Abstract
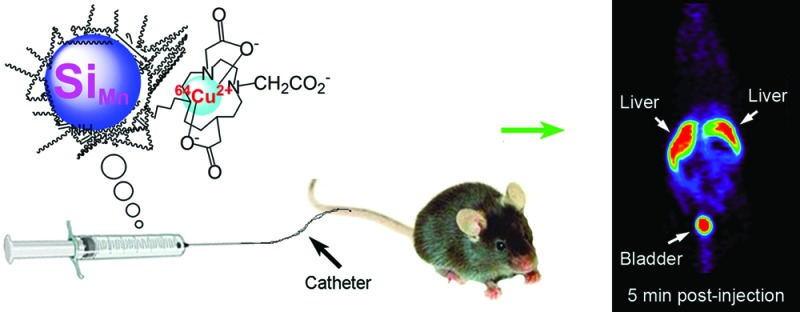
Investigation of nanomaterial disposition and fate in the body is critical before such material can be translated into clinical application. Herein a new macrocyclic ligand−64Cu2+ complex was synthesized and used to label dextran-coated silicon quantum dots (QD), with an average hydrodynamic diameter of 15.1 ± 7.6 nm. The chelate showed exceptional stability, demonstrated by no loss radiolabel under a ligand competition reaction with EDTA. The QDs’ biodistribution in mice was quantitatively evaluated by in vivo positron emission tomography (PET) imaging and ex vivo gamma counting. Results showed that they were excreted via renal filtration shortly postinjection and also accumulated in the liver.
Keywords: Biodistribution, positron emission tomography, imaging, silicon, quantum dot
Over the past decade, quantum dots (QDs) have been touted as promising agents for in vivo biomedical imaging due to their desirable properties of high quantum yield, resistance to photobleaching, narrow emission peak, and tunable emission wavelength.1−3 Investigation of nanomaterial disposition and fate in the body, i.e. biodistribution and plasma clearance, is critical before such material can be translated into clinical application. The biodistribution of group II−VI QDs has been investigated in rodents via both fluorescence and radioactivity-based techniques.1,4−6 However, to date, such studies have not been reported for recently emerged, fluorescent silicon QDs, which are expected to be an ideal candidate for many biological applications because of their biocompatibility.7−11
Taking advantage of the QD’s intrinsic luminescence properties, optical imaging techniques can be used to investigate QDs in vitro and offer the benefits of noninvasiveness, high sensitivity, and relatively low cost. However, for in vivo use, tissue absorption and scattering of light impair excitation of QDs in deeper lying tissues, and the fluorescence signal detected from deeper structures is significantly attenuated as a function of tissue depth, which makes optical tracking and quantification of QDs in living systems difficult.6 For example, there are studies showing that current fluorescence imaging systems significantly underestimate QD mass amounts in living animals, especially in deep tissues, and therefore are not robust enough to accurately measure in vivo biodistribution.4,12
Positron emission tomography (PET) is a noninvasive, highly sensitive nuclear imaging technique that can produce three-dimensional images.13−15 We have used PET to track the biodistribution of a wide array of imaging probes. The results demonstrated that this noninvasive imaging technique is capable of providing a robust and reliable measure of in vivo probe distribution.16−18 PET has been employed to study biodistribution in living animals of group II−VI CdSe QDs radiolabeled with a positron-emitting tracer.4,19 Herein, we report the evaluation of Si QDs biodistribution in mice using 64Cu labeled Si QDs for both in vivo PET imaging and gamma counting of ex vivo tissues.
