Abstract
Cancer vaccines consisting of intact tumor cells genetically modified to secrete the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) have undergone extensive preclinical development. These vaccines induce the massive accumulation of dendritic cells at the injection site, which engulf, process, and present tumor antigens to activate tumor-specific T cells. Early phase clinical testing demonstrated promising evidence of safety and bioactivity, although initial Phase III clinical trials were unsuccessful. Together, the preclinical and clinical data argue for the continued clinical development of these vaccines, integrating them with standard and novel cancer therapeutics that enhance vaccine activity by overcoming immune tolerance and suppression, and or augmenting co-stimulatory pathways of T cell activation.
Introduction
Immunotherapy, including monoclonal antibodies, adoptive cellular therapy, and vaccines, is becoming increasingly important in cancer treatment. Antibody therapy is a critical component of the treatment for many cancers, particularly lymphoma and HER-2-driven breast cancer. Adoptive cellular therapy is also commonly used in the treatment of hematologic malignancies after bone marrow transplantation, and is under active investigation alone for both solid and liquid tumors. As an alternative to reconstituting immunity with these passive immunotherapies, cancer vaccination can actively harness the intrinsic power of the immune system to target and destroy tumors. The primary objective of cancer vaccination is to actively generate an antigen-specific immune response to proteins differentially expressed by tumor cells. Tumor vaccines can coordinately activate distinct components of the immune system, including dendritic cells (DCs), antibodies and T cells. Compared to traditional cancer therapies, the antitumor immune response elicited by cancer vaccines stands out by having the advantages of high specificity, minimal toxicity, and the possibility of a lasting treatment effect due to immunologic memory. Therapeutic cancer vaccines have been developed and tested in phase I, II and III clinical trials both as single agents, or in combination with other cancer treatments (Emens and Jaffee, 2003). The majority of phase III cancer vaccine trials, however, have been largely unsuccessful due to poor trial design and a lack of understanding of the host-tumor interaction. Here we will review progress in the development of GM-CSF-secreting cancer vaccines for solid tumors to date, and touch on some new strategies for cancer vaccine development.
GM-CSF-Secreting Tumor Vaccines: Mechanism of Action
In a seminal preclinical study, Dranoff and colleagues used a B16 melanoma mouse model to systematically evaluate the immunologic potency of a panel of ten distinct B16 melanoma vaccines in preventing the outgrowth of a subsequent tumor challenge (Dranoff et al., 1993). These ten vaccines consisted of B16 tumor cells modified to incorporate immune modulators either presented on the surface of or secreted by the B16 melanoma cells. This study demonstrated that the immune modulator which most effectively induced long-lasting, specific anti-tumor immunity was granulocyte-macrophage colony-stimulating factor (GM-CSF), and laid the foundation for the subsequent clinical development of GM-CSF-secreting tumor vaccines.
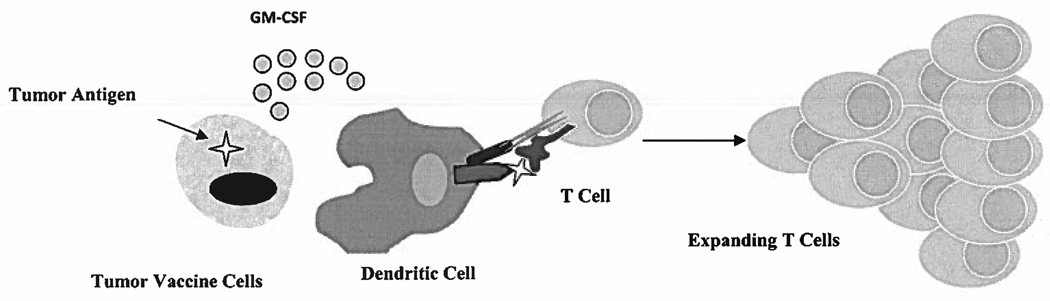
These vaccines consist of whole tumor cells genetically-modified to secrete GM-CSF in a paracrine fashion, while simultaneously delivering a variety of tumor-associated antigens (TAAs) (Figure 1). Local GM-CSF secretion causes the influx and activation of bone marrow-derived DCs, which process and present TAAs delivered by the tumor vaccine cells. These DCs prime tumor-specific CD4+ and CD8+ T cells to mediate direct tumor lysis (Huang et al., 1996). Because the tumor-specific T cells are primed by antigens in the context of MHC alleles present in host bone marrow-derived APCs (DCs) and not the vaccine cells themselves, there is no need to match the MHC haplotypes of the vaccine and the patient with this vaccine strategy (Huang et al., 1994).
