Abstract
Background
Curcumin is a plant-derived dietary spice with various biological activities, including anti-tumoral and anti-inflammatory. Its therapeutic applications have been studied in a variety of conditions, including rheumatoid arthritis, colon cancer and depression; but no studies evaluated the effects of curcumin on periodontal disease in vivo.
Methods
Experimental periodontal disease was induced in rats by placing cotton ligatures around both lower first molars. Curcumin was given to the rats intragastrically daily in two doses (30 and 100 mg/Kg) during 15 days. Control animals received ligatures but only the corn oil vehicle by gavage and no treatment negative control animals were included. Bone resorption was assessed by microcomputer tomography and the inflammatory status was evaluated by stereometric analysis. RT-qPCR and ELISA were used to determine the expression of interleukin (IL)-6, tumor necrosis factor (TNF)-alpha and prostaglandin E2 (PGE2) synthase on the gingival tissues. Modulation of p38 mitogen-activated protein kinase (MAPK) and NK-kB activation was assessed by western blot.
Results
Bone resorption was effectively induced in the experimental period, but it was not affected by either dose of curcumin. Curcumin effectively inhibited cytokine gene expression at mRNA and protein levels and dose-dependently inhibited activation of NF-kB in the gingival tissues. p38 MAPK activation was not inhibited by curcumin. Curcumin-treated animals also presented a marked reduction on the inflammatory cell infiltrate and increased collagen content and fibroblastic cell numbers.
Conclusions
Curcumin did not prevent alveolar bone resorption, but its potent anti-inflammatory effect suggests it may have a therapeutic potential in periodontal diseases.
Keywords: Curcumin, inflammation, periodontal disease, NF-kB, p38 MAPK
Introduction
Phytochemicals are naturally occurring substances found in plants. There has been considerable public and scientific interest in the use of phytochemicals derived from dietary components to combat human diseases, especially the two major causes of death in the developed world: cardiovascular diseases and cancer (1). Curcumin (diferuloymethane), is one such phytochemical, which is a major constituent of the yellow spice turmeric derived from the rhizomes of Curcuma spp. (2).
Curcumin has potent anti-inflammatory, anti-carcinogenic and antioxidant activities, and a number of pre-clinical trials have been conducted to assess its therapeutic potential (3–5). Following oral administration, curcumin has been shown to prevent cancer in the colon, skin, stomach, liver, lung, duodenum, soft palate and breasts of rodents (6, 7). In a rat model of acute ulcers, curcumin potently attenuates the ulcer activity by preventing glutathione depletion, lipid peroxidation, and protein oxidation. Both, oral and intraperitoneal administration of curcumin blocked gastric ulceration in a dose-dependent manner (8). In animal studies of arthritis, oral administration of curcumin decreased the levels of inflammatory glyprotein, Gp A72, with a reduction in inflammatory response in the paws (9).
The anti-inflammatory properties of curcumin seem are mediated by the modulating the activity of signaling pathways and transcription factors, especially NF-KB, AP-1 and MAPKinases (10). Down regulation of the activation of NF-kB and MAPKinases by curcumin suppresses the expression of IL-6, IL-1β, TNF-α, MMP-2 and MMP-9 in the late phase of experimental acute pancreatitis (11), in the modulation of arthritis (12–15) in the prevention and healing of indomethacin-induced gastric ulcer (8), in the treatment of inflammatory bowel disease and Crohn’s disease (16–18).
Periodontal disease initiation and progression occurs as a consequence of the host response to microorganisms of the dental biofilm. Besides their role as stimulants of the host response, periodontal pathogens release harmful by-products and enzymes that break down extracellular matrix components, such as collagen, as well as host cell membranes. Once the host response is initiated, various inflammatory molecules, such as cytokines and prostaglandins are released from leukocytes, fibroblasts or other host tissue-derived cells (19–21). Chronic periodontal inflammation perpetuates and amplifies itself through numerous autocrine and paracrine regulatory loops of the inflammatory mediators, acting on cells within the periodontal microenvironment. An improper immune response leads to overproduction of inflammatory cytokines and consequently periodontal attachment loss and bone resorption. Host modulation therapeutic strategies aimed at inhibition of the progression of inflammatory bone loss associated with periodontitis include the blockage of inflammatory cytokines. Recently, the cell signaling pathways that regulate the expression of inflammatory mediators have become promising therapeutic targets (19).
Although a range of biological and pharmacological activities of curcumin have been reported, its therapeutic potential for destructive periodontal disease is poorly understood. To the best of our knowledge, this is the first study evaluating the effect of curcumin on the modulation of periodontal disease in vivo.
Besides its anti-inflammatory properties, curcumin was also shown to improve wound healing by increasing collagen deposition, angiogenesis and the density of fibroblasts, reducing the radiation-induced delay in wound repair (22). Interestingly, curcumin-treated wounds presented not only a greater number of fibroblasts but also more infiltrating macrophages and neutrophils compared to untreated wounds (23, 24). These studies demonstrated that treatment with curcumin resulted in faster closure of wound, better regulation of granulation tissue formation and induction of growth factors (24), all features that can be extremely useful in the setting of periodontal disease.
These findings prompted us to investigate the effect of systemically administered curcumin on the inflammatory response during the course of ligature-induced periodontal disease in rats. The present study was undertaken to determine whether curcumin could inhibit connective tissue breakdown in ligature-induced periodontitis in rats. To obtain greater insight into the anti-inflammatory effects of curcumin, we assessed the modulation of signaling pathways (p38 MAPK and NF-kB) and the expression of IL-6, COX-2 and TNF-α in the periodontal tissues.
Materials and methods
Experimental design
All the experimental protocols were approved by the Ethical Committee for Animal Experimentation (CEEA) of the School of Dentistry at Araraquara – UNESP and performed in accordance with the guidelines from the Brazilian College for Animal Experimentation (COBEA).