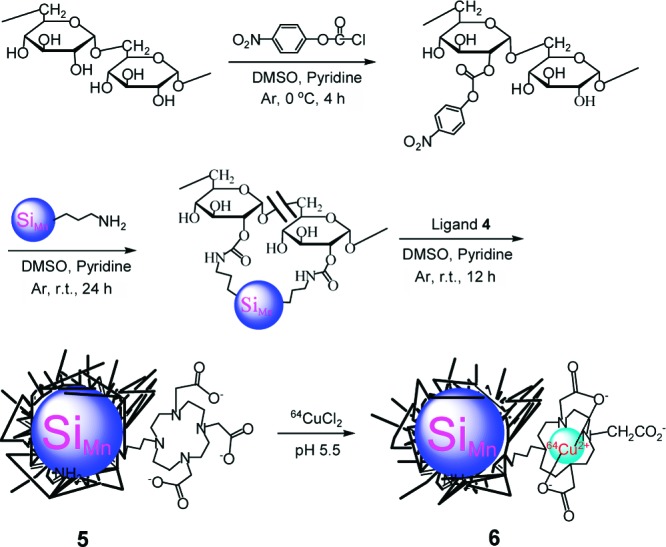
Silicon QDs were synthesized from the precursor sodium silicide through a solution-phase reduction, as previously reported, except that SiMn QDs (SiMn QDs = 1% manganese doped Si QDs) were coated with neutral dextran instead of anionic dextran sulfate.11 The dextran sulfate coated Si QDs are targeted to macrophages while the dextran coated Si QDs are nonspecific nanoparticles. That is, they do not interact specifically with any type of cells and therefore report the clearance of Si QDs from the body mainly based on the particle size.11,20 DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) is a frequently used ligand for chelation of 64Cu ion that forms a complex of high thermodynamic stability (DOTA-Cu log K = 20−28).4,21−24 However, in practice we found that attachment of commercially available p-SCN-Bn-DOTA to the particles using traditional bioconjugation methods was synthetically difficult, probably due to the steric hindrance caused by the short and rigid arm of SCN-Bn.17 Therefore, we synthesized a new bifunctional DO3A (1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid) derivative 4 that has a more flexible functional arm for chelation of 64Cu. The conjugation of ligand 4 with the dextran coating of SiMn QDs was realized by polarity inversion of dextran hydroxyl groups.
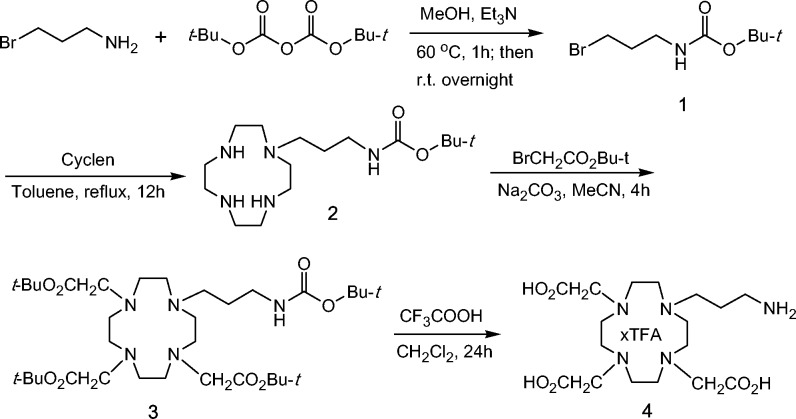
The synthetic route for ligand 4 is shown in Scheme 1. Di-tert-butyl dicarbonate and 3-bromopropylamine hydrobromide were stirred in methanol in the presence of triethylamine to afford compound 1 in 79% yield. After cyclen was monoalkylated with 1, the resultant compound 2 was further alkylated with 3 equiv of tert-butyl bromoacetate to produce compound 3.25,26 Compound 3 was hydrolyzed with trifluoroacetic acid in dichloromethane to give the free ligand 4 as an adduct of trifluoroacetic acid to free base, which was confirmed by 1H and 13C NMR and the strong absorption at −74.25 ppm in 19F NMR of 4.
Scheme 1. Synthesis of Ligand 4 for 64Cu Chelation.
The polarity of dextran was partially inverted by esterification with 4-nitrophenyl chloroformate and 4-(dimethylamino)pyridine (DMAP) (catalyst) in DMSO/pyridine.27 The following nucleophilic addition−elimination reactions of polar, partially inverted dextran with propylamine capped SiMn QDs and ligand 4 generated DO3A conjugated, dextran coated SiMn QDs 5,11 as shown in Scheme 2. The average hydrodynamic diameter of the purified particle 5 was 15.1 ± 7.6 nm. Particle 5 contained 1.5% silicon. Its emission peak was at 440 nm and the intensity of emission was maximal for excitation at 360 nm, similar to our previous results for the unconjugated particles.11 The results support that particle 5 is dextran coated silicon QDs, which is important because the dextran alone can form different sizes of nanoparticles.28 DO3A conjugated SiMn QDs 5 were metalated with 64CuCl2 in pH 5.5 acetate buffer solution followed by the extraction of possible uncoordinated free copper ions by applying excess ethylenediaminetetraacetic acid (EDTA). After purification by centrifuge filtration, the radiolabeling yield was found to be 78%.
Scheme 2. Synthesis of 64Cu-DO3A Conjugated Dextran SiMn QDs 6.
