Abstract
Although left hemisphere language specialization is one of the most widely reported findings in human neuropsychology, some studies have found evidence for more bilateral language organization in women. We report findings of a large scale, multi-task investigation of sex differences in both structural asymmetries and lateralization of word reading. Two hundred participants were tested in eight divided visual field lexical tasks, and each received a structural MRI scan. We examined whether there was evidence for sex differences in overall measures of neuroanatomical and behavioral lateralization, in specific language tasks and brain regions, and in variation in asymmetry within and across tasks and brain regions. There was very little evidence for sex differences on any behavioral measure. The few indications of sex differences in the current report accounted for 2% or less of the individual variation in asymmetry and could not be replicated in independent subsamples. No sex differences were observed in the asymmetry of structures in Broca’s and Wernicke’s area such as pars triangularis, pars opercularis, the planum temporale, planum parietale, or Heschl’s gyrus. There were also no sex differences in the variability of neuroanatomical asymmetries within or between brain regions. However, a significant relationship between planum temporale and behavioral asymmetry was restricted to men.
Keywords: sex differences, cerebral asymmetry, word reading, planum temporale, neuroanatomical asymmetry
Are there sex differences in the lateral organization of the human brain? This question has beguiled both scientists and the lay public, yet despite much effort over the past few decades there is little consensus on this matter. As our notions about brain-behavior relationships are still evolving, it is perhaps not surprising that questions about individual differences in such relationships remain unsettled. However, one of the most well-established findings in the field is the superiority of the left cerebral hemisphere for many aspects of language processing, whether this is examined by the effects of unilateral lesions (Kertesz & Sheppard, 1981), behavioral investigations using lateralized stimulus presentation (Chiarello, 1988; Hugdahl, 2003), or functional neuroimaging (Binder, et al., 1995). Similarly, structural asymmetries have also been found for brain regions implicated in language function, such as the planum temporale (PT) in the supratemporal plane (Geschwind & Levitsky, 1968) and pars triangularis in the inferior frontal lobe (Foundas, Leonard, & Heilman, 1995). The repeated findings of left hemisphere superiority pose an interesting challenge for investigators of sex differences who have argued that women have a more bilateral language organization (McGlone, 1977). One the one hand, methodological tools for examining language are well-developed and there is no question that lateralization is a fundamental organizing principle of language representation in the brain. This should provide a firm footing for explorations of possible sex differences. On the other hand, the ease of demonstrating left-hemisphere language specialization would seem to reduce the likelihood of finding significant sex differences: would findings of LH specialization have been obtained so uniformly if they primarily apply to only half of the population?
The examination of potential sex differences is also complicated by the fact that there is an uncertain relationship between sex differences in behavior and in neural substrates. Although it is often assumed that sex differences in brain structure can give rise to sex differences in behavior, it is also possible that sex differences in brain structure can prevent or minimize sex differences in behavior by “compensating” for the effects of differing physiology (Cahill, 2006; De Vries, 2004). A variety of animal studies indicate that both possibilities may co-exist (De Vries, 2004). Thus it is important to examine neuroanatomy and behavior concurrently in order to fully understand sex differences and similarities.
Here we report relevant findings from the Biological Substrates for Language project, which gathered both neuroanatomical and behavioral data from a sample of 200 young adults. To our knowledge this is the largest sample available for the investigation of sex differences in both anatomy and behavior, and anatomical-behavioral relations. The current study focuses on processes involved in reading, particularly single word reading. Although this represents just one domain of language function, a thorough examination of one domain may provide more reliable findings than a more broadly based approach that relies on single measures for a variety of language functions. We begin by summarizing prior research on behavioral and neuroanatomical lateralization and then outline the specific objectives of the current study.
Investigations of Sex Differences in Functional Language Lateralization
A number of widely cited studies support the view that women have a more bilateral language representation as compared to men (McGlone, 1977; Springer, & Deutsch, 1997). McGlone (1977) reported that men were much more likely to become aphasic after LH injury than were women, suggesting a more bilateral language organization for women. Some early behavioral examinations of language lateralization found reduced LH advantages for women using dichotic listening (Lake & Bryden, 1976) or divided visual field methods (Bradshaw, Gates, & Nettleton, 1977). Functional magnetic resonance imaging (fMRI) has indicated that women demonstrated a more bilateral pattern of activation for rhyme decisions while men showed greater activation in the left hemisphere (Pugh, et al., 1996; Shaywitz, et al., 1995). Although some subsequent reports have observed similar patterns of sex differences (Clements, et al., 2006; Coney, 2002; Inglis & Lawson, 1981), literature reviews, meta-analyses, and large-scale investigations have found negligible or no sex differences in language lateralization (Boles, 2005; Fairweather, 1982; Frost, et al., 1999; Hugdahl, 2003; Kertesz & Sheppard, 1981; Pujol, Deus, Losilla, & Capdevila, 1999; Sommer, Aleman, Bouma, & Kahn, 2004; Sommer, Aleman, Somers, Boks, & Kahn, 2008; Voyer, 1996). It is interesting that in Sommer, et al.’s (2004) meta-analysis of functional neuroimaging studies, investigations obtaining sex differences had, on average, smaller sample sizes (mean = 31) than those not obtaining such differences (mean = 76). This raises the question as to whether inadequate sampling may account for some of the reported sex differences. One large meta-analysis of behavioral asymmetries, however, did provide evidence of reduced auditory and visual verbal asymmetries in women (Voyer, 1996; but see also Sommer, et al., 2008). However, the effect size for the sex difference was extremely small (d = .06), and Voyer (1996) noted that the findings were “susceptible to the file drawer problem” (i.e., failure to publish null findings of sex differences, pg. 70).
Large-scale studies or reviews also have some limitations. In general, studies with large samples have included only a single task (Frost, et al., 1999; Hugdahl, 2003; Knecht, et al., 2000; Springer, et al., 1999), or have included multiple tasks with different individuals contributing data across tasks (Boles, 2005; Fairweather, 1982; Sommer, et al., 2004, 2008; Voyer, 1996). If sex differences in language lateralization are task- or process-specific (Shaywitz, et al., 1995), a small sampling of tasks might miss true differences. Further, examining variability in asymmetry within the same individuals requires a multi-task approach. A more optimal method would be to examine a large sample of individuals across multiple verbal tasks. In this paper, we report the results of such an investigation.
Investigations of Neuroanatomical Sex Differences in Asymmetry
Reported sex differences in neuroanatomy include highly reliable differences in cerebral volume (Andreasen, 1993; Willerman, 1991), cerebellar volume (Filipek, 1994) and white matter proportion (smaller amounts in women) (Gur, et al., 1999; Allen, Damasio, Grabowski, Bruss, & Zhang, 2003) and we have replicated these effects in the current sample (Leonard, et al., 2008a). There are few reports of sex differences in grey and white matter asymmetry. A recent review includes a graph from Gur, et al. (1999) showing that men, but not women, have a significant leftward asymmetry of grey matter and a significant rightward asymmetry of cerebral spinal fluid (Halpern, Benbow, Geary, Gur, Hyde, & Gernsbacher, 2007). Allen, et al. (2003), however, did not find such a sex difference. Sex differences in asymmetries of specific cortical regions are also unreliable (reviewed by Beaton, 1997; Shapleske, 1999; Sommer, et al., 2008). Potential sex differences in asymmetry of the planum temporale (PT, larger in the left hemisphere in approximately 70% of cases) have been investigated in numerous studies, following a report that atypical PT asymmetries were found more frequently in women, than in men (Wada, Clarke, & Hamm, 1975). An early review of these studies concluded that “…there is very little strong evidence one way or the other as to whether males and females differ in degree of planum asymmetry” (Beaton, 1997, pg. 307). A later, more extensive review reported a meta-analysis of 11 studies that included 186 women and 246 men (Shapleske, Rossell, Woodruff, & David, 1999). The laterality coefficient for the PT in this combined sample was nearly identical for women and men (F < 1), providing little support for the conclusion that “PT asymmetry does appear to be influenced by gender but the sample sizes studied to date lack adequate statistical power to detect differences” (Shapleske, et al., 1999, pg. 41). A more recent meta-analysis of 13 studies (N = 807) also found equivalent PT asymmetries in men and women (Sommer, et al., 2008). Individual studies published more recently have continued to be equivocal. One very large (N = 465) study did observe reduced leftward asymmetry of Heschl’s gyrus and the PT in women as compared to men (Good, Johnsrude, Ashburner, Henson, Friston, & Frackowiak, 2001). Using a novel MRI texture analysis procedure, Kovalev, Kruggel, & von Cramon (2003) observed greater asymmetry in male brains in several regions including the superior temporal gyrus and Heschl’s gyrus (N = 290). Others have failed to observe sex differences in asymmetry (Luders, et al., 2006, N = 60; Dos Santos Sequeira, et al., 2006, N = 104;Watkins, et al., 2001, N = 142). One study found leftward PT asymmetry in women, but not in men (Knaus, Bollich, Corey, Lemen, & Foundas, 2004, n = 24), although this finding was not replicated in a later study that found no sex differences in the asymmetry of PT, pars triangularis, and pars opercularis (Knaus, Bollich, Corey, Lemen, & Foundas, 2006, N = 48). Again the issue of sample size may be significant, although the very largest studies both support (Good, et al, 2001; Kovalev, et al., 2003) and refute (Shapleske, et al., 1999; Sommer, et al., 2008) the sex differences hypothesis.
