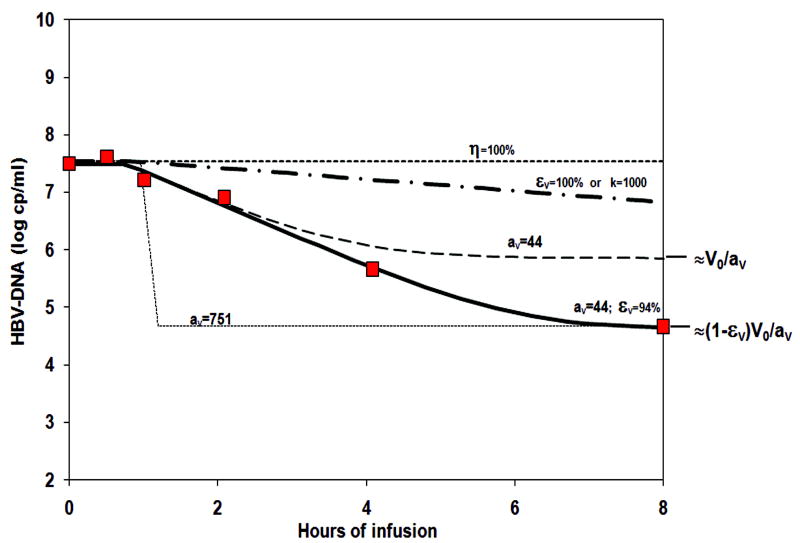
Non-linear fitting of the HBV-DNA and of HBsAg kinetics to a viral dynamics model (Supp material Equations 1-4) allowed us to test various hypotheses for the anti-viral mechanism of HepeX-B™ (Figure 2). First, blocking de-novo infection (1≥η>0) cannot be the major anti-viral mechanism since it can only result in a viral decline slope of the order of the loss rate of infected cells, half-life larger than 1 day, which is not in agreement with the rapid viral decline observed here. Second, accelerated loss of infected cells (k>1) or blocking of virion production or release from infected cells (0<εV≤1) by themselves are also not sufficient explanations, since the expected decline would follow the clearance rate of serum HBV virions. The half-life of HBV virions (ln(2)/cv) ranges between 3-24 hours, as found in previous studies of HBV kinetics (15, 23-26), which is too slow compared to the very rapid decline observed during HepeX-B™ infusion (Figure 2). Third, assuming an accelerated clearance of HBV virions from circulation (aV >1) cannot by itself explain the rapid decline in serum HBV-DNA, as shown in Figure 2. The observed half-life of order of 0.33-0.53 hours gives a minimal (maximal) estimate of accelerated clearance of HBV particles of aV =5.7 (72.7), by assuming the most rapid (slow) observed virion half-life of 3 (24) hours. However, such acceleration of clearance can give rise to a decline of only log (1/aV)= 0.75-0.86 log10copies/ml (Figure 2), since viral load during accelerated clearance will reach a new steady state at V0/a (supp material Equation 6). This is significantly (P<0.01) less than the viral decline observed here, and thus accelerated clearance is a necessary but not sufficient explanation of the antiviral mechanism.
Figure 2. Simulation of various models for potential anti-viral mechanism of Hepex-B™ in comparison to HBV-DNA decline.
When the anti-viral mechanism is assumed to be blocking infection (η>0, as expected from neutralizing antibodies), the resulting decline slope (upper most dotted line) is maximally the loss rate of infected cells (half-life longer than 1 day), and thus cannot explain the fast decline observed in-vivo (red squares). Also when assuming that the mechanism is blocking virion release from infected cells (εv >0) or increased loss of infected cells (k>1), the resulting slope (dash-dotted line), which corresponds to the intrinsic half-life of serum HBV virions (longer than 3 hours), is still too slow. Only when assuming that Hepex-B™ accelerates (here av =44) the clearance of virions from circulation, can the simulated decline slope match that of the in-vivo data (dashed and solid lines). However, if acceleration of the clearance is the only mechanism assumed (or if it is combined with blocking de-novo infection, not shown) then the viral load at end of infusion is expected to be only 44 fold lower than baseline (dashed line), instead of 751 fold. On the other hand, if an acceleration of av=751 is assumed, the expected slope of decline (bottom dotted line) would have been much faster than that observed. The in-vivo decline can be simulated (solid line) only if one assumes that Hepex-B™ provides both - acceleration of virion clearance from serum (av =44), as well as partial blocking εv =94%) of release or virion production from infected cells.