To confirm that 64Cu ions remain attached to the QDs 6 during imaging and verify that distribution is not due to free dissociated copper, the kinetic stability of QDs 6-64Cu was tested by subjecting QDs 6-64Cu to a competitive ligand exchange with EDTA, which is known to have a high affinity for Cu (log KCu-EDTA = 18.7),29 over the 48-h time period. Figure 1 shows that, over time, the QDs 6-64Cu in pH 5.5 sodium acetate−acetic acid buffer solution do not lose radiolabel, confirming the exceptional stability of the chelator.
Figure 1.
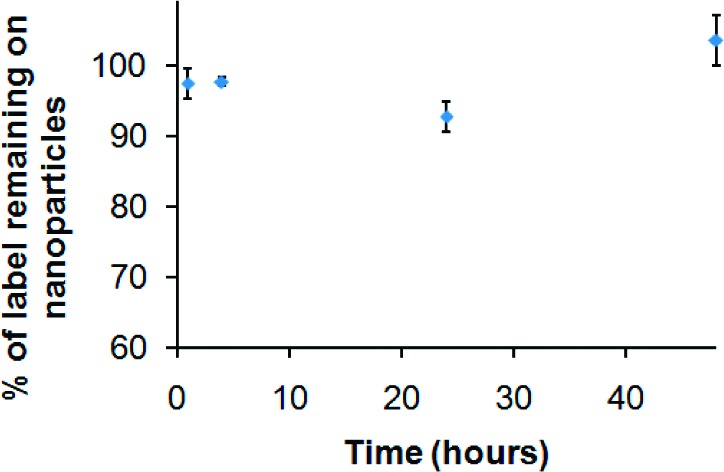
Stability of QDs 6-64Cu in pH 5.5 sodium acetate−acetic acid buffer solution over the 48 h time period.
The clearance of SiMn QDs 6 from the blood circulation is rapid, as shown in Figure 2. By 2 min post tail-vein injection, 95.7%ID/g (percentage of injected dose per gram blood or tissue) has already been cleared out from the bloodstream. The rapid clearance continued until 10 min postinjection, when <2.5%ID/g was left in the bloodstream. Thereafter, the clearance slowed and ∼1.5%ID/g remained in the blood 120 min postinjection. The fast blood clearance of SiMn QDs 6 is similar to that of polyethylene glycol coated CdSe QDs (12 or 21 nm, respectively) measured by PET imaging and gamma counting in mice, which were also cleared in minutes,4 but differs from reports of polymer-coated QDs or dextran-coated iron oxide nanoparticles measured by fluorescence imaging, which had similar hydrodynamic diameters to SiMn QDs 6 but much longer blood circulation times of up to a few hours.1,6,16
Figure 2.
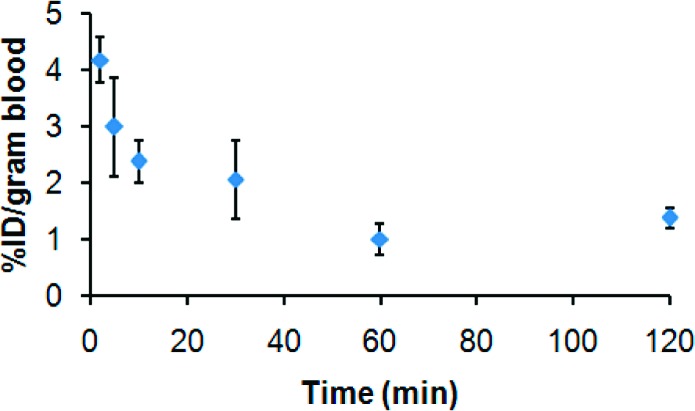
Blood clearance of 64Cu-DO3A conjugated dextran SiMn QDs 6 in mice (n = 3).
The concentrations of particles radiolabeled with positron-emitting tracer can be determined semiquantitatively from the PET images and used to analyze the biodistribution of SiMn QDs 6 in living mice over time. As shown in Figure 3, the main sites for accumulation of 6 are the urinary bladder and the liver after 5 min and 1 h post tail-vein injection. After 4 h, 24 h, and 48 h postinjection, SiMn QDs 6 were found to mainly accumulate in the liver. Only a weak signal was seen in the bladder after 4 h postinjection.
Figure 3.