Prior studies of neuroanatomical asymmetries rarely included measures of language skill or lateralization, making it impossible to examine sex differences in structure-function relationships. Relevant investigations that have attempted to correlate structure and function are reviewed below.
Neuroanatomical-Behavioral Studies of Language Lateralization
In a preliminary investigation of 20 male college students we observed a positive correlation between planum temporale asymmetry and a composite reaction time (RT) asymmetry across five lexical tasks (Chiarello, Kacinik, Manowitz, Otto, & Leonard, 2004). No anatomical-behavioral correlations were obtained for either Heschl’s gyrus or the planum parietale. This finding suggests that larger RT asymmetries for visual word recognition are associated with larger PT asymmetries, but it is unclear if similar results would be obtained for female subjects, or replicated in a larger sample. Dos Santos Sequeira, et al. (2006) investigated the relation between PT asymmetry and a dichotic listening measure in a larger sample including both men (N=46) and women (N=58). They observed a reliable correlation between dichotic listening and PT asymmetries only in consistently right-handed men – for this group greater leftward PT asymmetry was associated with larger REA/left hemisphere advantages. In contrast, an fMRI study using a complex semantic decision task did not observe any relationship between PT and functional asymmetry in either men (N = 48) or women (N = 51) (Eckert, Leonard, Possing, & Binder, 2006). Other studies attempting to correlate PT and functional asymmetries (using either dichotic listening, divided visual field methods, or fMRI) have either not included women (Hellige, Taylor, Lesmes, & Peterson, 1998; Heiervang, et al., 2000; Moffat, Hampson, & Lee, 1998) or have used samples too small to detect reliable sex differences (Dorsaint-Pierre, et al., 2006; Jancke & Steinmetz, 1993). Hence there is too little evidence to draw firm conclusions about whether there are sex differences in the relationship between neuroanatomical and behavioral asymmetries. Furthermore, it is unknown whether any association exists for asymmetries in frontal regions that are implicated in language processing (pars triangularis, pars opercularis) (Foundas, Eure, Luevano, & Weinberger, 1998; Knaus, Corey, Bollich, Lemen, & Foundas, 2007). In the current study, we explore these issues in a relatively large sample of male and female participants.
Objectives of the Current Study
Our study attempted to answer several questions. First, considering the behavioral data, we investigated whether sex differences in standardized measures of reading could be observed in order to provide a measure of reading skill, independent of lateralization. Second, we explored whether there was evidence for an across-the-board sex difference in visual lexical lateralization (combined across multiple tasks). Third, we sought evidence for more limited sex differences that might be specific to certain tasks or measures. Fourth, we examined whether female participants were more variable in their asymmetries both within tasks and across tasks. If women demonstrate more variability in asymmetry this could contribute to the lack of replicability across studies, even if most women do not have bilateral language representation. To address these questions, we examined 200 healthy young adults (100 female), each of whom was tested in eight divided visual field lexical tasks. The tasks were selected to include a variety of lexical processes, including phonological encoding (nonword naming), rapid visual processing (masked word recognition), semantic and lexical discrimination (semantic and lexical decision), and semantic generation (verb and category generation). Response requirements also varied across tasks, and included both vocal and manual responses, open-ended and forced-choice responses, and tasks with a single correct response and with multiple correct responses. Due to the anatomy of the visual system, stimuli briefly presented to the left visual field (LVF) are directly transmitted to the right visual cortex, and vice versa. An overall RVF/left hemisphere advantage is routinely obtained in verbal tasks (Chiarello, 1988). If women have more bilateral language representation we would expect them to obtain reduced or negligible RVF advantages, and/or more variable asymmetries, relative to men.
We also examined a similar set of issues for structural brain asymmetries. Using magnetic resonance imaging (MRI) we measured the asymmetries of grey matter, white matter, and CSF, and five perisylvian areas that, based on prior studies, demonstrate left-right differences in surface area: the planum temporale, planum parietale, Heschl’s gyrus, pars triangularis and pars opercularis. The strength and robustness of hemispheric asymmetries in these areas varies. Virtually all research groups find leftward asymmetry for the planum temporale (reviewed in Eckert, et al., 2006; Sommer, et al., 2008) and Heschl’s gyrus (Penhume, Zatorre, MacDonald, & Evans, 2008; Dorsaint, et al., 2006), and rightward asymmetry for the planum parietale (Chiarello, et al., 2004; Foundas, Leonard, & Hanna-Pladdy, 2002; Jancke, Schlaug, Huang, & Steinmetz, 1994). Asymmetries are weaker and less reliable in the frontal regions (Foundas, Leonard, Gilmore, Fennell, & Heilman, 1996; Foundas, et al., 1998; Knaus, Corey, Bollich, Lemen, & Foundas, 2007). We considered whether there was evidence for sex differences in asymmetry within and across these brain regions, and whether men and women differed in the variability of their structural asymmetries. If women rely on more bilaterally symmetrical language structures we would expect them to demonstrate reduced and/or more variable asymmetries in at least some of these regions, relative to men.
Finally, we examined whether there could be sex differences in the relationship between anatomical and visual field asymmetries. Our prior study that included men only demonstrated a positive association between lexical task and planum temporale asymmetries (Chiarello, et al., 2004). We attempt to replicate this result here, and determine whether women show similar anatomical-behavioral relationships.
Method
Participants took part in five sessions of behavioral testing, and then received a structural MRI scan in their final session. Behavioral testing and preliminary analyses of these data were conducted at the University of California, Riverside with the experimenters blind to the status of the brain measurements. Similarly, brain measurements were made at the University of Florida by anatomists who were blind to the identity and behavioral findings of the participants. The behavioral and anatomical data were pooled only after the data were scored and brain measurements completed.
Participants
Campus-wide electronic messages and announcements were used to recruit potential participants. One hundred male and 100 female university student volunteers participated, receiving $100 payment for their participation. Subjects with a history of brain injury or disease or conditions incompatible with an MRI scan were excluded. The neuroradiologist (R.O.) reviewed all scans for pathology, and four additional participants were excluded from the final sample due to abnormal findings on the MRI. The male and female participants did not differ in mean age (21.7 vs 21.5 years), socio-economic status (3.40 vs 3.25)1, hand preference (+.67 vs +.74) (scale ranges between +1.0 strong right handedness to −1.0 strong left handedness, Bryden, 1982), or Verbal (108.7 vs 108.8) or Performance (110.3 vs 107.3) IQ (Wechsler, 1999). All were native speakers of English with normal or corrected-to-normal vision. Using a cut-off score of +.30, 28 of the participants (13 female) were ambidextrous or left-handed. All of the analyses reported here were conducted with and without the non-right-handers. As the inclusion of non-right-handers did not alter any of the results, we report findings for the entire sample. Based on self-reports of their majors, it appears the participants were a representative sample of the campus population, with somewhat more females than males majoring in the humanities and social sciences (57% vs 44%), and fewer females than males majoring in the sciences and engineering (31% vs 47%) (the remainder majored in business or were undeclared).