Sixty male Holtzman rats (Rattus norvegicus albinus Holtzman), weighing between 100 and 200 g, were randomly distributed into the following six experimental groups comprising 10 animals each: 1) vehicle control (no ligatures), 2) vehicle PD (with ligatures), 3) curcumin 30 mg/Kg control (no ligatures), 4) curcumin 30 mg/Kg PD (with ligatures), 5) curcumin 100 mg/Kg control (no ligatures) and 6) curcumin 100 mg/Kg PD (with ligatures). The rats were kept in a room with controlled temperature (21 ± 1°C) and humidity (65–70%) and a 12 h–12 h light–dark cycle. Animals were fed standard rat chow and water ad libitum. To induce periodontitis, three groups of rats were anesthetized by intramuscular administration of ketamine (Francotar, Virbac of Brazil Ind. and Com. Ltd, São Paulo, Brazil; 80 mg/kg body weight) and xylazine (Virbaxil, Virbac of Brazil Ind. And Com. Ltd, São Paulo, Brazil; 20 mg/kg body weight), and cotton threads were tied around the first molars bilaterally.
Administration of curcumin started the day before the placement of ligatures for the induction of periodontal disease. Curcumin was administered daily intragastrically by gavage in two different doses: one group received the lower dose of 30 mg/Kg/body weight, and the other group received 100 mg/Kg/body/weight. Control animals were given the same volume of the corn oil vehicle. Fifteen days after the placement of ligatures the animals were sacrificed and the mandibular jaws were hemisected. This period was selected based on a previous publication (25) demonstrating that 15 days corresponds to the peak of inflammatory severity in this experimental model. Eight block sections including 1st and 2nd molars with their surrounding tissues were submitted to routine histological processing for descriptive and stereometric evaluation, whereas in the remaining block sections the soft tissues surrounding the teeth and overlying the bone from other blocks were carefully dissected and used for the extraction of total RNA and protein.
These tissue blocks were immersed directly in 10% buffered formalin fixative solution for 48 h and decalcified in tetrasodium-EDTA aqueous solution (0.5 M, pH 7.4) during 2–3 months, under agitation at room temperature. Each specimen consisted of a section containing the 1st and 2nd molars and their surrounding alveolar process and was included in paraffin blocks. Serial 4 μM sections were obtained in the bucco-lingual direction and stained with hematoxylin-eosin (HE).
Stereometry
The analysis was conducted by a single examiner that was blind to the experimental groups using an optical microscope (Diastar Cambridge) set at 200 X magnification. Semi-serial sections of 4 μM were obtained from the tissue blocks on a buccal-lingual orientation. A total of 3 sections, spaced 100 μM from each other, were evaluated per tooth. A 50 × 50 μM grid was overlayed the histological images allowing the analysis of an ‘area of interest’ of 2,500 μM2. Two grids composed of 5 × 5 10 μM squares were used in each histological image: one was positioned with its lower border 25 μM below the top of the alveolar bone crest and perpendicularly to the root surface, and the other was positioned with its upper border at the base of the junctional epithelium, representing the ‘bone crest’ and ‘submarginal’ areas, respectively. In both cases, the lateral border of the grids was always positioned over the most prominent part of the root surface in the area. Each one of the 25 points of the grid projected on each section was counted and the proportion of collagen, fibroblastic cells and inflammatory cells (distinguished by the morphological characteristics) in the area of interest was determined as percentage of the total points counted. A total of three images obtained from equally spaced slides (spanning 900 uM of the buccal-lingual aspect of the molars) were evaluated from each animal. Slides from at least three different animals in each experimental group were used.
Micro computed tomography and bone volume fraction analysis (μCT)
After dissection of the soft tissues used for RT-qPCR and western blot experiments, the mandibles were tumbled in 10% formalin at 4°C overnight, transferred to a 70% ethanol solution and stored at 4°C. Specimens were scanned using micro-computed tomography (μCT) with 18 μm-thick sections at the Orthopaedic Research Laboratories of the University of Michigan (Ann Arbor, MI, USA). After 3D reconstruction, the results were analyzed using GE Microview software to quantify bone volume fraction. In each scan, a tridimensional standardized region of interest (ROI) was defined by the following landmarks: the apex of the distal root of the 1st molar (apical limit), bottom of the furcation of 1st molar (coronal limit), most distal aspect of mesial root of the 2nd molar (posterior limit), most mesial aspect of the mesial root of the 1st molar (anterior limit). The analysis of bone volume fraction (BVF) was calculated with a threshold of 1621 arbitrary units for the detection of mineralized tissue within the ROI.
Evaluation of cytokine gene expression at the mRNA level (RT-qPCR)
Total RNA was extracted from tissue samples using Trizol reagent (Invitrogen Corp) according to the manufacturer’s instructions. Concentration of RNA was determined by spectrophotometry and 700 ng of total RNA were reverse-transcribed into cDNA using random hexamers as primers (High Capacity cDNA synthesis kit, Applied Biosystems). The relative abundance of the transcripts of the candidate inflammatory genes were measured by real-time reverse transcription-PCR (RT-PCR) using Taqman chemistry and pre-designed sets of primers and probes (TaqMan Gene Expression Assays, Applied Biosystems) on a StepOne Plus Real-Time PCR System (Applied Biosystems). The reactions were carried out in a 96-well plate on a final reaction volume of 30 μL that included Taqman Universal PCR Master Mix (Applied Biosystems), Taqman Gene Expression Assays (Applied Biosystems) for each target gene: TNF-α (tumor necrosis factor alpha), NM_013693; IL-6 (interleukin 6), NM031168; Ptgs-2 (prostaglandin-endoperoxide synthase-2), NM_011198; Gapdh (glyceraldehyde-3-phosphate dehydrogenase), NM_008084; and cDNA template (corresponding to 30 ng of cDNA). Optimized thermal cycling conditions were: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. For each sample, analyses of gene expression were performed in duplicate. The experiments were with samples isolated from at least three different animals in each experimental group. To normalize the amount of mRNA present in each reaction, the expression of GAPDH, which was not altered by the experimental conditions, was used as a housekeeping gene. To compare the expression levels among different samples, the relative expression level of the genes was calculated using the comparative ΔCT method using the thermocycler’s software.
Activation of signaling pathways (Western-blot) and determination of cytokine gene expression at the protein level (ELISA)
Total proteins were extracted from gingival tissue samples using a detergent-based extraction buffer (T-PER, Tissue Protein Extraction Reagent – Pierce Biotechnology) containing a protease inhibitor cocktail (Protein Stabilizing Cocktail – Santa Cruz Biotechnology) according to manufacturer’s instructions (Pierce Biotechnology). The tissue samples were macerated in the buffer (50 μL/mg of tissue) and centrifuged for 5 min at 13,000 RPM at 4°C. The proteins were quantified using Lowry method (DC assay, Bio-Rad Laboratories) and 40 μg of total protein were added to a SDS sample buffer containing 2% SDS, DTT as a reducing agent, glycerol and bromophenol blue dye (Cell Signaling), heated-denaturated at 97° C for 5 min and chilled on ice of 5 min before loading on 10% SDS-polyacrylamide gels.