Figure 1. Mechanism of Action of GM-CSF-Secreting Vaccines.
The intradermal injection of tumor cells genetically-modified to secrete GM-CSF results in the infiltration of dendritic cells at the injection site. The dendritic cells engulf, process, and present tumor antigens delivered by the vaccinating tumor cells to the patient’s T cell, resulting in T cell activation and expansion.
Phase I/II Clinical Trials of Autologous GM-CSF-Secreting Tumor Vaccines
Early studies evaluated GM-CSF-modified autologous tumor cell vaccines in patients with advanced kidney cancer, melanoma, or prostate cancer (Table 1). The first clinical trial to provide proof of principle for GM-CSF vaccines in humans evaluated their safety and bioactivity in patients with metastatic renal cell carcinoma (RCC) (Simons et al., 1997). This phase I trial enrolled 18 RCC patients in a randomized, double-blind, dose-escalation study with equivalent doses (4 × 106 to 4 × 108 cells) of autologous, irradiated unmodified RCC vaccine cells alone (n=9), or autologous, irradiated RCC vaccine cells modified by retroviral gene transfer to secrete GM-CSF (n=9). Infiltrates of eosinophils developed at the DTH sites of only those who received the GM-CSF-transduced vaccine cells, not those who received the control unmodified vaccine. One vaccinated patient who developed the largest DTH conversion also displayed an objective partial clinical response.
Table 1.
Seminal Clinical Trials of GM-CSF-Secreting Vaccines Alone
| Patient population |
Number of patients |
Vaccine dose | Clinical observations |
Ref |
|---|---|---|---|---|
| Metastatic renal cell carcinoma |
18 treated 16 evaluable |
4 × 106, 4 × 107, or 4 × 108 cells every 4 weeks for 4 cycles |
1 partial response at a dose of 4 × 107 cells |
1 |
| Metastatic melanoma |
26 treated 21 evaluable |
1 × 107 cells once per week for 12 cycles, or every 2 weeks for 6 cycles, or every 4 weeks for 3 cycles |
1 partial, 1 mixed, 3 minor responses 2 patients disease free after surgery and 1 after radiation survived > 20 months |
2 |
| Metastatic melanoma |
38 treated 28 evaluable |
5 × 106 or 5 × 107 cells every 3 weeks for 3 cycles |
6 patients with disease free survival >5 years 2/6 developed vitiligo |
3 |
| Metastatic prostate cancer |
8 evaluable | 1 × 107 or 5 × 107 cells every 3 weeks for at least 3 cycles |
Not reported | 4 |
| Stage 2/3 pancreas cancer |
14 treated 14 evaluable |
1 × 107, 5 × 107, 1 × 108, or 5 × 108 cells every 4 weeks for 3 cycles, with a boost at 6 months |
3/14 patients disease free >10 years later |
5 |
Two subsequent studies administered GM-CSF-secreting melanoma vaccines to metastatic melanoma patients. In both studies, DTH responses of ≥1 cm were observed in 100% of vaccinated patients (Soiffer et al., 1998; Luiten et al., 2005). Another trial in metastatic prostate cancer patients demonstrated DTH of 1 cm in 5 of 6 vaccinated patients (Simons et al., 1999). Together, these three clinical trials showed that this type of vaccine is biologically active, and that the vaccine sites themselves were typically infiltrated by DCs, macrophages, eosinophils, neutrophils and T cells. In addition, these trials showed that vaccine-related toxicities are generally limited to local injection-site reactions, including swelling, redness, and itching. Vaccine-related side effects also included rash, urticaria, eosinophilia, low-grade fever and other transient flu-like symptoms.
Early phase, second generation trials tested autologous GM-CSF-secreting vaccines modified by adenoviral transduction, and enrolled patients with metastatic melanoma or early-stage/metastatic non-small cell lung cancer (NSCLC). One trial of autologous GM-CSF-secreting melanoma cells in 26 evaluable patients with metastatic melanoma demonstrated DTH ≥ 1 cm in 68% of patients, as well as 1 complete, 1 partial, and 1 mixed response, and 5 cases of stable disease (Soiffer et al., 2003). In two studies conducted in patients with metastatic NSCLC, DTH ≥ 1 cm developed in 82% of vaccinated patients in one study, and increased from 13% to 34% of patients post-vaccination in the other (Salgia et al., 2003; Nemunaitis et al., 2006). In the former study, the vaccine consisted of GM-CSF-secreting tumor cells modified by adenoviral transduction; of 25 evaluable patients, 1 patient had a mixed response and 5 displayed stable disease (Salgia et al., 2003). In the latter study, the vaccine consisted of autologous tumor cells admixed with GM-CSF-secreting bystander cells; clinical outcomes included 4 mixed responses and 7 cases of stable disease (Nemunaitis et al., 2006). In a third study of 43 patients with early-stage or metastatic NSCLC, longer survival was observed in vaccinated, advanced-stage NSCLC patients who developed serum levels of GM-CSF≥ 40 ng compared to those who developed lower peak GM-CSF levels (Nemunaitis et al., 2004). This provides initial clinical evidence that a threshold level of GM-CSF is required for the induction of effective immunity.