Behavioral Stimuli and Procedure
In an initial 2-hour session participants completed a 5-item hand preference questionnaire (Bryden, 1982), questionnaires regarding language and family background, and standardized measures of reading skill and intelligence (Wechsler, 1999; Woodcock, 1998). Following this session, four test sessions were held on separate days in which participants completed 8 lateralized word recognition tasks. All participants received tasks and test sessions in the same order, however, the amount of time separating the test sessions differed in order to individually accommodate participants’ schedules.
Experimental stimuli consisted of 3–6 letter concrete nouns and/or pronounceable nonwords. Nonwords were created by replacing a single letter of a concrete noun, with each position of replacement occurring equally often. No stimuli were repeated within an experimental session, and no stimulus was used more than twice throughout the entire study. Word lists for each task were equated for word length and log-transformed word frequency based on the Hyperspace Analogue to Language corpus (Lund & Burgess, 1996). Mean word length for each task ranged from 4.44 to 4.64 and mean log word frequency ranged from 4.16 to 4.71. Within each task, items were matched across visual field conditions on the basis of length, log frequency (Lund & Burgess, 1996), familiarity (Wilson, 1988) and imageability (Wilson, 1988).
All stimuli were presented in uppercase, black 20 point Helvetica font on a white background on an Apple Studio Display M7649 monitor. Macintosh computers were used for stimulus presentation and recording of manual responses in the visual field tasks. Psyscope programming software (Cohen, MacWhinney, Flatt, & Provost, 1993) was used to control experimental events and record responses. Participants were seated 60 cm in front of the monitor, using a headrest to stabilize head position. For those experiments requiring manual responses (Lexical Decision, Masked Word Recognition, and Semantic Decision), participants used the index fingers of each hand on the ‘.’ and ‘x’ keys to indicate one response and the middle fingers of each hand on the ‘/’ and ‘z’ keys to indicate the other response. This configuration was designed to accommodate both left- and right-handed participants. A Sony ECM-MS907 microphone was used to register vocal responses. Vocal responses were entered into the data file by an experimenter. Special codes were entered for spurious vocal responses (a cough, for example), or failure to respond, and such trials were not analyzed.
The 8 tasks were administered across 4 testing sessions, in the following order:
Lexical Decision: 90 word and 90 nonword trials, keypress discrimination response, 125 ms exposure.
Word Naming 1: 90 trials, pronounce word, 125 ms exposure.
Category Generation: 82 trials, produce exemplar of stimulus noun category (e.g., FRUIT), 155 ms exposure.
Nonword Naming: 90 trials, pronounce nonword, 150 ms exposure.
Masked Word Recognition: 100 trials, recognize word preceded and followed by 60 ms pattern mask (@#@#), two-alternative forced choice key press response, 30 ms exposure. The response alternatives differed by only a single letter.
Verb Generation: 100 trials, pronounce verb associated with stimulus noun, 150 ms exposure.
Word Naming 2: 90 trials, pronounce word, 125 ms exposure.
Semantic Decision: 120 trials, determine whether stimulus noun represents a naturally occurring or manmade object, keypress response, 120 ms exposure.
On average, each session was separated by 4 days. Each task was preceded by 30–48 practice trials.
Stimuli were randomly presented to the left or right visual field (LVF, RVF), 1.8 degrees eccentric from a central fixation “+”. At the onset of each trial, the fixation marker appeared for 600–805 ms and flickered just prior to the onset of the stimulus. Participants were instructed to maintain central fixation and respond as quickly and accurately as possible.
Imaging Procedure
After the images were reviewed for neuropathology they were transferred to compact discs at the Imaging Center and sent to the McKnight Brain Institute at the University of Florida. Preprocessing the images was performed using FSL scripts (http://www.fmrib.ox.ac.uk/) (Smith et al., 2004). Extraction of the brain parenchyma from scalp and skull was performed with BET (Smith, 2002) before registration (FLIRT) (Jenkinson and Smith, 2001) to a 1 mm isovoxel study-specific template image aligned into the Talairach planes. No nonlinear warping was performed on the images. Hence, changes in the images were restricted to the translation and rotation necessary to align the midline and the anterior commissure-posterior commissure axis with the standard Talairach planes. Segmentation into separate grey matter, white matter and cerebrospinal fluid (CSF) volumes was performed using FAST (Zhang, Brady & Smith, 2001). In these volumes, each voxel is represented as a partial volume estimate of a particular tissue type. The volume of each tissue type was calculated by multiplying the number of voxels times the average partial volume estimate of those voxels as described on the FSL website. Volumes, surface areas, means, standard deviations, and average asymmetries were automatically accumulated in a data file for statistical analysis. Each structure was measured twice by at least two different investigators who were blind to hemisphere and subject characteristics. When there was more than 15% disagreement between the average values for the two measurements, the experimenters conferred and identified the reason for disagreement and then remeasured until the two measures agreed.
Grey, white and cerebrospinal fluid (CSF) volumes of each hemisphere were estimated by outlining every fifth sagittal image starting at the midline. The brainstem was excluded by transection in the midcollicular plane. The midsection was traced twice and half the slab volume added to each hemisphere. The inter-rater reliability of this measure is > .98 (intraclass correlation). Preliminary studies showed that the accuracy of volumes sampled in this way was equivalent to that in which every section was measured.
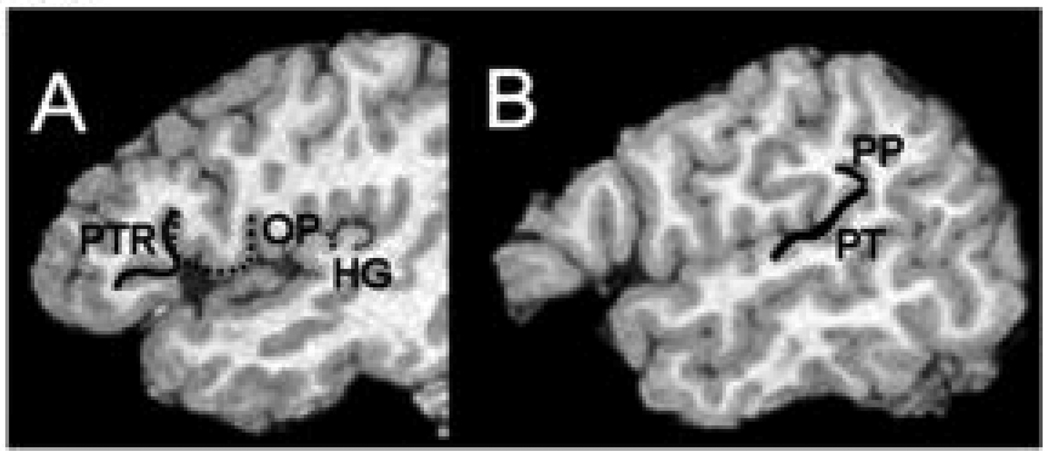
Figure 1 displays the locations of the five perisylvian areas we measured. Surface areas of the planum temporale and planum parietale were calculated between x = 47 and 56 (sagittal coordinates adjusted for hemisphere width and chosen to maximize lateral asymmetry as well as reliability) (Chiarello, et al., 2004; Eckert, et al., 2001; Leonard et al., 1996). In individuals with one clearly defined Heschl's gyrus, the anterior border of the planum temporale was defined as the depth of the sulcus that formed the posterior border of Heschl's gyrus (Heschl's sulcus). When Heschl's gyrus is indented by an intermediate sulcus, the tracing includes the gyri on both banks of the sulcus (as shown in Figure 1). When an independent gyrus appears posterior to Heschl’s gyrus, this gyrus is included in the planum measurement (Eckert, et al., 2006). The posterior boundary of the planum temporale was defined as the origin of the posterior ascending ramus or the termination of the Sylvian fissure. At medial positions, the origin of the planum parietale is absent or difficult to distinguish, whereas in more lateral positions the anterior border of the planum temporale frequently becomes indistinct. The anterior border of the planum parietale is defined as the point where the Sylvian fissure angles superiorly. The sulcus is traced to its termination in the parietal lobe. Inter-rater reliability for these measurements is .85. A comparative study of techniques to measure the planum temporale (Best and Demb, 1999) found that asymmetry measures using this index agreed well with those gained using other techniques. The surface area of the primary Heschl’s gyrus was measured between Talairach x = 34 to x = 48. Inter-rater reliabilities were .9 for H1. The pars triangularis in the inferior frontal gyrus (part of Broca's area on the left) was measured from Talairach x = 39 to x = 48 by tracing the surface formed by the anterior ascending ramus (AAR) and the anterior horizontal ramus (AHR) of the Sylvian fissure. The surface was traced from the tip of the AAR, down to the Sylvian fissure and the following the AHR to the end (Foundas,et al., 1998). Inter rater reliability for these measurements is .85. The pars opercularis in the inferior frontal gyrus (part of Broca's area on the left) was measured from Talairach x = 35 to x = 44. The surface was measured by tracing the convolutions on sagittal sections, starting at the anterior ascending ramus of the Sylvian fissure and ending at the anterior subcentral sulcus. This method was developed by (Foundas and others, 1998). Inter-rater reliability for these measurements is .85. Asymmetry coefficients for all structures described above were calculated by subtracting the left measure from the right and dividing by the average, so that leftward asymmetries yielded positive coefficients.