Electrophoresis on discontinuous acrylamide gels were carried out at 100 V for 90 min and subsequently electro-transferred to 0.2 μM nitrocellulose membranes using 300 mA constant current for 1 hour. The membranes were blocked for 1h in Tris-buffered saline containing 5% non-fat dry milk and 0.1% Tween-20 and subsequently washed for 5 min (3 times) with TBS-0.1% Tween at room temperature. The membranes were then incubated with primary antibodies overnight at 4°C (1:100 dilution in PBS – phospho-p65 and phospho-p38 – Cell signaling). Membranes were washed in TBS-T buffer for 5 minutes (3 times) and incubated with secondary antibodies conjugated to horseradish peroxidase (1:1000 dilution in the blocking buffer) for 1 hour at room temperature, washed again with TBS-T buffer (5 min, 3 times). Detection of bands was carried out on radiographic film by using a chemiluminescence system (Lumi-Glo, Cell Signaling).
Proteins from these same samples were also used to in the ELISA assays to determine the concentration of PGE2, IL-6 and TNF-α. These assays were performed according to the manufacturer’s instructions (R&D Systems) and the results were normalized to the total concentration of protein in the samples. Samples from at least three different animals in each experimental group were used and assayed in duplicate.
Data analysis
The purpose of data analysis was to compare the results from curcumin-treated animals with those of vehicle treated control animals. Considering the two experimental groups treated with different doses of curcumin (30 and 100 mg/kg body weight) as independent variables, we used non-paired t-tests for the comparisons between all the groups (control × 30 mg/Kg of curcumin, control × 100 mg/Kg of curcumin and 30 × 100 mg/Kg of curcumin) with a significance level of 5%.
Results
Alveolar bone loss associated with ligature-induced periodontal disease is not prevented by curcumin administration
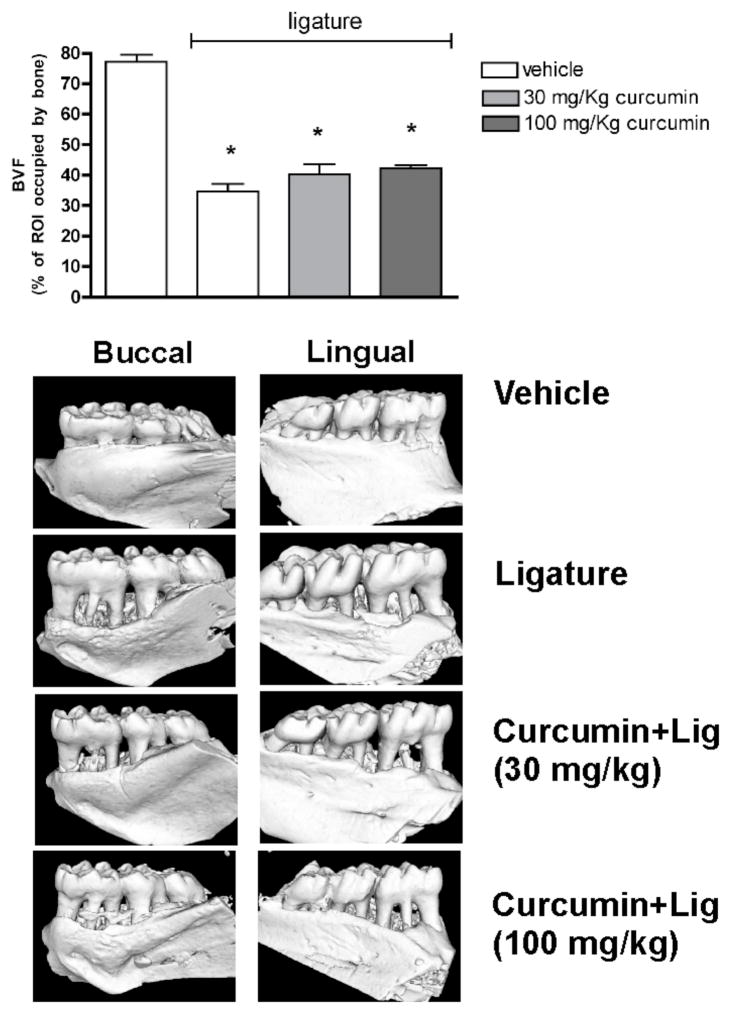
On the tridimensional reconstructions, the fraction of the region of interest (ROI) that is occupied by bone tissue (BVF) was significantly reduced (>50% reduction of BVF in comparison with control and vehicle-treated animals without the ligatures, p<0.05) in the vehicle-treated animals, indicating that the ligatures induced alveolar bone resorption in the 15-day experimental period. The administration of curcumin had no effect on the extent of alveolar bone resorption, since animals treated with both doses of curcumin still presented a significant decrease on BVF in comparison with control animals without ligatures and no differences were detected in comparison with the BVF of vehicle-treated animals with ligatures (Fig. 1). Administration of curcumin or of the corn oil vehicle had no effect on alveolar bone in the absence of ligature-induced periodontal disease.
Figure 1.
μCT analysis of mandible specimens obtained from the experimental animals shows that neither dose of intragastrically-administered curcumin prevented alveolar bone loss in the ligature-model of periodontal disease. The tridimensional images are representative of 8 samples in each group (4 animals/group) and depict the significant resorption of alveolar bone on both the buccal and lingual aspects of the first molars. The bar graphs represent the quantification of the area occupied by bone tissue in a standardized region of interest (ROI) defined by anatomic landarks including the first molar and the interproximal area between first and second molars. Bars represent means and vertical lines standard deviations of the percentage of the ROI occupied by bone tissue. *indicates a significant (p<0.05) difference in comparison with negative control specimens from animals treated with vehicle and without ligatures.