Allogeneic GM-CSF-Secreting Cancer Vaccines
These early clinical studies required manufacturing times for autologous tumor vaccine cells of up to 6–10 weeks. During this processing period, patients could develop progressive disease and become ineligible for vaccination. In addition, it is often difficult to obtain sufficient quantities of autologous tumor cells for full vaccine protocols. To overcome these practical problems, the use of allogeneic tumor cells is an attractive alternative. The use of allogeneic tumor cells as a generalized vaccine is supported by two observations. First, the patient’s own DCs, not the vaccine cells themselves, activate tumor immunity (Huang et al., 1994; Huang et al., 1996). Second, many tumor antigens are shared with a given histologic tumor type (van der Bruggen et al., 2002), and some are universal tumor antigens thought to be expressed by all tumors (Nadler and Schultze, 2002). Allogeneic GM-CSF-secreting vaccines were first evaluated as single agents in pancreatic and prostate cancer. Two studies in stage 2/3 pancreatic cancer patients delivered a GM-CSF-secreting pancreatic cancer cell vaccine after primary surgical therapy (Jaffee et al., 2001; Laheru et al., 2007). The first phase I study tested four different vaccine cell dose levels (1 × 107 to 5 × 108 cells) in 14 patients, and demonstrated new DTH≥ 1 cm to the patient’s own pancreas tumor cells in 1 of 3 patients at 1 × 108 cells and 2 of 4 patients at 5 × 108 cells (Jaffee et al., 2001) (Table 1). Disease-free survival of over 10 years was demonstrated in 3 out of 14 vaccinated patients. The follow up phase II study tested the vaccine at the highest 5 × 108 cell dose in 60 patients (Laheru et al., 2007). The overall survival rate of vaccinated patients was 26 months, compared to a historical rate of 21 months with similarly treated non-vaccinated patients. Based on the development of the allogeneic GM-CSF-secreting pancreas tumor vaccine, an allogeneic GM-CSF-secreting breast tumor vaccine was developed and tested alone in 6 patients with metastatic breast cancer, with evidence of new vaccine-induced immunity after vaccination (Emens et al., 2009). The side effect profiles observed in all of these trials were similar to those observed in the autologous GM-CSF-secreting vaccine studies.
Multiple clinical trials have tested allogeneic GM-CSF-secreting vaccines in patients with prostate cancer. Two studies enrolled patients with metastatic hormone-naïve prostate cancer (Simons et al., 2006; Urba et al., 2008). One study of 19 patients demonstrated a median time to disease progression of 9.7 months (Urba et al., 2008), and the other study of 21 patients revealed 1 partial response and 16 minor responses, and 14 cases of stable disease (Simons et al., 2006). Another study was conducted in patients with metastatic hormone-refractory prostate cancer (Small et al., 2007). It enrolled 55 patients, 34 who had evaluable disease, and 21 who had only a biochemical relapse (rising PSA only). This trial documented a median survival of 26.2 months in patients with evaluable disease. Another trial demonstrated new antibody to filamin-B in 29 of 80 vaccinated patients with a median survival of 43 months for a cohort with stable disease (35% of patients) and 19 months for a cohort with progressive disease (Higano et al., 2008).