Figure 1.
Two sagittal MRI images depicting outlines of perisylvian structures. A. Frontal structures pars triangularis (PTR), solid line, and pars opercularis (OP), large dashed line, and posterior structure Heschl’s gyrus (HG). B. Posterior structures planum temporale (PT) and planum parietale (PP).
Results
1. Standardized Reading Test Findings
There were no reliable sex differences for either the Passage Comprehension (M = 67.0%, F = 63.0%) or Word Attack (nonword reading, M = 50.7%, F = 45.2%) reading subtests (Woodcock, 1998). However, a sex difference was observed on the Word Identification (word reading) subtest, t(198) = 2.77, p < .01, d = 0.39 ± 0.28, with better average performance for men (52.9%) than for women (46.5%).
2. Divided Visual Field Task Findings
Mean reaction time (RT) and percent correct scores were computed for each participant in each task and visual field. Visual field asymmetry scores for RT were also calculated for each task using a standard laterality index: (LVF-RVF)/(LVF+RVF). The selection of an asymmetry measure for accuracy is more problematic because floor or ceiling effects can artificially truncate the degree of asymmetry that can be observed. Several different accuracy asymmetry indices have been proposed, but no standard has emerged (Birkett, 1977; Bryden & Sprott, 1981; see recent discussion by Boles, Barth, & Merrill, 2008). The size of our database allowed us to take an empirical approach to this issue, by calculating several different indices and examining their psychometric properties. The lambda z-score (Bryden & Sprott, 1981) was chosen because it proved to be the measure with the best reliability (both test-retest and split-half), and the least affected by floor and ceiling effects2. For both the RT and accuracy asymmetry measures, positive scores indicate a RVF/LH advantage.
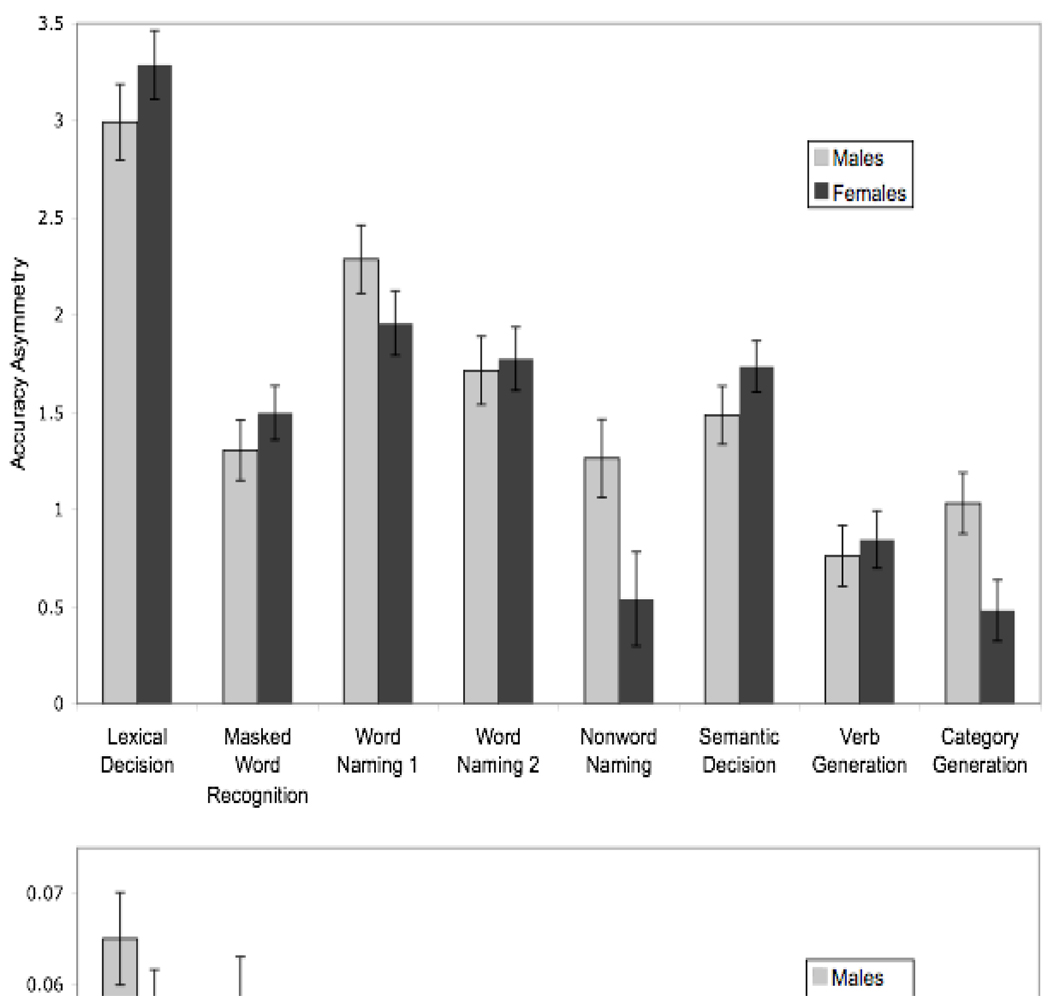
Figure 2 displays the mean asymmetry scores for males and females by task. Findings are presented below as they address our experimental questions.
Figure 2.
Mean asymmetry score (+/− SEM) by task for male and female participants for accuracy (above) and reaction time (below). Positive scores indicate RVF/left hemisphere advantages. For accuracy there was a significant RVF/left hemisphere advantage for all tasks, and for reaction time the RVF/left hemisphere advantage was significant for all tasks except nonword naming.
Is there a sex difference in measures of overall visual lexical lateralization?
Composite measures will be more reliable than any single assessment (Rosenthal, 2005), and this may provide the strongest test of the sex difference hypothesis. Therefore, a composite lexical asymmetry score was computed for each participant by z-scoring the asymmetry value for each task and then averaging across the seven tasks (Word Naming 2 was omitted from this measure as it was the only repeated task), separately for percent correct and RT (see Chiarello, Kacinik, Manowitz, Otto, & Leonard, 2004 for a similar measure). A z-score of 0 indicates the average asymmetry for a given task – if women are less lateralized they should obtain more negative z-scores relative to men. Even in our large sample men and women did not differ in this composite asymmetry measure, either for RT (.015 vs −.015, t < 1) or percent correct (.024 vs −.024, t < 1). Hence, an overall measure of visual lexical lateralization did not reveal any evidence of sex differences.
Are there task-specific sex differences in visual word recognition or visual lexical lateralization?