Curcumin inhibits the ligature-induced inflammatory process and enhances the deposition of collagen
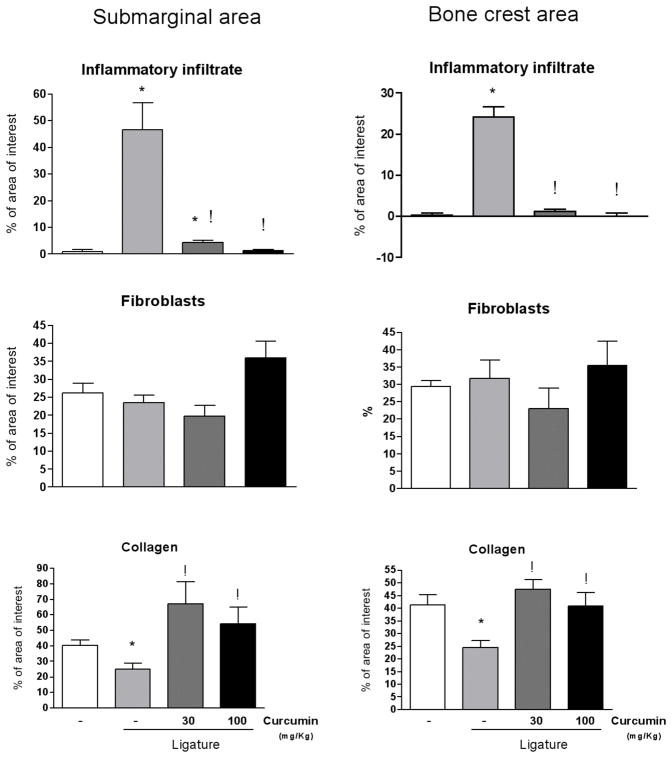
A stereometric analysis was performed to correlate the information on the effects of curcumin on the activation of signaling pathways and on the expression of pro-inflammatory cytokines with the overall modulation of the inflammatory status in the periodontally diseased tissues. Ligature-induced periodontitis induced a clear and obvious inflammatory process, characterized by increased cell and vascular densities and reduced collagen content. Administration of curcumin had a suppressive effect on the inflammation, as evidenced by the absence of inflammatory cells in the submarginal and bone crest areas (Figs. 2 and 3). Collagen content in the tissues was also increased in curcumin-treated animals, which may be due to the inhibition of inflammation and, consequently, of the collagen degradation associated (Figs. 2 and 3). These results indicate a clear correlation between the histological inflammation status and the expression of pro-inflammatory mediators.
Figure 2.
Stereometry analysis of the histological characteristics of gingival tissues subjected to ligature-induced periodontal disease according to the dose of curcumin (30 or 100 mg/Kg) administered for 15 days intragastrically. Control animals were administered vehicle and negative control animals also were given vehicle but no ligature was placed around the first molars. Two 50 × 50 μM grids were overlayed on histological images captured from H/E stained slides at 200 X magnification, allowing the analysis of two ‘areas of interest’ of 2,500 μm2 each: one was positioned 25 μM below the top of the alveolar bone crest, and another at the base of the junctional epithelium, representing the ‘bone crest’ and ‘submarginal’ areas, respectively. The proportion of collagen, fibroblastic cells and inflammatory cells (distinguished by the morphological characteristics) in the area of interest was determined. A total of three images obtained from equally spaced slides (spanning 900 uM of the buccal-lingual aspect of the molars) were evaluated from each animal. Slides from at least three different animals in each experimental group were used. Both doses of curcumin markedly reduced the inflammatory infiltrate in periodontally-diseased tissues. The lower dose of curcumin (30 mg/Kg) induced a greater increase in the collagen content, whereas the higher dose (100 mg/Kg) was associated with increased proliferation of fibroblastic cells. Bars indicate averages and vertical lines the standard deviations. Student’s t-test was used for pairwise comparison and (*) indicates a significant (p<0.05) difference in comparison with healthy (no ligature) control, whereas (!) indicates significant difference (p<0.05) between curcumin-treated and vehicle-treated in periodontal disease (ligature) sites.
Figure 3.
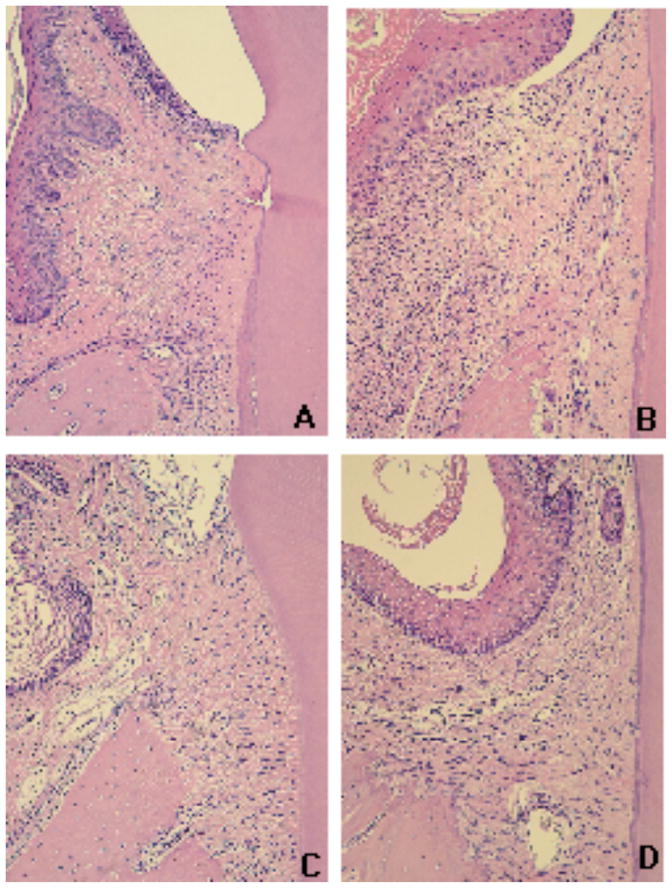
Histological aspect of the gingival tissues according to the experimental group (control X ligature) and administration of curcumin (vehicle, 30 mg/Kg and 100 mg/Kg). Tissues were harvested 15 days after ligatures were placed and administration of curcumin was started. Semi-serial sections with 5 μM thickness were routinely processed and stained with hematoxylin and eosin. A total of three sections spaced 100 μM were evaluated for each first molar in a minimum of four animals in each experimental group. An epithelial layer of regular thickness, dense connective tissue with reduced number of cellular infiltrate and a smooth bone crest surface characterize the gingival tissues of control (non-ligated) animals (A). Placement of ligatures (B) produced an increase of the thickness of the epithelium layer, an intense cellular infiltrate and irregular bone crest surface with the presence of multinucleated osteoclast-like cells; whereas in animals treated with 30 mg/Kg (C) and 100 mg/Kg (D) of curcumin, the epithelial layer presents normal thickness and reduced cellular infiltrate. All images were obtained at 100 X magnification.