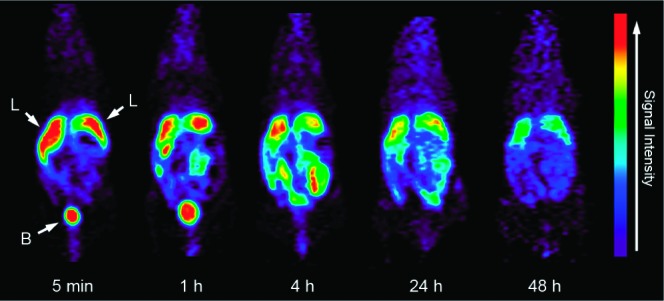
In vivo PET images of mice (n = 4) at 5 min, 1 h, 4 h, 24 h, and 48 h postinjection of 64Cu-DO3A conjugated dextran SiMn QDs 6: L, liver; B, bladder.
The absolute quantification of biodistribution and deposition of SiMn QDs 6 in living mice was obtained via gamma counting of ex vivo tissues. The four mice used for PET imaging were sacrificed after 48-h PET scan, and organs were harvested and analyzed quantitatively by a gamma-counter detector. The mean and SD of %ID/g have been corrected for natural decay of 64Cu. Liver was found to be the major organ where SiMn QDs 6 accumulated (Figure 4). In detail, the liver accumulated 12.4 ± 1.0%ID/g of SiMn QDs 6, whereas the left and right kidneys took up 4.1 ± 1.3%ID/g and 3.0 ± 0.3%ID/g of 6, respectively. Heart (4.2 ± 0.4%ID/g), intestine (3.8 ± 0.8%ID/g), spleen (2.4 ± 0.1%ID/g), and lung (2.2 ± 0.6%ID/g) also showed localization of SiMn QDs 6, and a small amount of 6 was found in the bladder (0.6 ± 0.5%ID/g) and brain (0.5 ± 0.1%ID/g).
Figure 4.
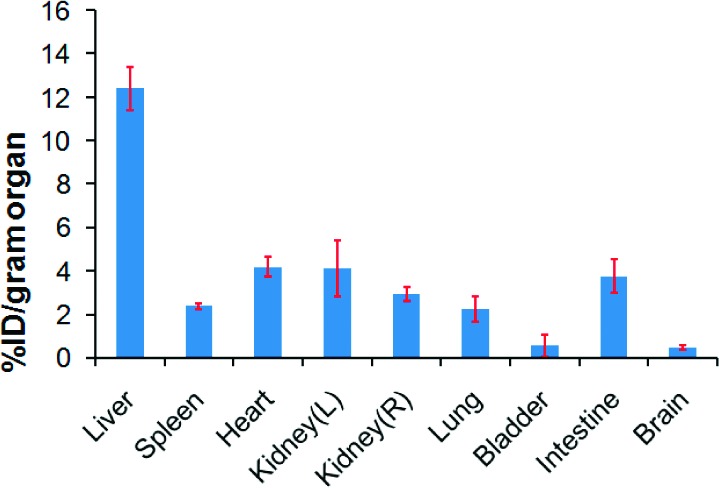
Ex vivo biodistribution of 64Cu-DO3A conjugated dextran SiMn QDs 6 in mice. Mice (n = 4) were sacrificed 48 h post intravenous injection. Organs were harvested and measured by well gamma counting.
Among many factors that may affect the biodistribution of systemically administered QDs in the body, the size of nanoparticles is critical.30−33 Immediately upon exposure to blood, QDs may be quickly adsorbed by opsonins and in turn flagged for phagocytosis, an obligate response of the immune system when encountering a nanoparticulate foreign body.30,34 Small nanoparticles (<7 nm) may be rapidly and efficiently eliminated via renal filtration with excretion into the urine, while larger particles are taken up nonspecifically by the reticuloendothelial system (RES) and end up in the liver, spleen, and lymphatic system, where they are excreted into the biliary system after hepatobiliary (HB) processing and enter the gall bladder and intestine.20,30,35−37 Our observations for biodistribution are consistent with expectations based on size (Figure 5). We conjecture that the fraction of SiMn QDs 6 with small hydrodynamic diameter was rapidly excreted from the body through renal filtration and urinary bladder, while the fraction of SiMn QDs 6 with larger hydrodynamic diameter was taken up by the RES and accumulated in the liver.
Figure 5.