To address these questions, 2 (Sex) X 2 (VF) X 8 (Task) mixed-design ANOVAs were conducted using the Greenhouse-Geisser correction, for both RT and percent correct. Sex differences that are independent of lateralization would be indicated by a main effect of Sex or Sex X Task interactions. Sex differences in lateralization would be supported by significant Sex X VF or Sex X VF X Task interactions3. For RT, there were main effects of VF F(1,197) = 353.9, p < .0001, η2=0.64± 0.06, and Task F(7,1379) = 1342.2, p < .0001, η2=.87±0.00, but not Sex (F < 1). There were no significant interactions with sex.
For accuracy, there were also main effects of VF F(1,197) = 435.0 p < .0001, η2=0.69±0.06, and Task F(7,1379) = 201.4, p < .0001, η2=0.51±0.03 No main effect of sex was obtained (p > .25). A Sex X Task interaction, F(7,1385) = 2.54, p < .05, η2= 0.010±0.009, was due to a sex difference in the Masked Word Recognition task only, F(1,198) = 6.33, p < .05, η2= .031±.029, where men were more accurate than women (76.0% vs 73.0%). The only other interaction was a reliable Sex X VF X Task effect, F(7,1379) = 2.62, p < .05, η2 = .013±0.010. The Sex X VF interaction was significant for two tasks - Nonword Naming, F(1,198) = 4.14, p < .05, η2=.021±.020, and Category Generation F(1,198) = 5.20, p < .05, η2=.03±.00. For both tasks, respectively, there were significant RVF/LH advantages for both men (F(1,99)=36.4, p<.0001, d=.42±.28; F(1,99) = 39.9, p < .0001, d=.46±.28) and women (F(1,99)=5.4, p < .05, d=.18±.08; F(1,99) = 11.2, p < .005, d=.26±.23). However, asymmetries for women were reduced relative to men for both Nonword Naming (3.7% vs 8.0%) and Category Generation (2.8% vs 5.6%).
We further explored these task-specific sex differences in two ways. First, to assess issues of replicability, we randomly divided our sample in half (but with equal numbers of men and women in each half). We were able to observe the accuracy Sex X VF interaction for Nonword Naming, F (1,98) = 10.1, p < .005, η2=.09±.08, and Category Generation, F(1,98) = 8.9, p < .005, η2=.08±.07, for one half of the sample, but not the other half (Fs < 1). A different randomization yielded the same results. Second, we performed multiple regressions for the accuracy asymmetry scores for these two tasks. When Sex was entered as a sole predictor it accounted for approximately 2% of the variance in asymmetry for both Nonword Naming, t = −2.32, p < .05, R2 = .028±.022, and Category Generation, t = −2.38, p <.05, R2 = .021±.021. To summarize, RT asymmetries did not differ by Sex for any task. Accuracy asymmetries were significantly reduced for women for Nonword Naming and Category Generation, although these sex differences accounted for little variance and could not be replicated across independent subsamples.
Are women more variable in behavioral asymmetry?
We first assessed whether, within each task, there was greater variation in asymmetry for female than for male participants. There were no sex differences in homogeneity of variance (Levene, 1960) for RT or accuracy asymmetries for any task. Because one of our tasks, Word Naming, was administered twice we also assessed whether there were sex differences in the test-retest reliability of asymmetry scores. There was no difference in this measure between men (r = .57) and women (r=.56) for accuracy (p > .7), or for RT (r = .32 vs .41, respectively, p > .4).
If asymmetries differ more across tasks for female than for male participants, then the standard deviations of their asymmetries across tasks should be larger. To investigate this, we computed the standard deviation across the z-scored asymmetries for our seven unique tasks. There was no sex difference in this measure for either RT (male sd = .89, female sd = .92, t <1) or percent correct (male sd = .88, female sd = .84, p < .25).
It appears that women are not more variable in visual lexical asymmetry across different tasks, within any given task, or within a task across two different administrations.
Anatomical Findings
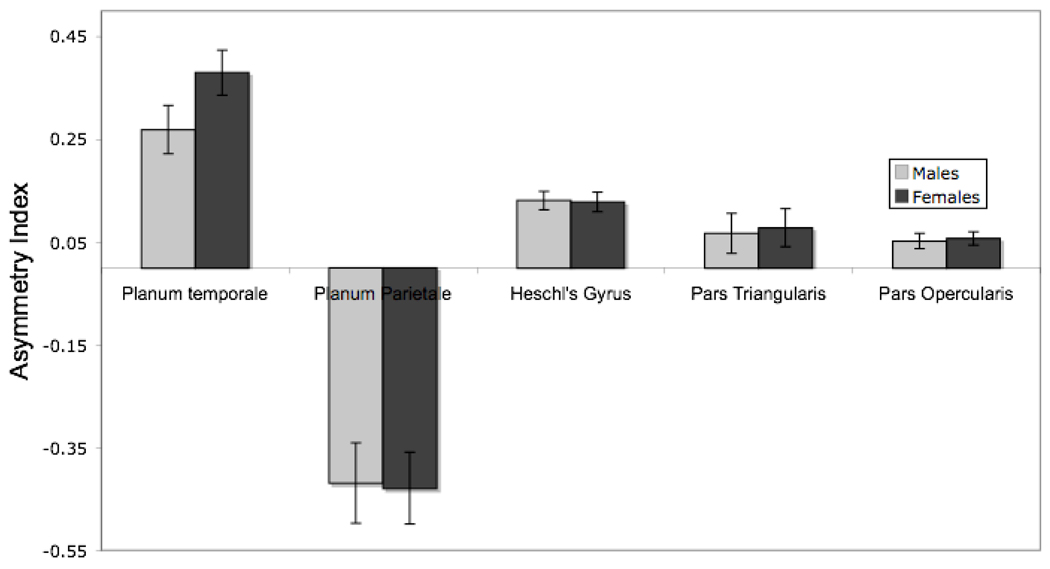
Figure 3 displays the group averaged anatomical asymmetries for men and women across the five cortical regions. Analyses of these asymmetries are presented below as they address our experimental questions.
Figure 3.
Mean asymmetry coefficient (+/− SEM) for five cortical regions for male and female participants. Values are positive for asymmetries favoring the left hemisphere.
Is there a sex difference in neuroanatomical asymmetry across brain regions?
To address this question, a 2 (Sex) X 5 (Region) mixed-design ANOVA was conducted on neuroanatomical asymmetry scores using the Greenhouse-Geisser correction. Sex differences in lateralization would be supported by significant effects of Sex or a Sex X Region interaction. A main effect of Region was observed, F(4,792) = 75.23, p < .0001, η2= .28±.05. As indicated in Fig. 3, asymmetries were substantial for the planum parietale (rightward asymmetry) and planum temporale (leftward asymmetry), but smaller in magnitude for Heschl’s gyrus (although highly reliable) and the frontal areas (leftward asymmetries). There was no effect of Sex and no Sex X Region interaction (Fs < 1). As indicated in Table 1, asymmetries were significantly different from zero for all five regions in women, and for all regions except the pars triangularis in men. The direction of asymmetry was as expected for all regions, being leftward for the frontal areas and Heschl’s gyrus and the PT, but rightward for the planum parietale. The results of within-region t-tests of sex differences are also given in Table 1 and confirm that asymmetries did not differ by sex for any region.
Table 1.