Curcumin inhibits ligature-induced activation of NF-kB, but not of p38
Since NF-kB is considered a prime target of curcumin and also based on the relevance of both NF-kB and MAPKinase signaling pathways for inflammatory cytokine expression, we next evaluated the modulation of these pathways in periodontally diseased tissues by orally administered curcumin.
The placement of ligature elicited the inflammatory process and increased activation of NF-κB. Administration of curcumin produced a marked, dose-dependent inhibition of NF-kB activation in periodontally-diseased tissues. Surprisingly, activation of p38 MAPK was reduced in periodontally-diseased tissues. The lower dose of curcumin (30 mg/Kg) did not change the reduced activation status of p38 MAPK associated with ligature-induced periodontal disease, whereas the higher dose (100 mg/Kg) restored the activation status observed in healthy periodontal tissues. (Fig 4).
Figure 4.
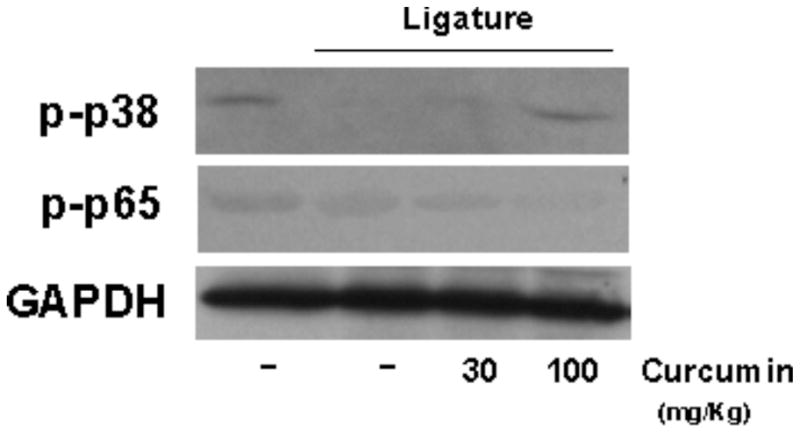
Modulation of NF-kB and p38 MAPK activation in gingival tissues by systemic administration of curcumin. The animals were sacrificed after 15 days of curcumin (30 and 100 mg/Kg) or vehicle administration and the tissues surrounding the lower first molars were harvested. Total protein was isolated from gingival biopsies with a detergent-containing buffer (T-Per, Pierce) supplemented with protease and phosphatase inhibitor cocktails (Complete and Phos-Stop, Roche). Protein concentration was determined with a Lowry-based microassay (DC assay, Bio-Rad) and 60 μg of each sample were diluted in LDS-containing running buffer supplemented with DTT, heat-denatured, electrophoresed in 10% Tris-Acrilamide gels and transferred to 0.2 μM nitrocellulose membranes in a semi-dry transfer apparatus. The activation of the signaling pathways was assessed by the detection of phosphorylated forms of p65 (NF-kB) and p38 MAPKinase. Expression levels of constitutive housekeeping GAPDH are shown to confirm equal protein loading. The images are representative of samples from three different animals in each experimental group.
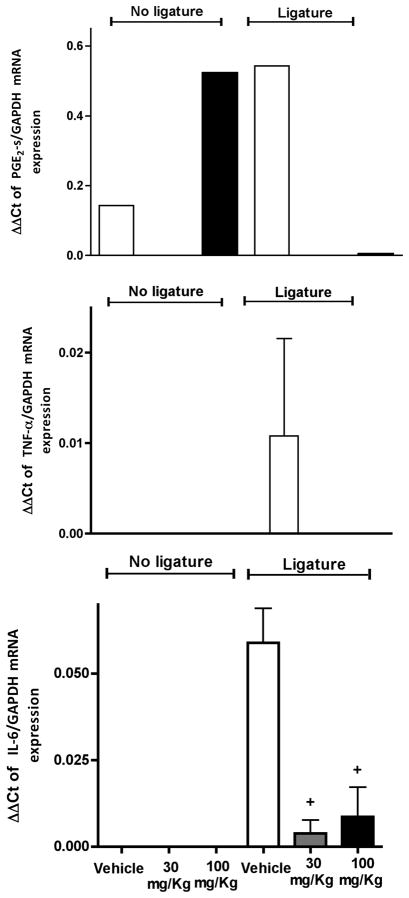
Curcumin completely abrogates PGE2-synthase (murine Cox-2 analog) mRNA expression in ligature-induced periodontal disease
To investigate the effect of curcumin on ligature-induced inflammatory gene expression the total RNA and protein were extracted from gingival tissues surrounding the lower first molars of rats with or without ligature placement. These samples were used for RT-qPCR and ELISA experiments, respectively. Interestingly, animals treated with the higher dose of curcumin (100 mg/Kg) presented a marked increase on PGE2-s mRNA levels in comparison to control animals. Ligature placement increased PGE2-s mRNA in the tissues, but treatment with curcumin completely inhibited this increase (Fig. 5). We could not detect PGE2 expression at the protein level in our samples, probably due to the reduced level of expression.
Figure 5.
Curcumin inhibits cytokine gene expression in experimental periodontitis. In the absence of periodontal disease, only PGE2-synthase mRNA was significantly induced by the higher dose of curcumin in the absence of ligature-induced periodontal disease. The animals were treated with 30 or 100 mg/Kg of curcumin by oral gavage daily for 15 days. Control animals received the same volume of the vehicle by oral gavage. Cotton ligatures were placed around the first molars of rats bilaterally to induce periodontal disease. After 15 days, the animals were sacrificed and total RNA was isolated from gingival biopsies with (‘Ligatures’) and without (‘No ligatures’) periodontal disease and used for RT-qPCR performed with pre-designed primers and probes for the indicated target genes and to the housekeeping GAPDH using TaqMan reagents. The results were analyzed by the delta-Ct method and expression of target genes was normalized to GAPDH expression. (+) indicates significant reduction (p< 0.05) in comparison to vehicle control with ligatures. Bars indicate means and vertical lines standard error of mean of at least three animals in each experimental group, except for PGE2-s mRNA analysis in which the samples of three animals in each experimental group were pooled to enable detection and there are no error bars.