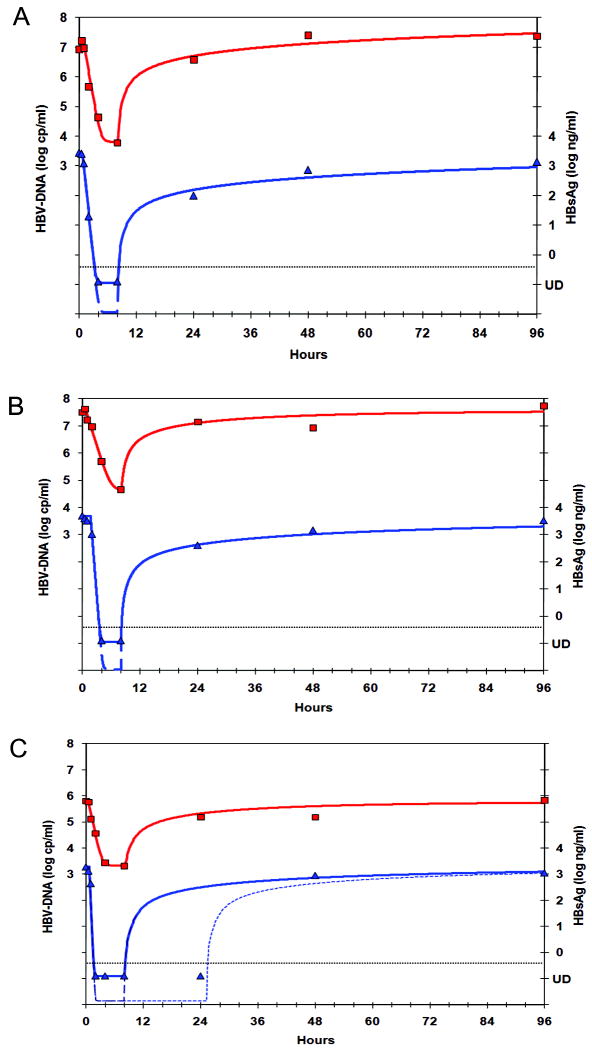
The observed HBV-DNA kinetics during HepeX-B™ infusion can be explained by assuming a combination of two mechanisms of action: an accelerated HBV clearance from the circulation (aV>1), mediated by the infused antibodies, together with partial blocking of virion release from infected cells (1>εV>0), as shown in Figure 2. Using this assumption, we performed non-linear fitting for all patients (Figure 3 and Table 1), assuming either a slow or a rapid intrinsic half-life of HBV virions, thus giving, accordingly, low and high estimates of the effectiveness in blocking virion release of εV =76.2-96.1% (97-99.5%).
Figure 3. Non-linear fitting of HBV-DNA and HBsAg kinetics with a model assuming a combined effect of blocking virion release together with acceleration of virus clearance from circulation.
This model yields good non-linear fitting simultaneously for HBV-DNA (squares, left Y-axis) and HBsAg (triangles, right Y-axis) in the 3 patients with frequent samples (A-C) and allows us to estimate the viral dynamics parameters (Table 1). Since HBsAg became undetectable in all patients, the equivalent parameters are only minimal estimates corresponding to the solid blue curves, but in fact could be faster (e.g. the dashed curves). In one patient (C) we observe a slower relapse, thus indicating a gradual decline in the effectiveness of blocking viral release (dotted line).
The analysis of HBsAg kinetics following HepeX-B™ infusion yields similar conclusions with higher acceleration of clearance and effectiveness in blocking release (Figure 3). Analysis of HBsAg decline during treatment with lamivudine shows decline with half life of 38 days, which we use as a maximal estimate of HBsAg half-life, with a minimal estimate of 1 day (data not shown). Non-linear fitting of HBsAg decline during HepeX-B™ gives half-life of 0.09-0.19 hours, thus corresponding to a minimal (maximal) estimate of the acceleration of HBsAg clearance from circulation of aA=126-282 (4,800-10,729) times an intrinsic half-life of 1 (38) days. As for the HBV-DNA kinetics, accelerated clearance of HBsAg cannot by itself explain the total decline and thus a maximal (minimal) estimate of effectiveness in blocking release of HBsAg particles from infected cells is εA=98.6-99.5% (46.2-82.4%). Note, that since all patients reached undetectable levels of HBsAg, the decline is probably larger and thus the estimates of effectiveness of blocking HBsAg release are most probably underestimated here.