Two phase III randomized, controlled clinical trials (VITAL-1 and VITAL-2) tested the allogeneic GM-CSF-secreting prostate cancer vaccine (Higano et al., 2009; Small et al., 2009). These studies enrolled 626 and 408 hormone-refractory metastatic prostate cancer patients respectively. In the VITAL-1 trial, the vaccine alone was compared to active chemotherapy with an established clinical benefit (Higano et al., 2009). Patients were randomized to receive either the vaccination regimen alone (12 cycles) or standard chemotherapy (docetaxel plus prednisone for 9 cycles). In the VITAL-2 trial, the vaccine was integrated into standard dose chemotherapy. Patients were randomized to receive the vaccination regimen plus docetaxel for 10 cycles, or standard treatment with docetaxel plus prednisone for 10 cycles (Small et al., 2009). The primary endpoint of both trials was median overall survival. In August 2008, the VITAL-2 trial was terminated after an Independent Data Monitoring Committee noted an imbalance of deaths, with 67 in the experimental arm and 47 in the control arm. Importantly, with further follow up, this imbalance lessened to 85 and 75 deaths respectively (Small et al., 2009). All deaths were the result of disease progression and death from prostate cancer. Subsequently in October 2008, the VITAL-1 trial was terminated after futility analysis predicted a < 30% chance of meeting the primary endpoint (Higano et al., 2009).
Lessons Learned from GM-CSF-Secreting Vaccine Trials to Date
The failure of these Phase III clinical trials, together with information gained from the early stage trials of a range of GM-CSF-secreting vaccines for diverse solid tumor types, greatly informs the way forward in developing GM-CSF-secreting vaccines. First, initial results of the VITAL-1 trial demonstrated that immunotherapy was better tolerated than chemotherapy in terms of the rate and range of adverse events, without a significant difference in clinical outcome (Higano et al., 2009). Second, the initial imbalance of deaths on the VITAL-2 study lessened with further follow up (Small et al., 2009), a finding consistent with an emerging appreciation that immunotherapy responses occur more slowly and later than responses to conventional cytotoxic chemotherapy (Hales et al., 2010). Third, it is likely that utilizing cancer vaccines earlier in the natural history of cancer, before it becomes metastatic, will be more effective. This principle is reflected in the choice of Stage 2/3 pancreas cancer patients in the first clinical trials of the GM-CSF-secreting pancreatic cancer vaccine (Jaffee et al., 2001; Laheru et al., 2007). Fourth, the development of DTH responses to autologous tumor cells has been an informative measure of the immune response in small trials, but is not practical to implement on a larger scale. Finally, there is an undeniable impact of conventional therapy on the vaccine-induced immune response (Emens, 2010). This was observed in the Phase I study of the GM-CSF-secreting pancreas vaccine (Jaffee et al., 2001; Thomas et al., 2005), and a negative effect of chemotherapy on vaccine activity could (in part) account for the lack of success of VITAL-2.
Monitoring Immune Responses to GM-CSF-Secreting Cancer Vaccines
For the effective clinical development of cancer vaccines, monitoring the magnitude and quality of the vaccine-induced immune response is essential. Perhaps the most effective indicator of the ability of endogenous T cells to migrate to and recognize autologous tumor cells is by monitoring the development of DTH to the patient’s own tumor cells. However, this is not practical for large scale testing, and autologous tumor cells may not always be available for DTH testing. In contrast to cancer vaccines that deliver a specific antigen, whole cell vaccines pose a challenge for immune monitoring since they deliver a battery of antigens. Identifying one of these antigens as a sentinel measure of vaccine activity is one approach for monitoring vaccine-induced immunity, and correlating it with potential clinical benefit from the whole cell vaccine. This strategy has been developed for the pancreas, prostate, and breast cancer GM-CSF-secreting vaccines by measuring immunity to mesothelin (Jaffee et al., 2001; Thomas et al., 2004; Laheru et al., 2007), filamin B (Simons et al., 2006; Small et al., 2007; Urba et al., 2008), and HER-2 respectively (Emens et al., 2009).
Conventional Cancer Drugs Have Immune-Modulating Activity
Historically, successful cancer therapy requires the integration of distinct treatment modalities, including surgery, radiation therapy, and systemic chemotherapy. Most chemotherapy regimens combine drugs with non-overlapping toxicities in a complementary and synergistic fashion to target multiple pathways involved in tumor growth and survival, thus surmounting resistance pathways intrinsic to the cancer cell. Consistent with these historical concepts, recent advances in molecular and cellular immunology reveal that the basic host immune response as well as soluble factors secreted by tumor and host cells generate a local tumor microenvironment that is immunologically complex, and an overriding inhibitory systemic milieu. In addition, the immunologic mechanisms by which some conventional chemotherapy drugs kill tumors are now emerging (Zitvogel et al., 2008). For example, anthracyclines and oxaliplatin work in part by cross-priming the tumor-specific immune response through the uptake of dying tumor cells by DCs (Apetoh et al., 2007; Tesniere et al., 2009). This new framework of knowledge has allowed researchers to begin testing combinatorial immunotherapy in which drugs such as conventional cytotoxic agents, monoclonal antibodies, and novel immune modulators are used in conjunction with cancer vaccines to boost the antitumor response by targeting different elements of tumor progression, including the host-tumor interaction.