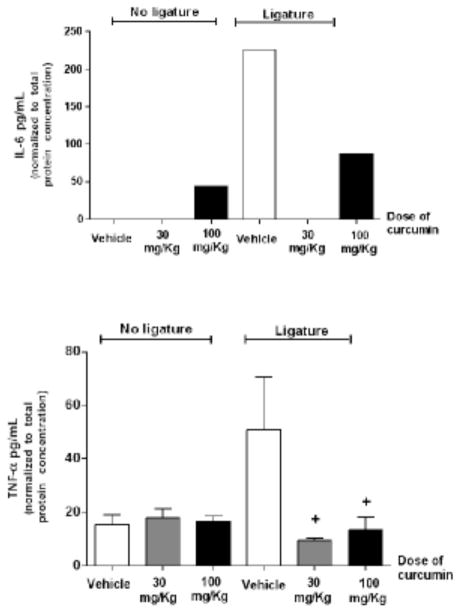
Curcumin effectively inhibits IL-6 and TNF-α gene expression
At protein level, the treatment with 100 mg/Kg of curcumin induced IL-6 protein expression in non-diseased periodontal tissues; however, there was a dose-independent and significant inhibition of IL-6 mRNA (Fig. 5) and protein (Fig. 6) expression in periodontally-diseased tissues of animals treated with both doses of curcumin. Interestingly, greater inhibition of IL-6 was associated with the lower dose (30 mg/Kg) of curcumin. TNF-alpha mRNA expression in ligature-induced periodontal disease was completely inhibited by curcumin (Fig. 5). Both doses of curcumin inhibited TNFα protein expression in periodontal tissues surrounding ligated teeth; however similarly to the regulation of IL-6 this inhibition reached statistical significance only for the 30 mg/Kg dose (Fig 6).
Figure 6.
Effective inhibition of both IL-6 and TNF-α production in periodontally diseased tissues by curcumin. In the absence of ligatures, the higher dose of curcumin was associated to increased production of IL-6, whereas TNF-α production was not affected. Gingival tissues around both lower first molars with (‘Ligature’) and without (‘No ligature’) experimentally induced periodontal disease were collected after 15 days of administration of curcumin (30 or 100 mg/Kg) by oral gavage. Control animals received the same volume of vehicle. Total protein was extracted and used in ELISA tests to quantify the expression of the target cytokines. These tests were performed according to the manufacturer’s instructions and the results for each sample were normalized to the concentration of total proteins determined by a Lowry-based microassay (DC assay, Bio-Rad). (+) indicates significant difference (p< 0.05) in comparison to vehicle control in animals with ligature-induced periodontitis. Bars indicate means and vertical lines standard error of mean of at least three animals in each experimental group. IL-6 concentration was determined in pooled samples from three animals in each experimental group due to the low level of expression, and the bar indicate the average of the triplicate measurement from these pooled samples.
Discussion
In this report, we investigated the anti-inflammatory effect of curcumin in ligature-induced periodontitis in rats. Curcumin did not affect body weight or the behavior of the experimental animals in comparison to vehicle-treated controls. No animal died during the experimental period, suggesting the lack of severe adverse effects associated with curcumin administration, which is supported by studies using similar or higher doses of curcumin in other rat models that did not observe any changes in lymph nodes, pancreas, liver, kidneys or lungs (11, 26).
The current study, for the first time, demonstrated that intragastrically administered curcumin effectively reduces inflammation and connective tissue breakdown in this experimental periodontitis model. Moreover, we provide evidence that this effect might be explained, at least in part, by the inhibition of IL-6, PGE-2 and TNF-α expression, as a result of the modulation of NF-κB activation. Surprisingly, however, we did not observe any effect of curcumin administration on alveolar bone resorption.
The relevance of these cytokines is supported by studies showing that these cytokines have all been found to be significantly elevated in diseased periodontal sites compared with healthy or inactive sites (27–33) and have been positively correlated with increased probing depth and attachment loss (19, 30, 32).
Recent studies have found that curcumin have the ability of suppress NF-κB (1, 34–37) and MAP kinases (1, 34, 37) thus reducing the production of inflammatory cytokines such as IL-6 (11, 26, 34, 36), TNF-α (11, 26, 34, 38) and PGE-2 (37). Anti-inflammatory properties of curcumin protected the liver of rats against injury caused by carbon tetrachloride (CCL4) by dramatically suppressing serum levels of TNF-α, IL-6 and IFN-γ. The doses of daily doses of curcumin used by these authors were higher than the doses used in our study, but the authors did not observe a dose-dependent effect. This may be related to the different experimental models or the maximum effective dose may have been reached. Curcumin has also been shown to possess beneficial effects in alleviating arthritic symptoms in both animal and human models (39). Indeed, there are clinical trials for the treatment of patients with rheumatoid arthritis and osteoarthritis with high doses of curcumin (40). Due to their nontoxic and antimutagenic (41) nature, curcumin and other spice-derived principles hold promise in the treatment of arthritis. In animals models, carrageenan-induced paw inflammation is reduced in rats fed with curcumin at 30 mg/kg body weight (9). Histological changes including infiltration of immune cells, synovial hyperplasia, cartilage destruction, and bone erosion in the hind paw sections were extensively suppressed by oral administration of curcumin (4 –100 mg/kg) in a dose-dependent manner (13). MMP-1 and MMP-3, critical factors in the degradation of joint cartilages, whose levels were prominently increased in the vehicle-treatment group, were also reduced by curcumin. In regard to the modulation of intracellular signaling pathways, activation of JNK, but not ERK and p38 MAPK, was inhibited in a dose-dependent manner (13).