Viral dynamics after multiple anti-HBs infusions
In addition to the 3 patients that received a single infusion of 40 mg HepeX-B™, 6 patients received multiple HepeX-B™ doses of 40 and 80 mg (Figure 1C-1F), but those patients had less frequent sampling and the duration of infusion was only 4 hours. We have therefore tested in the first 3 patients with frequent samples whether the estimation of decline half-life and total decline from 0.5 to 4 hours is accurate enough. We have found that although both values were slightly underestimated with less frequent samples, still the estimate of effectiveness in blocking release with the 4 hours sample was accurate within 90-110% of the non-linear fitting (Table 1). In 5 of 6 patients who received multiple doses of HepeX-B™ a rapid decline with half-life 0.31-0.66 hours and magnitude of 1.6-3.3 log10copies/ml was observed. The mathematical analysis, with low and high estimates of the accelerated clearance and effectiveness, indicates also in these patients that the antiviral activities of HepeX-B™ antibodies include both antibody-mediated accelerated clearance and partial blocking of viral particles release from infected cells (Table 1). One patient with relatively high baseline levels of HBV-DNA and HBsAg did not show significant declines during HepeX-B™ infusions.
Both HBV-DNA and HBsAg levels returned to baseline levels ±0.5 log10 within 24-48 hours after the infusion in the 3 patients with frequent samples, and within 1-7 days in the 6 patients with less-frequent samples. There was no cumulative effect of HepeX-B™ on the decline of HBV-DNA or HBsAg in the patients that received 4 weekly infusions. However, HBV-DNA and HBsAg levels at 24 hours after infusion were in general lower than expected from the rebound kinetics predicted by the model , if the anti-viral effect of HepeX-B™ disappears immediately after the end of the infusion (supp material Equation 9). Notably, for the 80 mg dose (Figure 1E-1F), at 24 hours after 5 of 12 infusions HBsAg was still undetectable and HBV-DNA was at least 3 log10 lower than baseline. Simulation of the slow rebound kinetics indicates a delay (10-16 hours) in release of viral particles after infusion and a prolonged effect of the antibodies after the end of infusion with half-life of order of 1-10 days (Figure 3C). Due to the infrequent sampling after the infusion it is not possible to quantify these effects precisely.
In vitro effects of anti-HBs
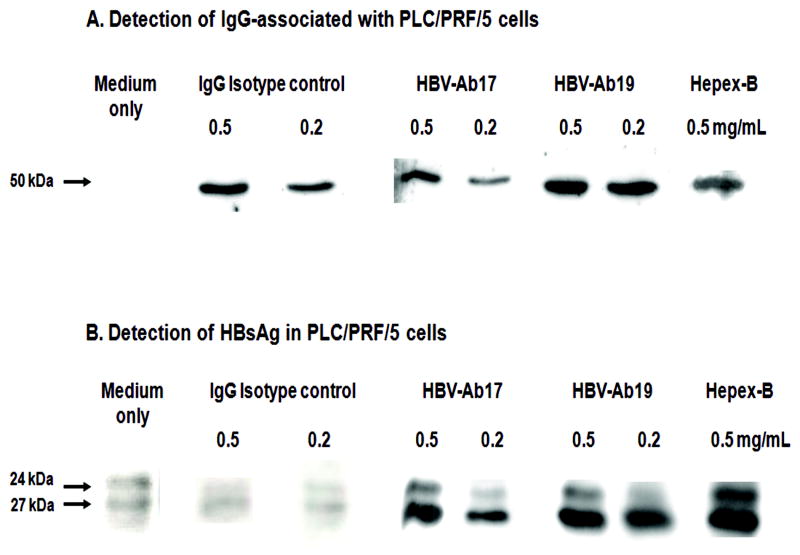
The assumption that HepeX-B™ can block the release of viral particles from cells was tested in a series of in-vitro experiments using PLC/PRF/5 cells, which are known to have stable production of HBsAg (16-19, 27). The Western blot analysis of cell lysates after 48 hour culture showed dose-dependent internalisation of both control IgG (non-specific for HBV), as well as of IgG with anti-HBs specificity (Figure 4A), which is in line with our previous findings (10). The cellular uptake of HBV-Ab19 appears to be higher than HBV-Ab17, as indicated by the different density of the Western blot bands (Figure 4A). In the same cytoplasmic extracts, the Western blot revealed a marked intracellular accumulation of HBsAg, which was observed only in cells cultured in the presence of anti-HBs, but not in control cells (Figure 4B). The combination of HBV-Ab17 and HBV-Ab19 (HepeX-B™) had a greater effect for HBsAg retention within the cells, than each of these two antibodies alone.