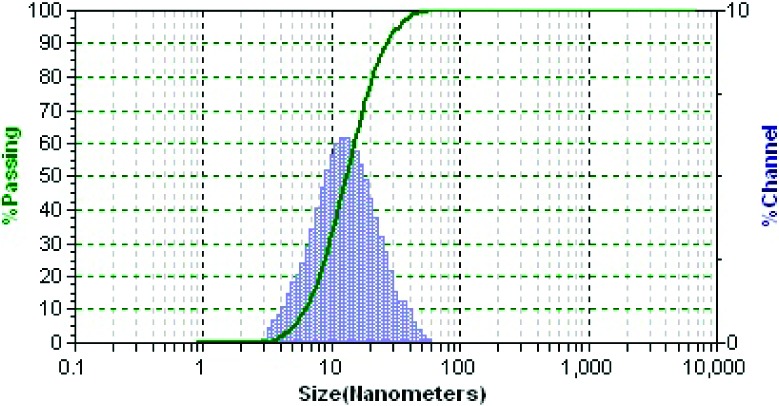
Hydrodynamic diameter of DO3A conjugated dextran SiMn QDs 5 measured by dynamic light scattering (DLS).
In conclusion, these results present the first analysis of in vivo biodistribution for Si QDs radiolabeled with a positron-emitting tracer. Knowledge of detailed biodistribution of QDs in living animals is of paramount importance before diagnostic or therapeutic application in the clinic is possible.4,6,32 The present study shows rapid clearance of dextran coated Si QDs from mouse bloodstream, and the QDs were excreted from the body via both renal filtration and urinary bladder, and accumulated in the liver, which may provide useful information for the future design of new QDs and nanoparticles for biomedical applications.
Acknowledgments
The authors wish to acknowledge the National Institute of Health (HL081108-01 and EB008576-01) and the Center for Molecular and Genomic Imaging at the University of California, Davis (U24 CA 110804) for support of this work. We thank Tonya M. Atkins, Michelle Connell, and Jennifer Fung for their assistance with ICP-MS measurement and gamma counting.
Author Contributions
C.Q.T. developed Si QDs coating synthesis, executed 64Cu radiolabeling and in vivo PET imaging, and wrote manuscript. X.C.M. synthesized Si cores. A.H. handled animals. S.M.K. and A.Y.L. conceived of design, syntheses, experiments and edited manuscript.
Supporting Information Available
Synthetic procedures and characterization of compounds 1−4 and nanoparticles 5 and 6, details of the stability measurements of 64Cu labeled dextran SiMn QDs, and in vivo PET imaging and ex vivo gamma counting procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ballou B.; Lagerholm B. C.; Ernst L. A.; Bruchez M. P.; Waggoner A. S. Noninvasive imaging of quantum dots in mice. Bioconjugate Chem. 2004, 15, 79–86. [DOI] [PubMed] [Google Scholar]
- Allen P. M.; Liu W. H.; Chauhan V. P.; Lee J.; Ting A. Y.; Fukumura D.; Jain R. K.; Bawendi M. G. InAs(ZnCdS) Quantum Dots Optimized for Biological Imaging in the Near-Infrared. J. Am. Chem. Soc. 2010, 132, 470–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila L. A.; Ebenstein Y.; Weiss S. Quantum Dots for In Vivo Small-Animal Imaging. J. Nucl. Med. 2009, 50, 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper M. L.; Cheng Z.; Lee S. W.; Bentolila L. A.; Iyer G.; Rao J. H.; Chen X. Y.; Wul A. M.; Weiss S.; Gambhirl S. S. MicroPET-based biodistribution of quantum dots in living mice. J. Nucl. Med. 2007, 48, 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley J. L.; Daar A. S.; Saner M. A. State of Academic Knowledge on Toxicity and Biological Fate of Quantum Dots. Toxicol. Sci. 2009, 112, 276–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pic E.; Bezdetnaya L.; Guillemin F.; Marchal F. Quantification Techniques and Biodistribution of Semiconductor Quantum Dots. Anti-Cancer Agents Med. Chem. 2009, 9, 295–303. [DOI] [PubMed] [Google Scholar]
- Warner J. H.; Hoshino A.; Yamamoto K.; Tilley R. D. Water-soluble photoluminescent silicon quantum dots. Angew. Chem., Int. Ed. 2005, 44, 4550–4554. [DOI] [PubMed] [Google Scholar]
- Zhang X. M.; Neiner D.; Wang S. Z.; Louie A. Y.; Kauzlarich S. M. A new solution route to hydrogen-terminated silicon nanoparticles: synthesis, functionalization and water stability. Nanotechnology 2007, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erogbogbo F.; Yong K. T.; Roy I.; Xu G. X.; Prasad P. N.; Swihart M. T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudeep P. K.; Page Z.; Emrick T. PEGylated silicon nanoparticles: synthesis and characterization. Chem. Commun. 2008, 6126–6127. [DOI] [PubMed] [Google Scholar]