Although many studies (42–46) have investigated the effect of curcumin on modulation of different signaling pathways and transcription factors such as AP-1 and MAP kinases, the best-characterized and studied molecular target of curcumin is NF-κB. Inhibition of NF-κB may be one of main mechanisms for the anti-inflammatory effects of curcumin. In the present study, we demonstrated that curcumin inhibited the activation of NF-κB in ligature-induced periodontal disease in rats. Nevertheless, it is possible that the inhibition of pro-inflammatory gene expression by curcumin is partially dependent on the modulation of signaling pathways other than NF-κB. Even though we have not observed modulation of p38 MAPK, another signaling pathway involved in the expression of pro-inflammatory cytokines and bone resorption (47–53), it is important to bear in mind that our data is derived from protein from the multiple cell types present in the periodontal microenvironment. Curcumin may have differential effects on signaling pathways according to the cell type, e.g., macrophages, lymphocytes, fibroblasts and osteoblasts.
Besides its anti-inflammatory properties, curcumin is also investigated for its therapeutic potential in cancer, as an angiogenesis inhibitor in the treatment of corneal diseases (54) and in wound healing. Recent studies show that topical application of curcumin, incorporated in collagen vehicle accelerated healing in experimental surgical wounds of 2 cm2, as indicated by enhanced cell proliferation and improved free radical scavenging in comparison with the wounds of negative control and collagen vehicle-treated rats (55). In agreement with these reports, we observed a more organized and higher collagen content in periodontally diseased tissues of animals treated with both doses of curcumin in comparison to the tissues of the vehicle control group. Importantly, we also evaluated intragastric administration of curcumin in the LPS model of experimental periodontal disease, which we have previously shown to present important differences in comparison with the ligature model used in this study (25), and observed very similar results (Guimaraes et al., in press), with a dose-independent inhibition of inflammatory cytokine gene expression, NF-kB activation and collagen degradation. This demonstrates the consistency of curcumin as an anti-inflammatory substance; however the lack of inhibition of bone resorption was also consistent in the LPS model of experimental periodontal disease (Guimaraes et al., in press). We speculate that this lack of effect on bone resorption may be related with a time-delay to reach levels high enough for the biological effects of curcumin, since bone loss in the ligature model has been recently shown to occur already three days after placement of ligatures and stabilizes after the initial 11 days (56). These results are in contradiction with in vitro studies demonstrating that curcumin inhibits RANKL-induced osteoclastogenesis (57, 58) and in striking contrast with the inhibition of bone resorption assessed in the distal femur in rats with experimentally-induced type 1 diabetes (59). We could not compare the dose of curcumin in this study, since the authors added curcumin directly to the rat chow and even though there is no objective quantification of the dose of curcumin, it was estimated in 120 mg/day (59), which is almost three times higher than our 100 mg/Kg/day (or approximately 45 mg/day in a 450 g rat). The discrepancies may also be related with peculiarities of each experimental model, but interestingly these authors did not find regulation of RANKL mRNA expression by curcumin (59), similarly to what we have observed at the protein level by immunohistochemistry in the diseased gingival tissues (data not shown). Thus, it is possible that the direct effects of curcumin on osteoclast precursor cells inhibiting osteoclastogenesis are offset in vivo by the lack of regulation of RANKL expression by stromal and immune cells. The modulatory effects of curcumin on alveolar bone turnover will be addressed in future studies.
Curcumin has a long history as a traditional herbal medicine and has established anti-inflammatory properties in animals following systemic administration (38, 60, 61). This compound is particularly interesting for therapeutic applications because its anti-inflammatory and anti-proliferative effects are potent, occurring at micromolar concentrations. Moreover, clinical trials have shown no significant toxicity even when administered at 8 g per day (40). Although there are no reports of curcumin-induced toxicity associated with systemic administration, topical application may not only increase the potency of its effects but also prevent possible unwanted secondary effects in conditions associated with local chronic inflammation, such as periodontal diseases and rheumatoid arthritis. It is important to understand this as a proof-of-principle type of study in which the potential of curcumin to modulate inflammation associated with periodontal disease induced by ligatures in vivo was assessed for the first time. There are a number of important aspects to be considered that were not addressed in this initial study, such as the pharmacokinetics of curcumin, serum levels, alternative routes of administration (e.g., topical), vehicle used and its use with a therapeutic (i.e., after periodontal disease is established) or preventive (i.e., before induction of periodontal disease) emphasis. In summary, our results confirm and extend our prior in vitro observations (not shown), as well as our in vivo observations in a different experimental model of periodontal disease (Guimaraes et al., in press) and suggest that curcumin is be a candidate as an anti-inflammatory therapeutic strategy for periodontal diseases.
Acknowledgments
Financial support was provided by Brazilian Federal Government through the National Council for Scientific and Technological Development (CNPq) and Coordination for Improvement of Higher Education Personnel (CAPES, #4638-05), and by the National Institutes of Health – National Institute of Dental and Craniofacial Research (1R01DE018290) and National Center for Research Resources (P20RR017696). The authors are also very grateful to the laboratory technicians Jose Antonio Sampaio Zuanon (Dept. of Physiology and Pathology, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista, UNESP) and Ana Claudia Gregolin Costa Miranda (Dept. of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista, UNESP) for their collaboration with the histological processing and to Steven A. Goldstein, PhD (Director, Orthopaedic Research Laboratories, University of Michigan, Ann Arbor, MI) and Jaclynn Mary Kreider (Associate Research Laboratory Technician, Orthopaedic Research Laboratories, University of Michigan, Ann Arbor, MI) for their collaboration on the microcomputer tomography analysis.
Contributor Information
Morgana R. Guimarães, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Leila S. Coimbra, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Sabrina Garcia de Aquino, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Luis C. Spolidorio, Department of Physiology and Pathology, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Keith L. Kirkwood, Department of Craniofacial Biology, College of Dental Medicine – Medical University of South Carolina (MUSC), Charleston, SC, USA.