Figure 4. Immunoblot analysis of cytoplasmic extracts from PLC/PRF/5 cells cultured in the presence of human monoclonal anti-HBs antibodies.
HBV-Ab17 and HBV-Ab19 were used individually at two concentrations (0.5 and 0.2 mg/ml), or as mixture (Hepex-B™). Human IgG isotype and medium only served as controls. A) Detection of cell-associated IgG γ chain (50 kDa). Both IgG isotype control and IgG anti-HBs show dose-dependent uptake in the cells. B) Detection of HBsAg (24 and 27 kDa). The cellular uptake of specific anti-HBs antibodies leads to dose-dependent increase of HBsAg within the cells in contrast to cell-associated isotype IgG or medium only control.
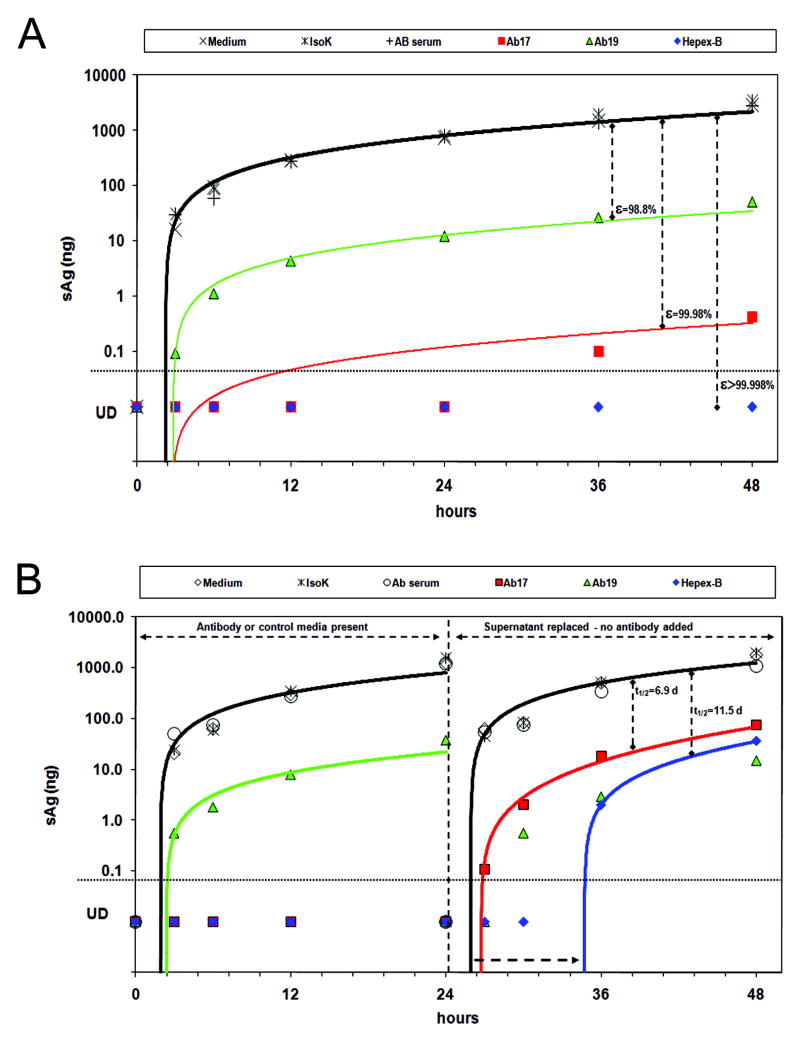
We also determined the effect of anti-HBs (HBV-Ab17 or HBV-Ab19 alone, or in combination as HepeX-B™) on the kinetics of HBsAg secretion (Figure 5A). In the control supernatants, the HBsAg levels rose rapidly in the first hours and then continued with a slower increase to an average level of 3054±342 ng. However, in the presence of HBV-Ab17 (or HBV-Ab19) the HBsAg levels were markedly reduced to only 0.4 (50.5) ng, which is 3.9 (1.8) log10ng lower than the controls. Moreover, in the presence of HepeX-B™ , HBsAg remained undetectable (<0.1 ng) throughout the 48 hours of the experiment. Fitting of the full kinetics data with the in-vitro model (Supp material Equations 10-11) indicated that HBV-Ab17 or HBV-Ab19 gives rise to a partial blocking of HBsAg release with effectiveness of 99.98% or 98.8%, respectively. A minimal estimate of the effectiveness in blocking release in the presence of HepeX-B™ is 99.998%. Similar results were obtained by estimation of HBsAg at 48 hours using the analytical solution (Supp material Equations 12-13).