Chemotherapy and GM-CSF-Secreting Cancer Vaccines
Conventional chemotherapy drugs, depending on type of drug, dosage, and schedule, can inhibit or augment the tumor immunity induced by cancer vaccines (reviewed in Emens et al., 2001). Vaccination after high dose chemotherapy (as in after bone marrow transplantation) can re-boot the immune system, and skew the T cell population toward the desired tumor-specificity (reviewed in Emens, 2010). Conversely, low dose chemotherapy also has immunologic effects (reviewed in Emens, 2010). Low dose Cyclophosphamide (CY) given 1–3 days prior to vaccination can inhibit the suppressive activity of regulatory T cells, allowing an effective antitumor T cell response to emerge. Low dose CY also promotes T helper type 1-mediated immunity, enhances DC activation, maturation and cytokine secretion, and upregulates type 1 interferons for immunologic memory. Low dose paclitaxel (PTX) also favors T helper type 1-mediated immunity, and promotes DC activation (Machiels et al., 2001; Pfannenstiel et al., 2010). Low dose doxorubicin favors the emergence of tumor-specific CD8+ T cells (Nigam et al., 1998; Machiels et al., 2001). Proof of principle for the use of these low doses of chemotherapy to enhance the activity of GM-CSF-secreting tumor vaccines has been demonstrated in the HER-2/neu transgenic mouse model of breast cancer, where giving the vaccine in sequence with CY one day prior to vaccination and DOX given one week after was more effective than either vaccine alone, or combined with only one chemotherapy agent (Machiels et al., 2001). Other chemotherapy drugs that can be effectively combined with GM-CSF-secreting vaccines in preclinical models include docetaxel and cytosine arabinoside (ara-C) (reviewed in Emens, 2010).
Two clinical trials have been published that combine immune-modulating doses of chemotherapy with GM-CSF-secreting cancer vaccines to enhance the immune response (Table 2). In one study, 50 patients with metastatic pancreatic cancer were given an allogeneic GM-CSF-secreting pancreas tumor cells (5 × 108 cells) (Laheru et al., 2008). Thirty patients were given vaccine alone, while 20 were given vaccine with 300 mg/m2 CY one day before vaccination. The median survival rates of patients who received vaccination alone and those who received vaccination with CY were 2.3 and 4.3 months respectively. The addition of CY did not potentiate vaccine-related toxicities, and patients who received CY-modulated vaccination were more likely to develop mesothelin-specific T cell responses. Another study administered an allogeneic GM-CSF-secreting HER2+ breast tumor cell vaccine to 28 metastatic breast cancer patients along with varying low doses of CY (0, 200, 250 and 250 mg/m2) one day prior to vaccination and DOX (0, 15, 25, 35, mg/m2) one week later to determine the doses of CY followed by DOX optimized the immunologic outcome (Emens et al., 2009). The vaccine as a single agent induced HER2-specific DTH. The DTH response was maintained, and the HER2-specific antibody response enhanced, when the vaccine was sequenced with doses of 200 mg/m2 CY one day prior and 35 mg/m2 DOX one week later. Interestingly, CY doses of 250 mg/m2 or higher abrogated vaccine-induced immunity.
Table 2.