Means and standard deviations (SD) for coefficients of asymmetry (means with positive signs indicate leftward asymmetry) in 100 female and 100 male participants, and test of sex difference for each structure (effect size is Cohen’s d).
| Region | Sex | Mean | SD | t | p | Effect Size |
|---|---|---|---|---|---|---|
| Planum Temporale | F | 0.38**** | 0.44 | |||
| M | 0.27**** | 0.47 | 1.73 | .08 | .25 | |
| Planum Parietale | F | −0.43**** | 0.70 | |||
| M | −0.42**** | 0.78 | −0.10 | .92 | −.01 | |
| Heschl’s Gyrus | F | 0.13**** | 0.189 | |||
| M | 0.13**** | 0.186 | −0.13 | .90 | −.02 | |
| Pars Triangularis | F | 0.08* | 0.37 | |||
| M | 0.07 | 0.39 | 0.22 | .83 | .03 | |
| Pars Opercularis | F | 0.06**** | 0.14 | |||
| M | 0.05*** | 0.15 | 0.27 | .79 | .04 | |
| Grey Matter | F | −0.008**** | 0.019 | |||
| M | −0.002 | 0.027 | −1.90 | .06 | −.26 | |
| White Matter | F | −0.016**** | 0.013 | |||
| M | −0.022**** | 0.014 | 3.43 | .001 | .44 | |
| Cerebrospinal Fluid | F | −.110**** | 0.085 | |||
| M | −.101**** | .078 | −.63 | .53 | .00 | |
| Total Hemisphere | F | −0.024**** | 0.02 | |||
| M | −0.024**** | 0.024 | −0.31 | .76 | .00 | |
Asymmetry significantly different from zero, p < .05
p < .001
p < .0001
As indicated in Table 1 significant rightward asymmetries were obtained for both men and female participants for white matter, cerebrospinal fluid and total hemispheric volume, and for female participants for grey matter. T-tests were also conducted to examine whether there were sex differences in these measurements. Men had greater rightward asymmetries for white matter than women (p < .001), while women tended to have greater rightward asymmetries for grey matter as compared to men (p = .06). Although the sex difference for white matter asymmetry was highly reliable, this reliability appears to be due to the low standard deviation and the large sample size rather than a substantial sex difference in asymmetry – both men and women had approximately 2% more white matter in the right hemisphere.4
In sum, no sex differences were detected in asymmetry of surface areas of five language-related regions. However, the magnitude of asymmetries in white and grey matter differed slightly by sex.
Are women more variable in neuroanatomical asymmetry?
If women’s asymmetries differ more across regions than men, then the standard deviations of their asymmetries across regions should be larger. To investigate this, we computed the standard deviation across the z-scored asymmetries for the five brain regions. There was no sex difference in this measure (male sd = .33, female sd = .35, t(198)=1.07, p > .25). We also examined whether, within each region, there was greater variation in asymmetry for female than for male participants. There were no sex differences in homogeneity of variance (Levene, 1960) for any region.
Behavioral-Anatomical Relationships
To investigate whether there are sex differences in the association between behavioral and brain asymmetries we correlated the RT and accuracy composite lexical asymmetries with asymmetries for each of the five brain regions (see Chiarello, et al., 2004 for a similar approach), separately for women and men. These correlation coefficients are given in Table 2. The only reliable correlation was between the planum temporale and the composite RT asymmetry, but only for the male participants. For this group (as in our earlier study, referred to above), larger PT asymmetries were associated, as predicted, with greater RVF/left hemisphere advantages. No correlation was observed for women. To test whether the male and female planum temporale correlation coefficients reliably differed from each other, the Fisher r-to-z transformation was used. Men had a significantly stronger correlation as compared to women, z = 1.85, p = .06 (two-tailed), p < .05 (one-tailed).
Table 2.
Correlation coefficients for composite lexical asymmetries (reaction time and accuracy) and asymmetry of five brain regions for female and male participants.
| Reaction Time | Accuracy | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Planum Temporale | −.038 | .224* | −.032 | .075 |
| Planum Parietale | −.032 | −.032 | .061 | .008 |
| Heschl’s Gyrus | −.064 | −.056 | −.034 | .194 |
| Pars Triangularis | .048 | −.046 | .135 | −.078 |
| Pars Opercularis | −.058 | .069 | .012 | .078 |
p < .05
Discussion
The current investigation represents a large-scale multi-task, multi-region investigation of sex differences in language lateralization. The findings revealed only very limited support for the view that women have a more bilateral language organization relative to men. None of the reaction time measures of asymmetry or variation in asymmetry revealed any sex differences. Most of the accuracy measures also failed to reveal sex differences. Women and men also had remarkably similar asymmetries and variation in asymmetry across five perisylvian regions. However, we obtained some evidence for a sex difference in the relationship between planum temporale and lexical task RT composite asymmetry, since a significant association only appeared for men. Before interpreting these findings, we discuss some limitations of the study.
Our sample, although large, was limited to college students. Hence we cannot ascertain how generalizable our results are to the adult population as a whole. We also only investigated visual word-level tasks, and it is unclear whether the findings would also hold for auditory language tasks or for investigations of syntax, discourse, or other higher level language processes. However, previous large sample investigations of auditory language lateralization have failed to detect sex differences (Boles, 2005; Hellige, Bloch, & Taylor, 1988; Hugdahl, 2003; Sommer, et al., 2008), and many of the smaller sample studies finding significant sex differences did use visual language stimuli (Clements, et al., 2006; Coney, 2002; Jaeger, et al., 1998; Pugh, et al., 1996). Thus language modality is unlikely to be an important factor. Future studies will be needed to address sex differences in language tasks beyond the lexical level. Finally, this study used only an indirect behavioral measure of language lateralization. Using currently available neuroimaging techniques would be prohibitively expensive in such a large sample multi-task investigation. Nevertheless, recent meta-analyses of functional imaging studies failed to detect significant sex differences in language lateralization (Sommer, et al., 2004, 2008).
The manual nature of the brain structure measurements is also a limitation. There are wide variations in sulcal morphology among normal individuals (Cykowski, et al., 2008; Ono & Abernathy, 1990) and arbitrary decisions about structure boundaries are frequently necessary. The techniques used here produce measurements that correlate with various indices of language function in children and adults (Leonard, Eckert, & Kuldau, 2006; Leonard, et al., 2008), but these correlations are not always replicable between samples (Leonard, Eckert, Givens, Berninger, & Eden, 2006). We are currently processing the images in this sample with two different automated techniques (Makris, et al., 2006; Mangin, et al., 2004) in order to study the effect of method choice on asymmetry.
The male and female participants performed quite similarly on most of our reading/word recognition measures. Although female advantages in some verbal tasks have been reported (Maccoby & Jacklin, 1974; but see also Hyde & Linn, 1988), none were observed here. Nor were there any sex differences in response speed. However, in our sample, men performed more accurately in the standardized word identification measure, and in masked word recognition. We do not wish to overinterpret this finding, given the absence of sex differences in our other lexical tasks. Nevertheless, it is worth pointing out that sex differences in “unexpected” directions (i.e., male advantages on verbal tasks) can sometimes be obtained.
With respect to behavioral asymmetry, a composite measure of lateralization across our multiple language tasks produced no evidence for sex differences. Left hemisphere advantages were found in all tasks, regardless of sex. Hence we found no support for the view that women, in general, have a more bilateral language organization than men for word reading tasks. A more limited proposal would hold that sex differences in language organization are specific to only some linguistic processes. For example, there is some neuroimaging (Clements, et al., 2006; Pugh, et al., 1996l; Shaywitz, et al., 1995) and behavioral (Coney, 2002) evidence that women have more bilateral hemispheric participation in making nonword rhyme judgments than men. It is interesting, then, that one of the two tasks obtaining Sex X VF accuracy interactions in the present study was nonword naming. This could indicate that women recruit the right hemisphere more than men for phonological encoding of print. It is less likely that this hypothesis can account for a similar finding in the category generation task. Indeed that finding is particularly puzzling since there was no evidence for sex differences in the similar verb generation task. Accuracy asymmetries for our two semantic generation tasks are moderately correlated (r = .42). Although we know of no other sex differences studies employing category generation, neuroimaging investigations of verbal fluency or verb generation have not found evidence of sex differences (Buckner, Raichle, & Peterson, 1995; Pujol, et al., 1999; Weiss, et al., 2003). Given the absence of sex differences for word naming or verb generation, the hypothesis that only certain processes (phonological encoding, semantic generation) differentiate men and women becomes less tenable. In any case, these sex differences only accounted for approximately 2% of the variance in visual field differences, and the findings were not robust enough to replicate in independent subsamples. Other large-scale investigations have also reported only 1–2% of the variance in behavioral asymmetry attributable to sex differences (Boles, 1995; Hiscock, Inch, Jacek, Hiscock-Kalil, & Kalil, 1994).