Figure 5. Supernatant HBsAg kinetics in-vitro shows blocking of particle release by PLC/PRF/5 cells incubated with Hepex-B™.
A) Significantly lower HBsAg levels were observed when cells were incubated for 48 hours with HBV-Ab 17 (red squares), HBV-Ab 19 (green triangles) or Hepex-B™ (blue diamonds) as compared to medium (empty diamonds), isotype-control (stars) or human AB serum (empty circles). Fitting with the mathematical model (solid lines) indicates blocking of particle release. B) Cells were incubated with mAbs for 24 hrs, after which the supernatants were replaced with medium only. Blocking release continued in the absence of anti-HBs in the supernatant, with a gradual decline of its effectiveness.
In order, to verify whether the antibodies to HBsAg have a prolonged effect on HBsAg release from PLC/PRF/5 cells, we repeated the experiment, but after the first 24 hours, we replaced the supernatants with fresh medium and continued measuring the HBsAg levels in supernatants for additional 24 hours (Figure 5B). During the first 24 hours, when either control medium or antibodies to HBsAg were present, the kinetics were similar to the first experiment. After replacing the supernatants with fresh medium at 24 hours, the HBsAg levels in the new supernatants started from 0 and the kinetics of HBsAg secretion from control cells (treated with medium only) was slightly faster, probably due to a larger number of cells per well relative to the first 24 hours. In contrast, cells that were treated initially with HBV-Ab17 (undetectable HBsAg during first 24 hours), showed slower kinetics of HBsAg secretion (i.e. de-novo increase in supernatants), also after the antibodies were removed from the medium. Fitting of the kinetics with the model indicated a delay of 2.6 hours in HBsAg release from the cells and also a slow decline, with half-life of 6.9 days, of the effectiveness in blocking release from an initial 99.99% at 24 hours. Similarly, a delay of 10.7 hours and a slow decline of the blocking release effectiveness with half life of 11.5 days were observed for the cells treated initially with HepeX-B™.
Discussion
By modelling viral dynamics during in vivo and in vitro treatment with antibodies to HBsAg, the present study reveals a novel antiviral mechanism of antibodies to hepatitis B virus. Apart from the “conventional” antiviral activities against viral particles in the circulation, anti-HBs are internalised in liver cells and exert intracellular antiviral activities with prolonged blocking of viral particles release from infected cells. Both experimental approaches - in vivo infusions of HepeX-B™, and in vitro treatment of cells with the same antibodies produced concordant results with a reduction of viremia and/or HBsAg titres, as well as a prolonged antiviral effect after anti-HBs administration. The combination of two human antibodies - HBV-Ab17, recognizing a conformational epitope, and HBV-Ab19, recognizing a linear epitope on HBsAg, had an additive effect. Viral dynamics revealed that when used alone HBV-Ab17 had a stronger effect, but with a shorter duration when the antibody was not present (Figure 5B). Instead, HBV-Ab19 had a weaker effect in blocking the release of viral particles from the cells. However, its effect was more prolonged which may be due to a greater uptake within cells, as indicated by the Western blot (Figure 4).
Experimental data indicate that in some viral infections antibody binding to viral antigens expressed on the cell surface can modulate viral replication within cells. For example, treatment of alphavirus-infected rat neurons with monoclonal antibodies to E2 envelope protein was found to mediate viral clearance from the neurons (28); antibodies to measles virus added to virus-infected cells were shown to interfere with viral protein expresion inside the cells (29); the addition of pseudorabies-specific immunoglobulins to pseudorabies-infected monocytes induced internalization of plasma membrane-bound viral protein via endocytosis (30). A different type of antibody-mediated interaction with infected cells was observed by immunoglobulin A anti-haemagglutinin antibodies (31). Polymeric IgA anti-haemagglutinin was found to be actively transported into epithelial cells by polymeric immunoglobulin receptor and to mediate intracellular neutralization of influenza virus by binding to viral proteins within the cell and preventing viral assembly (31).