Clinical Trials of Combination Therapy with Allogeneic GM-CSF-Secreting Vaccines
| Patient population |
Number of patients |
Combination Drug Therapy |
Immunologic outcome |
Clinical observations | Ref |
|---|---|---|---|---|---|
| Metastatic pancreatic cancer |
50 | CY 0 mg/m2 (n = 20)vs. CY 300 mg/m2 (n =30) |
Increased mesothelin- specific immunity with CY 300 mg/m2 |
Median survival rates vaccination alone = 2.3 months vaccination + CY = 4.3 months |
1 |
| Metastatic breast cancer |
28 | CY 0,200,250,350 mg/m2 DOX 0,15,25,35 mg/m2 (factorial design of various dose combinations) |
HER-2-specific DTH, with HER-2- specific antibody levels enhanced by 200 mg/m2 CY and 35 mg/m2 DOX |
Not assessed | 2 |
| Prostate cancer patients |
12 | Ipilumimab 0.3,1,3,5 mg/kg | Antibodies to filamin B, PSMA and NY-ESO-1 |
Late-onset PSA declines >50% in 5 patients with response durations 6.7–23.1 months 4/5 had stable disease on bone scan for at least 12 months 5/6 patients with Grade 2/3 immune breakthrough events (hypophysitis, alveolitis) |
3 |
GM-CSF=granulocyte-macrophage colony-stimulating factor; CY=cyclophosphamide; DOX=doxorubicin; HER-2=human epidermal growth factor receptor-2; DTH=delayed type hypersensitivity; PSMA=prostate-specific membrane antigen; PSA=prostate-specific antigen; References;
Therapeutic Monoclonal Antibodies and GM-CSF-secreting Cancer Vaccines
Monoclonal antibodies are able to modulate the immune system and tumor-host response in a diverse ways (reviewed in Emens et al., 2005). Their Fc regions engage host immune effectors, thus facilitating tumor cell destruction via antibody-dependent cellular cytotoxicity (ADCC). They may enhance the lytic activity of antigen-specific CD8+ T cells against tumor cells by promoting the processing and presentation of the antigen. Monoclonal antibodies also may augment immune priming when used in conjunction with a cancer vaccine by engaging the Fc receptor on phagocytes at the vaccine site and its draining lymph nodes (Kim et al., 2008). Finally, monoclonal antibodies may also influence the tumor microenvironment. Trastuzumab and bevacizumab decrease VEGF levels, thus inhibiting the angiogenesis required for progressive tumor growth (reviewed in Emens and Jaffee, 2007). Consistent with these activities, HER-2-specific monoclonal antibodies can enhance CY-modulated vaccination in the HER-2/neu transgenic mouse model of breast cancer, resulting in greater numbers of T cells, improved tumor-free survival, and the evolution of a memory T cell response (Wolpoe et al., 2003; Kim et al., 2008). Based on these data, ongoing clinical trials are testing CY-modulated vaccination with GM-CSF-secreting tumor cells combined with cetuximab in pancreatic cancer patients and trastuzumab in breast cancer patients.
Targeted Immune Modulators and GM-CSF-Secreting Cancer Vaccines
Combining tumor vaccines with molecular immune checkpoint inhibitors is another potential strategy for altering pathways of immune tolerance and targeting the tumor microenvironment. Cell surface molecules like cytotoxic T-lymphocyte antigen 4 (CTLA-4), B7-H1, and B7-H4 control pathways that dampen tumor immunity, while CD28, B7, OX40 control regulatory pathways that amplify immunity (Pardoll, 2002).
Two clinical trials studied treatment with ipilimumab for metastatic melanoma or ovarian carcinoma patients previously vaccinated with autologous GM-CSF-secreting melanoma or ovarian cancer vaccines (Hodi et al., 2003; Hodi et al., 2008). Ipilumimab is a monoclonal antibody immune checkpoint inhibitor that antagonizes the activity of CTLA-4, a negative regulator of antitumor immunity (Fong and Small, 2008). Periodic infusions of ipilumimab stimulated extensive tumor necrosis, or the reduction/stabilization of cancer antigen-125 (CA-125) levels, suggesting that CTLA-4 blockade can unleash latent tumor immunity in patients who have been previously vaccinated (Hodi et al., 2003; Hodi et al., 2008).
Building on these observations, a Phase I clinical trial combined ipilimumab with allogeneic GM-CSF-secreting prostate tumor cells (Gerritson et al., 2008). Twelve patients were vaccinated every two weeks, and also received ipilumimab (0.3, 1, 3, or 5 mg/kg) once monthly. Five of 6 patients who received a 3 or 5 mg/kg dose of ipilumimab experienced grade Grade 2/3 immune breakthrough adverse events, including hypophysitis and alveolitis. Late onset PSA responses (declines >50%) were seen in these 5 patients with response durations of 6.7, 8.6, 9.5, 13.8 (ongoing) and 23.1 months. Four of these five patients had stable disease on bone scan for at least 12 months.
Conclusions
Immunotherapy is the next frontier of cancer treatment. Although GM-CSF-secreting tumor cell vaccines hold promise for new ways that cancers can be targeted and destroyed by using the power intrinsic to the host immune system, clinical trial results to date have only hinted at meaningful clinical activity. Strategically integrating these vaccines with conventional cancer drugs and novel immune modulators to break down immune tolerance will bring GM-CSF-secreting cancer vaccines to the forefront of cancer therapy.
Acknowledgments
This work was supported by funding from the Department of Defense (W81XWH-04-1-0595; W81XWH-07-1-0485), the Breast SPORE program (P50 CA88843), the American Cancer Society (RSG CCE-112685), Komen for the Cure (BCTR0707297), the AVON Foundation, the Gateway Foundation for Cancer Research, the V Foundation for Cancer Research, and Climb for Hope.