Our findings for anatomical asymmetries largely paralleled the behavioral results. Although we found the expected asymmetries in the structures we measured, evidence of sex differences was very weak. This was not unexpected, given the literature. Two large sample studies that used similar methods to examine asymmetries on a voxel by voxel level concluded that sex differences were either absent (Watkins, et al., 2001) or restricted to a small region near Heschl’s gyrus and the planum temporale (Good, et al., 2001). These findings differ notably from those of Kovalev, et al. (2003) who found that men were more asymmetrical in most brain regions. However, it is unclear how the asymmetry differences Kovalev, et al. (2003) report were affected by brain size. In a previous paper on the current sample we found that sex differences were very modest or undetectable when adjustments were made for brain size (Leonard, et al., 2008a).
The Kovalev, et al. findings are also difficult to reconcile with the small and unreliable sex differences in grey and white matter in whole brain analyses. Although Gur, et al. (1999) found that men had greater grey matter asymmetry (leftward) and cerebrospinal fluid asymmetry (rightward) than women, Allen, et al. (2003) and the present study found that men had less grey matter asymmetry than women, while there was no sex difference in cerebral spinal fluid asymmetry. We observed a greater rightward white matter asymmetry for men as compared to women, but this sex difference was very slight. Allen, et al. (2003), in contrast, found similar white matter asymmetries for both sexes. There does not appear to be any replicable sex differences in gray vs white matter asymmetries, and it is unlikely that such global measures relate to language representation.
Studies using morphometric methods to measure Hechl’s gyrus have been equally inconsistent regarding sex differences. In a small post-mortem sample Rademacher, et al. (2001) reported that men were more likely to have larger left Heschl’s gyri. Knaus, et al. (2006) found exactly the opposite – only women had significant asymmetries in Heschl’s gyrus. In our much larger sample, both men and women had leftward asymmetry that did not differ in degree.
Our findings do agree with previous reviews of planum temporale asymmetry that have documented similar asymmetries for men and women (Beaton, 1997; Shapleske, et al. 1999; Sommer, et al., 2008), as well as several other primary investigations (Dos Santos Sequeira, et al., 2006; Luders, et al., 2006; Watkins, et al., 2001). Although fewer studies have examined sex differences in asymmetries of the frontal regions (pars triangularis, pars opercularis), our findings concur with prior reports failing to detect sex differences in these areas (Knaus, et al., 2006, 2007). It seems that apparent sex differences in neuroanatomical asymmetry are neither large, stable, nor replicable.
One potential explanation for the inconsistent findings in the literature regarding sex differences in asymmetry could be greater variation for women, either within or across tasks, or within or across brain regions. However, women in our study were not more variable in their asymmetries than men, either anatomically or behaviorally.
The current investigation also considered sex differences in anatomical-behavioral relations. Here we did obtain some evidence for male-female differences. Replicating our prior report (Chiarello, et al., 2004), we found a positive, albeit small, association between composite RT lexical asymmetries and PT asymmetry in our male participants. – greater RVF/left hemisphere advantages were associated with greater leftward planum asymmetries. However, no such association was obtained for women. This result is similar to that reported by Dos Santos Sequeira, et al. (2006). In that study, a positive correlation was observed for dichotic listening and PT asymmetries, but only for consistent right-handed men. In addition, also similar to our findings, Dos Santos Sequeira et al. report no sex differences in asymmetry of the planum temporale, or their behavioral lateralization measure. This suggests that, although asymmetries (both behavioral and anatomical) may be similar for men and women, the way in which these asymmetries map onto each other may differ. This is an area that should be explored further in large sample studies.
In general, however, our findings echo those of most other studies or meta-analyses in finding little evidence to support the claim of more bilateral language representation in women. The few indications of sex differences in the current report accounted for very little of the individual variation in asymmetry, and/or could not be replicated in independent subsamples. As noted earlier, it would be surprising to obtain substantial evidence for sex differences in asymmetry, given the overwhelming evidence of left hemisphere language specialization obtained over the past 150 years. What then can account for the numerous reports of sex differences in lateralization that have been published, and for the continuing consideration of the sex differences hypothesis (Halpern, et al., 2007)?
Our own review of the behavioral and neuroimaging literature confirms Sommer, et al.’s (2004) finding that most of the studies obtaining sex differences in functional lateralization have much smaller sample sizes than studies that fail to find differences. There are several factors that may contribute to this situation. First, because there are individual differences in the degree and direction of asymmetry (regardless of how it is measured), it is possible that studies with small N’s have obtained “sex differences” which may in fact be due to individual differences that happen to be confounded with sex in smaller samples. Second, editors and authors are rightly conservative about publishing null findings when sample sizes are small. We suspect that many small investigations that do not obtain evidence for sex differences in lateralization, either are not published or the publications do not describe the lack of sex differences (see also discussion by Sommer, et al., 2008). Indeed, the first author’s laboratory has completed numerous language lateralization studies over the past 25 years, each of which had equal numbers of male and female participants, and each of which did not obtain evidence for sex differences. However, this fact was usually not mentioned in the published papers, and hence literature searches on sex differences would not discover these references.
A sampling size explanation might not entirely account for the inconsistent reports about sex differences in neuroanatomical asymmetries, as some large studies have obtained differences (Good, et al., 2001; Kovalev, et al.,) while others have not (current study; Watkins, et al., 2001; Sommer, et al., 2008). However, our impression of this literature is that the data apparently supporting bilateral language substrates in women has been overinterpreted. Studies that find greater asymmetries in women are frequently not mentioned in reviews (e.g., Knaus, et al., 2004; 2006), and conclusions are sometimes drawn from nonsignificant sex differences (e.g., Shapleske, et al., 1999).
We are not arguing against the possibility that there might be true sex differences in language lateralization for some very specific language functions and/or brain subregions. However, greater attention to sample size and replication is needed. The current study, as well as earlier reports (Boles, 1995; Frost, et al., 1999; Hiscock & MacKay, 1985; Shapleske, et al., 1999; Sommer, et al., 2004, 2008; Springer, et al., 1999), provides substantial evidence for what might be termed a “sex similarities” hypothesis (Hyde, 2005) of lateral brain organization. If little or none of the variation in asymmetry can be attributed to sex, this implies that most of the individual variability remains to be explained by other factors. It may be more fruitful to explore alternate dimensions of individual difference with at least as much vigor as heretofore has been devoted to documenting sex differences. However, our data suggest that even when brain structure and behavior appear similar for women and men, one should explore whether the mapping functions between them may differ.
Acknowledgments
This research was supported by NIH grant DC006957. We thank Vanessa Miller and Travellia Tjokro for assistance with data collection and analysis.
Footnotes
Parental education was used as a measure of socioecomonic status, using a 5-point scale (1 = “some high school”; 5 = post-graduate or professional degree).
We contrasted the following measures: percent of correct (POC, Bryden, 1982), laterality coefficient (LC, Birkett, 1977), lambda and zscore transform of lambda (Bryden & Sprott, 1977), and the arcsine transform of the left/right difference score. These scores were calculated for each participant for each of the eight tasks. We then computed the test-retest reliability of each measure across word naming 1 and 2, and the split-half reliability within each task. We also examined the distribution of scores, paying special attention to how each index behaved with slight changes in a visual field score (i.e., an index should not change dramatically due to a change in the accuracy of only a single response). LC and lambda had the lowest reliabilities, and these indices along with the arcsine difference score changed dramatically with small changes in the component scores. The POC measure had the peculiar property that asymmetries were magnified at lower levels of accuracy, relative to higher levels of accuracy, hence “correcting” for overall accuracy in the wrong direction. The lambda z-score measure was selected because it had the highest reliabilities without producing an unusual distribution of asymmetry scores. These data are available from the first author upon request.
These analyses were also conducted on asymmetry scores and the same results were obtained.
If the percentage difference is rounded off to two decimal points, then both men and women had 2% more white matter in the right hemisphere.