The present study reveals that the antiviral effect of anti-HBs against HBV involves not only binding of viral particles in the circulation, but it also involves intracellular antiviral activity by blocking viral particles release from the cells. We have previously demonstrated that anti-HBs is endocytosed into hepatocyte-derived cell lines irrespective of the presence or absence of HBsAg (10). This is likely to occur as a receptor-mediated endocytosis of IgG via the major histocompatibility complex class I-like Fc-receptor, FcRn. We have shown that FcRn is expressed on several liver cell lines and Fc elimination abrogated the IgG biding to the cells, as well as its effect on HBsAg secretion (10). FcRn is the transport receptor for IgG and protects IgG from catabolism after entry into cells (32, 33). This process is likely to operate during chronic HBV infection, as anti-envelope antibodies have been detected in the serum of virtually all patients with chronic hepatitis B when using sensitive assays (34). Intracellular binding and blocking the secretion of HBV particles may have a role for containment of HBV when at low level within cells, for example in subjects with spontaneously resolved HBV infection (35) or in liver transplant recipients having effective long-term HBIg prophylaxis without clinical HBV recurrence (9, 36).
The antiviral activity of HBV neutralizing antibodies may have clinical implications for treatment of chronic hepatitis B. Post-treatment rebound of HBV replication occurs frequently after stopping direct antivirals even after prolonged treatment for many years. Application of anti-HBs, in combination with a potent antiviral agent blocking directly HBV replication, will target different sites of HBV replicative cycle within cells and may reduce the relapse rates, analogous to the approach that was applied successfully after HAART withdrawal in patients with HIV infection (37). Furthermore, after prolonged and effective control of HBV replication in patients treated with potent antivirals, an add-on application of anti-HBs may facilitate HBsAg clearance in appropriately selected patients.
Supplementary Material
Acknowledgments
The authors thank Eithan Galun, principal investigator of the clinical investigation (Reference 13), that provided data for mathematical modelling, and Dov Terkieltaub for technical assistance.
Reference List
- 1.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6(3):231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 2.Fleury D, Barrere B, Bizebard T, Daniels RS, Skehel JJ, Knossow M. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat Struct Biol. 1999;6(6):530–534. doi: 10.1038/9299. [DOI] [PubMed] [Google Scholar]
- 3.Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, et al. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293(5532):1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 4.Emini EA, Kao SY, Lewis AJ, Crainic R, Wimmer E. Functional basis of poliovirus neutralization determined with monospecific neutralizing antibodies. J Virol. 1983;46(2):466–474. doi: 10.1128/jvi.46.2.466-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 6.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 7.Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000;32(6):1189–1195. doi: 10.1053/jhep.2000.19789. [DOI] [PubMed] [Google Scholar]
- 8.Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, et al. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135(9):796–800. doi: 10.7326/0003-4819-135-9-200111060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Naoumov NV, Lopes AR, Burra P, Caccamo L, Iemmolo RM, de Man RA, et al. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol. 2001;34:888–894. doi: 10.1016/s0168-8278(01)00039-3. [DOI] [PubMed] [Google Scholar]
- 10.Schilling R, Ijaz S, Davidoff M, Lee JY, Locarnini S, Williams R, et al. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003;77(16):8882–8892. doi: 10.1128/JVI.77.16.8882-8892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eren R, Lubin I, Terkieltaub D, Ben-Moshe O, Zauberman A, Uhlmann R, et al. Human monoclonal antibodies specific to hepatitis B virus generated in a human/mouse radiation chimera: the Trimera system. Immunology. 1998;93(2):154–161. doi: 10.1046/j.1365-2567.1998.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, et al. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology. 2000;32(3):588–596. doi: 10.1053/jhep.2000.9632. [DOI] [PubMed] [Google Scholar]