Footnotes
Conflict of Interest Statement
This review describes work using granulocyte-macrophage colony-stimulating factor-secreting tumor vaccines. Under a licensing agreement between Biosante, Incorporated and the Johns Hopkins University, the University is entitled to a share of royalty received by the University on sales of products described in this article. The terms of this agreement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri M, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat F, Saftig P, Levi F, Lidereau R, Nogues C, Mira J-P, Chompret A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens L. Chemoimmunotherapy. The Cancer Journal. 2010;16 doi: 10.1097/PPO.0b013e3181eb5066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens L, Asquith J, Leatherman J, Kobrin B, Petrik S, Laiko M, Levi J, Daphthary M, Biedrzycki B, Wolff A, Stearns V, Disis M, Ye X, Piantadosi S, Fetting J, Davidson N, Jaffee E. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: A chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens L, Jaffee E. Cancer Immunotherapy: Immune Suppression and Tumor Growth. London: Academic Press, Elsevier; 2007. Immunotherapy and cancer therapeutics: Why partner? pp. 207–233. [Google Scholar]
- Emens L, Machiels J, Reilly R, Jaffee E. Chemotherapy: Friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- Emens L, Reilly R, Jaffee E. Breast cancer vaccines: Maximizing cancer treatment by tapping into host immunity. Endocrine Rel Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- Emens L, Jaffee E. Cancer vaccines: An old idea comes of age. Cancer Biol Ther. 2003;2(4) suppl 1:S161–S168. [PubMed] [Google Scholar]
- Fong L, Small E. Anti-cytotoxic T-lymphocyte antigen-4 antibody: The first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- Gerritson W, Van Den Eertwegh A, De Gruijl T, Van Den Berg H, Scheper R, Sacks N, Lowy I, Stankevich E, Sacks N. Expanded Phase I combination trial of GVAX immunotherapy for prostate cancer and ipiliumumab in patients with metastatic hormone-refractory prostate cancer (mHRPC) Proc Amer Soc Clin Oncol 2008. 2008 abstr XXX. [Google Scholar]
- Hales R, Banchereau J, Ribas A, Tarhini A, Weber J, Fox B, Drake C. Assessing clinical benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010 doi: 10.1093/annonc/mdq048. epub PMID 20237404. [DOI] [PubMed] [Google Scholar]
- Higano C, Corman J, Smith D, Centeno C, Steidle C, M G, Simons J, Sacks N, Aimi J, Small E. Phase I/II dose escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- Higano C, Saad F, Somer B, Curti B, Petrylak D, Drake C, Schnell F, Redfern D, Schrijvers D, Sacks N. A phase III trial of GVAX immunotherapy for prostate cancer vs. docetaxel plus prednisone in asymptomatic castration-resistant prostate cancer (CRPC) presented at ASCO GU. 2009 [Google Scholar]
- Hodi F, Butler M, Oble D, Seiden M, Haluska F, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch M, Ramaiya N, Chen T, Neuberg D, Allison J, Mihm M, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen-4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F, Mihm M, Soiffer R, Haluska F, Butler M, Seiden M, Davis T, Henry-Spires R, Macrae S, Willman A, Padera R, Jaklitsch M, Shankar S, Chen T, Korman A, Allison J, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen-4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Bruce A, Pardoll D, Levitsky H. In vivo cross-priming of MHC Class I-restricted antigens requires tap transporter. Immunity. 1996;4:349–355. doi: 10.1016/s1074-7613(00)80248-4. [DOI] [PubMed] [Google Scholar]
- Huang A, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHD class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- Jaffee E, Hruban R, Biedrzycki B, Laheru D, Schepers K, Sauter P, Goemann M, Coleman J, Grochow L, Donehower R, Lillemoe K, O'Reilly S, Abrams R, Pardoll D, Cameron J, Yeo C. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: A phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- Kim P, Armstrong T, Song H, Wolpoe M, Weiss V, Manning E, Huang L, Murata S, Sgouros G, Emens L, Reilly R, Jaffee E. Antibody association with HER-2/neu-targeted vaccine enhances CD8+ T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;188:1700–1711. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemuanitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laheru D, Yeo C, Biedrzycki B, Solt S, Lutz E, Onners B, Tartakovsky I, Herman J, Hruban R, Piantadosi S, Jaffee E. A safety and efficacy trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene in combination with adjuvant chemoradiotherapy for the treatment of adenocarcinoma of the pancreas. Proc Amer Soc Clin Oncol. 2007;25(18S):3010. [Google Scholar]