References
- Alexander GM, Altemus M, Peterson BS, Wexler BE. Replication of a premenstrual decrease in right-ear advantage on language-related dichotic listening tests of cerebral laterality. Neuropsychologia. 2002;40:1293–1299. doi: 10.1016/s0028-3932(01)00220-2. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WTC. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of the planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, et al. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Archives of Neurology. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Boles DB. A large-sample study of sex differences in functional cerebral lateralization. Journal of Clinical and Experimental Neuropsychology. 2005;27:759–768. doi: 10.1081/13803390590954263. [DOI] [PubMed] [Google Scholar]
- Boles DB, Barth JM, Merrill EC. Asymmetry and performance: Toward a neurodevelopmental theory. Brain and Cognition. 2008;66:124–139. doi: 10.1016/j.bandc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Gates A, Nettleton NC. Bihemispheric involvement in lexical decisions: Handedness and a possible sex difference. Neuropsychologia. 1977;15:277–286. doi: 10.1016/0028-3932(77)90036-7. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Laterality: Functional asymmetry in the normal brain. New York: Academic Press; 1982. [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006 doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Chiarello C. Lateralization of lexical processes in the normal brain: A review of visual half-field research. In: Whitaker HA, editor. Contemporary Reviews in Neuropsychology. New York: Springer-Verlag; 1988. pp. 36–76. [Google Scholar]
- Chiarello C, Kacinik N, Manowitz B, Otto R, Leonard C. Cerebral asymmetries for language: Evidence for structural-behavioral correlations. Neuropsychology. 2004;18:219–231. doi: 10.1037/0894-4105.18.2.219. [DOI] [PubMed] [Google Scholar]
- Clements AM, Rimrodt SL, Abel JR, Blankner JG, Mostofsky SH, Pekar JJ, Denckla MB, Cutting LE. Sex differences in cerebral laterality of language and visuospatial processing. Brain and Language. 2006;98:150–158. doi: 10.1016/j.bandl.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Coney J. Lateral asymmetry in phonological processing: Relating behavioral measures to neuroimaged structures. Brain and Language. 2002;80:355–365. doi: 10.1006/brln.2001.2596. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov PV, Ingham RJ, Ingham JC, Mangin JF, Riviere D, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cerebral Cortex. 2008;18:571–583. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhume VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, Zatorre RJ. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhume VB, Ingham RJ, Mangin JF, Riviere D, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2008;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dos Santos Sequeira S, Woerner W, Walter C, Kreuder F, Leuken U, Westerhausen R, Wittling RA, Schweiger E, Wittling W. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry - A volumetric investigation using structural magnet resonance imaging. Neuropsychologia. 2006;44:622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Possing ET, Binder JR. Uncoupled lefteward asymmetries for planum morphology and functional language processing. Brain and Language. 2006;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather H. Sex differences. In: Beaumont JG, editor. Divided visual field studies of cerebral organization. London: Academic Press; 1982. pp. 148–194. [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex. 1994;5:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes: Evidence from functional MRI. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca’s area: The pars triangularis and pars opercularis. Brain and Language. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Loenard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: Do right- and left-handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in grey and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The science of sex differences in science and mathematics. Psychological Science in the Public Interest. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiervang E, Hugdahl K, Steinmetz H, Stevenson J, Lund A, et al. Planum temporale, planum parietale, and dichotic listening in dyslexia. Neuropsychologia. 2000;38:1704–1713. doi: 10.1016/s0028-3932(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Hellige JB, Bloch MI, Taylor AK. Multitask investigation of individual differences in hemispheric asymmetry. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:176–187. doi: 10.1037//0096-1523.14.2.176. [DOI] [PubMed] [Google Scholar]
- Hellige JB, Taylor KB, Lesmes L, Peterson A. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain and Cognition. 1998;36:158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Hugdahl K. Dichotic listening in the study of auditory laterality. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge: The MIT Press; 2003. pp. 441–476. [Google Scholar]
- Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60:581–592. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Inglis J, Lawson JS. Sex differences in the effects of unilateral brain damage on intelligence. Science. 1981;212:693–695. doi: 10.1126/science.7221560. [DOI] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, Van Valin RD, Jr, Kemmerer DL, Murphy BW, Wack DS. Sex differences in brain regions activated by grammatical and reading tasks. Neuroreport. 1998;9:2803–2807. doi: 10.1097/00001756-199808240-00022. [DOI] [PubMed] [Google Scholar]
- Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. Neuroreport. 1994;5:1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H. Auditory lateralization and planum temporale asymmetry. Neuroreport. 1993;5:169–172. doi: 10.1097/00001756-199311180-00019. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A. The epidemiology of aphasic and cognitive impairment in stroke: Age, sex, aphasia type and laterality differences. Brain. 1981;104:117–128. doi: 10.1093/brain/104.1.117. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Sex-linked differences in anatomy of the perisylvian language cortex: A volumetric MRI study of gray matter volumes. Neuropsychology. 2004;18:738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Variability in perisylvian brain anatomy in healthy adults. Brain and Language. 2006;97:219–232. doi: 10.1016/j.bandl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL. Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex. 2007;43:499–510. doi: 10.1016/s0010-9452(08)70244-2. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E-B, Hennington H. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. NeuroImage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- Lake DA, Bryden MP. Handedness and sex differences in hemispheric asymmetry. Brain and Language. 1976;3:266–282. doi: 10.1016/0093-934x(76)90022-5. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Givens B, Berninger V, Evans G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Kuldau JM. Exploiting human anatomical variability as a link between genome and cognome. Genes, Brain and Behavior. 2006;5 Supplement 1:64–77. doi: 10.1111/j.1601-183X.2006.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: Cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008a doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Kuldau JM, Maron L, Ricciuti N, Mahoney B, Bengston M, et al. Identical risk factors predict cognitive deficit in dyslexia and schizophrenia. Neuropsychology. 2008b;22:147–158. doi: 10.1037/0894-4105.22.2.147. [DOI] [PubMed] [Google Scholar]
- Levene H. In: Contributions to probability and statistics: Essays in honor of Harold Hotelling. Olkin I, et al., editors. Stanford University Press; 1960. pp. 278–292. [Google Scholar]
- Maccoby EE, Jacklin CN. The psychology of sex differences. Stanford University Press; 1974. [Google Scholar]
- Makris N, Kaiser J, Haselgrove C, Seidman LJ, Biederman J, Boriel D, et al. Human cerebral cortex: a system for integration of volume- and surface-based representations. Neuroimage. 2006;33:139–153. doi: 10.1016/j.neuroimage.2006.04.220. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Dushesnay E, Cointepas Y, Papadopoulos-Orfanos D, et al. A framework to study the cortical folding patterns. Neuroimage. 2004;23 Supplement 1:S129–S138. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in the cerebral organization of verbal functions in patients with unilateral brain lesions. Brain. 1977;100:775–793. doi: 10.1093/brain/100.4.775. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of planum temporale and corpus callosum in left-handers with evidence of left and right hemisphere speech representation. Brain. 1998;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Ono M, Jubik S, Abernathy CD. An atlas of cerebral sulci. New York: Thieme; 1990. [Google Scholar]
- Penhume VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cerebral Cortex. 1996;6:661–672. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Morosan P, Schleicher A, Freund HJ, Zilles K. Human primary auditory cortex in women and men. Neuroreport. 2001;12:1561–1565. doi: 10.1097/00001756-200106130-00010. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Conducting judgment studies: Some methodological issues. In: Harrigan JA, Rosenthal R, Scherer KR, editors. The new handbook of methods in nonverbal behavior research. Oxford, England: Oxford University Press; 2005. pp. 199–234. [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC. Sex differences in the functional organization for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS. Sex differences in handedness, asymmetry of the Planum Temporale and functional language lateralization. Brain Research. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Springer SP, Deutsch G. Left brain, right brain: Perspectives from cognitive neuroscience. Fifth Edition. New York: W. Freeman and Company; 1997. [Google Scholar]
- Voyer D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality. 1996;1:51–83. doi: 10.1080/713754209. [DOI] [PubMed] [Google Scholar]
- Wada JJ, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Archives of Neurology. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cerebral Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: A functional magnetic resonance imaging study. Neuroscience Letters. 2003;352:191–194. doi: 10.1016/j.neulet.2003.08.071. [DOI] [PubMed] [Google Scholar]
- Willerman L, Schultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence. Intelligence. 1991;15:223–228. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test-Revised Normative Update (WRMT-R) Circle Pines, MN: American Guidance Service, Inc; 1998. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]