- 13.Galun E, Eren R, Safadi R, Ashour Y, Terrault N, Keeffe EB, et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology. 2002;35(3):673–679. doi: 10.1053/jhep.2002.31867. [DOI] [PubMed] [Google Scholar]
- 14.Alexander JJ, Bey EM, Geddes EW, Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976;50(54):2124–2128. [PubMed] [Google Scholar]
- 15.Sypsa VA, Mimidis K, Tassopoulos NC, Chrysagis D, Vassiliadis T, Moulakakis A, et al. A viral kinetic study using pegylated interferon alfa-2b and/or lamivudine in patients with chronic hepatitis B/HBeAg negative. Hepatology. 2005;42:77–85. doi: 10.1002/hep.20738. [DOI] [PubMed] [Google Scholar]
- 16.Marion PL, Salazar FH, Alexander JJ, Robinson WS. Polypeptides of hepatitis B virus surface antigen produced by a hepatoma cell line. J Virol. 1979;32:796–802. doi: 10.1128/jvi.32.3.796-802.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 18.Daemer RJ, Feinstone SM, Alexander JJ, Tully JG, London WT, Wong DC, et al. PLC/PRF/5 (Alexander) hepatoma cell line: further characterization and studies of infectivity. Infect Immun. 1980;30(2):607–611. doi: 10.1128/iai.30.2.607-611.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNab GM, Alexander JJ, Lecatsas G, Bey EM, Urbanowicz JM. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ijaz S, Ferns RB, Tedder RS. A ‘first loop’ linear epitope accessible on native hepatitis B surface antigen that persists in the face of ‘second loop’ immune escape. J Gen Virol. 2003;84(Pt 2):269–275. doi: 10.1099/vir.0.18667-0. [DOI] [PubMed] [Google Scholar]
- 21.Tedder RS, Guarascio P, Yao JL, Lord RB, Eddleston AL. Production of monoclonal antibodies to hepatitis B surface and core antigens, and use in the detection of viral antigens in liver biopsies. J Hyg (Lond) 1983;90(1):135–142. doi: 10.1017/s0022172400063932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282(5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 23.Tsiang M, Rooney JF, Toole JJ, Gibbs CS. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology. 1999;29:1863–1869. doi: 10.1002/hep.510290626. [DOI] [PubMed] [Google Scholar]
- 24.Lewin SR, Ribeiro RM, Walters T, Lau GK, Bowden S, Locarnini S, et al. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology. 2001;34(5):1012–1020. doi: 10.1053/jhep.2001.28509. [DOI] [PubMed] [Google Scholar]
- 25.Wolters LM, Hansen BE, Niesters HG, Levi-Drummer RS, Neumann AU, Schalm SW, et al. The influence of baseline characteristics on viral dynamic parameters in chronic hepatitis B patients treated with lamivudine. J Hepatol. 2002;37:253–258. doi: 10.1016/s0168-8278(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 26.Neumann AU. Hepatitis B viral kinetics: a dynamic puzzle still to be resolved. Hepatology. 2005;42(2):249–254. doi: 10.1002/hep.20831. [DOI] [PubMed] [Google Scholar]
- 27.Tabor E, Copeland JA, Mann GF, Howard CR, Skelly J, Snoy P, et al. Nondetection of infectious hepatitis B virus in a human hepatoma cell line producing hepatitis B surface antigen. Intervirology. 1981;15(2):82–86. doi: 10.1159/000149217. [DOI] [PubMed] [Google Scholar]
- 28.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 29.Fujinami RS, Oldstone MB. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- 30.Van de Walle GR, Favoreel HW, Nauwynck HJ, Van OP, Pensaert MB. Antibody-induced internalization of viral glycoproteins in pseudorabies virus-infected monocytes and role of the cytoskeleton: a confocal study. Vet Microbiol. 2002;86(1-2):51–57. doi: 10.1016/s0378-1135(01)00490-4. [DOI] [PubMed] [Google Scholar]
- 31.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69(2):1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172(4):2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 33.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama T, McLachlan A, Iino S, Koike K, Kurokawa K, Milich DR. The serology of chronic hepatitis B infection revisited. J Clin Invest. 1993;91(6):2586–2595. doi: 10.1172/JCI116497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marusawa H, Uemoto S, Hijikata M, Ueda Y, Tanaka K, Shimotohno K, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31(2):488–495. doi: 10.1002/hep.510310232. [DOI] [PubMed] [Google Scholar]
- 36.Roche B, Feray C, Gigou M, Roque-Afonso AM, Arulnaden JL, Delvart V, et al. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBs passive immunoprophylaxis. Hepatology. 2003;38:86–95. doi: 10.1053/jhep.2003.50294. [DOI] [PubMed] [Google Scholar]
- 37.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 38.Danem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.