- Luiten R, Kueter E, Mooi W, Gallee M, Rankin E, Gerritsen W, Clift S, Nooijen W, Weder P, Van De Kasteele W, Sein J, Van Den Berk P, Nieweg O, Berns A, Spits H, De Gast G. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF transduced tumor cells in metastatic melanoma patients. J Clin Oncol. 2005;23:8978–8991. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- Machiels J, Reilly R, Emens L, Ercolini A, Lei R, Weintraub D, Okoye F, Jaffee E. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- Nadler L, Schultze J. From genomics to cancer vaccines: Patient-tailored or universal vaccines? Curr Opin Mol Ther. 2002;4:572–576. [PubMed] [Google Scholar]
- Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, Jablons D, Aimi J, Lin A, Hege K. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Sterman D, Jablons D, Smith J, 2nd, Fox B, Maples P, Hamilton S, Borellini F, Lin A, Morali S, Hege K. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- Nigam A, Yacavone R, Zahurak M, Johns C, Pardoll D, Piantadosi S, Levitsky H, Nelson W. Immunomodulatory properties of antineoplastic drugs administered in conjunction with GM-CSF-secreting cancer cell vaccines. Intl J Cancer. 1998;12:161–170. doi: 10.3892/ijo.12.1.161. [DOI] [PubMed] [Google Scholar]
- Pardoll D. Spinning molecular immunology into successful immunotherapy. Nature Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel L, Lam S, Emens L, Jaffee E, Armstrong T. Paclitaxel enhances early dendritic cell maturation and function through TLR-4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, Hodi F, Jaklitsch M, Mentzer S, Swanson S, Lukanich J, Bueno R, Wain J, Mathisen D, Wright C, Fidias P, Donahue D, Clift S, Hardy S, Neuberg D, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- Simons J, Carducci M, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift S, Hege K, Ando D, Piantadosi S, Mulligan R, Nelson W. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- Simons J, Jaffee E, Weber C, Levitsky H, Nelson W, Carducci M, Lazenby A, Cohen L, Finn C, Clift S, Hauda K, Beck L, Leiferman K, Owens A, Jr, Piantadosi S, Dranoff G, Mulligan R, Pardoll D, Marshall F. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- Simons J, Mikhak B, Chang J, Demarzo A, Carducci M, Lim M, Weber C, Baccala A, Goemann M, Clift S, Ando D, Levitsky H, Cohen L, Sanda M, Mulligan R, Partin A, Carter H, Piantadosi S, Marshall F, Nelson W. Induction of immunity to prostate cancer antigens: Results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59(20):5160–5168. [PubMed] [Google Scholar]
- Small E, Demkow T, Gerritson W, Rolland R, Hoskin P, Smith D, Parker C, Chondros D, Ma J, Hege K. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) GU ASCO. 2009 [Google Scholar]
- Small E, Sacks N, Nemunaitis J, Urba W, Dula E, Centeno A, Nelson W, Ando D, Howard C, Borellini F, Nguyen M, Hege K, Simons J. Granulocyte-macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- Soiffer R, Hodi Fs, Haluska F, Jung K, Gillessen S, Singer S, Tanabe K, Duda R, Mentzer S, Jaklitsch M, Bueno R, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Mihm M, Dranoff G. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger J, Hodi F, Liebster L, Lam P, Mentzer S, Singer S, Tanabe K, Cosimi A, Duda R, Sober A, Bhan A, Daley J, Neuberg D, Parry G, Rokovich J, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon J, Jooste V, Van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2009;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- Thomas A, Santarsiero L, Lutz E, Armstrong T, Chen Y, Huang L, Laheru D, Goggins M, Hruban R, Jaffee Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urba W, Nemunaitis J, Marshall F, Smith D, Hege K, Ma J, Nguyen M, Small E. Treatment of biochemical recurrence of prostate cancer with granulocyte-macrophage colony-stimulating factor secreting allogeneic, cellular immunotherapy. J Urol. 2008;180:2011–2017. doi: 10.1016/j.juro.2008.07.048. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz Es, Chapiro J, van den Eynde B, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human t cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- Wolpoe M, Lutz E, Ercolini A, Murata S, Ivie S, Garrett E, Emens L, Jaffee E, Reilly R. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in HER-2/neu-transgenic mice. J Immunol. 2003;171:2161–2169. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]