Carlos Rossa Junior, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
References
- 1.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 3.Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. British journal of pharmacology. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 5.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer letters. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 7.Anonymous. Clinical development plan: curcumin. J Cell Biochem Suppl. 1996;26:72–85. [PubMed] [Google Scholar]
- 8.Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. The Journal of biological chemistry. 2005;280:9409–9415. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 9.Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Molecular and cellular biochemistry. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- 10.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 11.Gulcubuk A, Altunatmaz K, Sonmez K, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. Journal of veterinary medicine. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 10:605–610. doi: 10.1016/j.intimp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Mun SH, Kim HS, Kim JW, et al. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: inhibition of the PKCdelta/JNK/c-Jun pathway. J Pharmacol Sci. 2009;111:13–21. doi: 10.1254/jphs.09134fp. [DOI] [PubMed] [Google Scholar]
- 14.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 15.Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899–908. doi: 10.1007/s00011-009-0063-1. [DOI] [PubMed] [Google Scholar]
- 16.Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 103:824–832. doi: 10.1017/S0007114509992510. [DOI] [PubMed] [Google Scholar]
- 17.Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 18.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Digestive diseases and sciences. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves DT, Oskoui M, Volejnikova S, et al. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. Journal of dental research. 2001;80:1875–1879. doi: 10.1177/00220345010800100301. [DOI] [PubMed] [Google Scholar]
- 21.Graves DT, Jiang Y, Valente AJ. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Front Biosci. 1999;4:D571–580. doi: 10.2741/graves. [DOI] [PubMed] [Google Scholar]
- 22.Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody gamma-irradiation. Plast Reconstr Surg. 2005;115:515–528. doi: 10.1097/01.prs.0000148372.75342.d9. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life sciences. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu GS, Singh AK, Thaloor D, et al. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–177. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia de Aquino S, Manzolli Leite FR, Stach-Machado DR, Francisco da Silva JA, Spolidorio LC, Rossa C., Jr Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life sciences. 2009;84:745–754. doi: 10.1016/j.lfs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Molecular pharmacology. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 27.Lee HJ, Kang IK, Chung CP, Choi SM. The subgingival microflora and gingival crevicular fluid cytokines in refractory periodontitis. J Clin Periodontol. 1995;22:885–890. doi: 10.1111/j.1600-051x.1995.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 28.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. Journal of periodontology. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 29.Ejeil AL, Gaultier F, Igondjo-Tchen S, et al. Are cytokines linked to collagen breakdown during periodontal disease progression? Journal of periodontology. 2003;74:196–201. doi: 10.1902/jop.2003.74.2.196. [DOI] [PubMed] [Google Scholar]
- 30.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. Journal of periodontology. 2000;71:1535–1545. doi: 10.1902/jop.2000.71.10.1535. [DOI] [PubMed] [Google Scholar]
- 31.Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of interleukin-6 in gingival crevicular fluid from adults with destructive periodontal disease. Journal of periodontology. 1993;64:980–983. doi: 10.1902/jop.1993.64.10.980. [DOI] [PubMed] [Google Scholar]
- 32.Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30:1046–1052. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 33.Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS. Tissue levels of bone resorptive cytokines in periodontal disease. Journal of periodontology. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 34.Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. American journal of physiology. 2003;284:G85–95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- 35.Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 36.Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150–156. doi: 10.1097/00024382-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacology and immunotoxicology. 2003;25:213–224. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- 39.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 40.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 41.Polasa K, Sesikaran B, Krishna TP, Krishnaswamy K. Turmeric (Curcuma longa)-induced reduction in urinary mutagens. Food Chem Toxicol. 1991;29:699–706. doi: 10.1016/0278-6915(91)90128-t. [DOI] [PubMed] [Google Scholar]
- 42.Chen D, Nie M, Fan MW, Bian Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82:264–269. doi: 10.1159/000161127. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian S, Eckert RL. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. The Journal of biological chemistry. 2007;282:6707–6715. doi: 10.1074/jbc.M606003200. [DOI] [PubMed] [Google Scholar]
- 44.Suh HW, Kang S, Kwon KS. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Molecular and cellular biochemistry. 2007;298:187–194. doi: 10.1007/s11010-006-9365-6. [DOI] [PubMed] [Google Scholar]
- 45.Kim SJ, Hellerstein MK. Pharmacological doses of dietary curcumin increase colon epithelial cell proliferation in vivo in rats. Phytother Res. 2007;21:995–998. doi: 10.1002/ptr.2053. [DOI] [PubMed] [Google Scholar]
- 46.Cho JW, Park K, Kweon GR, et al. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med. 2005;37:186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- 47.Rossa C, Jr, Liu M, Bronson P, Kirkwood KL. Transcriptional activation of MMP-13 by periodontal pathogenic LPS requires p38 MAP kinase. Journal of endotoxin research. 2007;13:85–93. doi: 10.1177/0968051907079118. [DOI] [PubMed] [Google Scholar]
- 48.Sartori R, Li F, Kirkwood KL. MAP kinase phosphatase-1 protects against inflammatory bone loss. Journal of dental research. 2009;88:1125–1130. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossa C, Jr, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK negatively regulates murine MMP-13 gene expression induced by IL-1beta and TNF-alpha in immortalized periodontal ligament fibroblasts. Matrix Biol. 2005;24:478–488. doi: 10.1016/j.matbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Rossa C, Ehmann K, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells. J Interferon Cytokine Res. 2006;26:719–729. doi: 10.1089/jir.2006.26.719. [DOI] [PubMed] [Google Scholar]
- 51.Patil C, Zhu X, Rossa C, Jr, Kim YJ, Kirkwood KL. p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunol Invest. 2004;33:213–233. doi: 10.1081/imm-120034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patil C, Rossa C, Jr, Kirkwood KL. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral microbiology and immunology. 2006;21:392–398. doi: 10.1111/j.1399-302X.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- 53.Mbalaviele G, Anderson G, Jones A, et al. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther. 2006;317:1044–1053. doi: 10.1124/jpet.105.100362. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 90:437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911–1917. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Li C, Cai X, Xiang J, Cao Z, Dong W. The temporal expression and localization of extracellular matrix metalloproteinase inducer (EMMPRIN) during the development of periodontitis in an animal model. J Periodontal Res. 45:541–549. doi: 10.1111/j.1600-0765.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 57.Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:5940–5947. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 58.Oh S, Kyung TW, Choi HS. Curcumin inhibits osteoclastogenesis by decreasing receptor activator of nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells. Mol Cells. 2008;26:486–489. [PubMed] [Google Scholar]
- 59.Hie M, Yamazaki M, Tsukamoto I. Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. European journal of pharmacology. 2009;621:1–9. doi: 10.1016/j.ejphar.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochemical and biophysical research communications. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 61.Miquel J, Bernd A, Sempere JM, Diaz-Alperi J, Ramirez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geriatr. 2002;34:37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]