- Tu C. Q.; Ma X. C.; Pantazis P.; Kauzlarich S. M.; Louie A. Y. Paramagnetic, Silicon Quantum Dots for Magnetic Resonance and Two-Photon Imaging of Macrophages. J. Am. Chem. Soc. 2010, 132, 2016–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. B.; Shin D. W.; Chen K.; Gheysens O.; Cao Q. Z.; Wang S. X.; Gambhir S. S.; Chen X. Y. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [DOI] [PubMed] [Google Scholar]
- Lecoq P. Molecular Imaging Challenges With PET. IEEE Trans. Nucl. Sci. 2010, 57, 1485–1491. [Google Scholar]
- Shokeen M.; Anderson C. J. Molecular Imaging of Cancer with Copper-64 Radiopharmaceuticals and Positron Emission Tomography (PET). Acc. Chem. Res. 2009, 42, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel F. M.; Higuchi T.; Javadi M. S.; Lautamaki R. Cardiac Positron Emission Tomography. J. Am. Coll. Cardiol. 2009, 54, 1–15. [DOI] [PubMed] [Google Scholar]
- Palko H. A.; Fung J. Y.; Louie A. Y. Positron emission tomography: A novel technique for investigating the biodistribution and transport of nanoparticles. Inhalation Toxicology 2010, 22, 657–668. [DOI] [PubMed] [Google Scholar]
- Jarrett B. R.; Gustafsson B.; Kukis D. L.; Louie A. Y. Synthesis of Cu-64-labeled magnetic nanoparticles for multimodal imaging. Bioconjugate Chem. 2008, 19, 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B.; Youens S.; Louie A. Y. Development of contrast agents targeted to macrophage scavenger receptors for MRI of vascular inflammation. Bioconjugate Chem. 2006, 17, 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. B.; Chen K.; Li Z. B.; Gambhir S. S.; Chen X. Y. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nucl. Med. 2007, 48, 1862–1870. [DOI] [PubMed] [Google Scholar]
- Geraldes C.; Laurent S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [DOI] [PubMed] [Google Scholar]
- Wadas T. J.; Wong E. H.; Weisman G. R.; Anderson C. J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg G.; Arnaud-Neu F.; Delgado R.; Felcman J.; Popov K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications. Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar]
- Glaus C.; Rossin R.; Welch M. J.; Bao G. In Vivo Evaluation of Cu-64-Labeled Magnetic Nanoparticles as a Dual-Modality PET/MR Imaging Agent. Bioconjugate Chem. 2010, 21, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. P.; Meyer L. A.; Capretto D. A.; Sherman C. D.; Anderson C. J. Receptor-binding, biodistribution, and metabolism studies of Cu-64-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother. Radiopharm. 2008, 23, 158–171. [DOI] [PubMed] [Google Scholar]
- Duimstra J. A.; Femia F. J.; Meade T. J. A gadolinium chelate for detection of beta-glucuronidase: A self-immolative approach. J. Am. Chem. Soc. 2005, 127, 12847–12855. [DOI] [PubMed] [Google Scholar]
- Wangler C.; Wangler B.; Eisenhut M.; Haberkorn U.; Mier W. Improved syntheses and applicability of different DOTA building blocks for multiply derivatized scaffolds. Bioorg. Med. Chem. 2008, 16, 2606–2616. [DOI] [PubMed] [Google Scholar]
- Herman S.; Persijn G.; Vandekerckhove J.; Schacht E. Synthesis of dextran derivatives with thiol-specific reactive groups for the preparation of dextran protein conjugates. Bioconjugate Chem. 1993, 4, 402–405. [DOI] [PubMed] [Google Scholar]
- Li Y. L.; Zhu L.; Liu Z. Z.; Cheng R.; Meng F. H.; Cui J. H.; Ji S. J.; Zhong Z. Y. Reversibly Stabilized Multifunctional Dextran Nanoparticles Efficiently Deliver Doxorubicin into the Nuclei of Cancer Cells. Angew. Chem., Int. Ed. 2009, 48, 9914–9918. [DOI] [PubMed] [Google Scholar]
- Jones-Wilson T. M.; Deal K. A.; Anderson C. J.; McCarthy D. W.; Kovacs Z.; Motekaitis R. J.; Sherry A. D.; Martell A. E.; Welch M. J. The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nucl. Med. Biol. 1998, 25, 523–530. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Duan H. W.; Mohs A. M.; Nie S. M. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Delivery Rev. 2008, 60, 1226–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis F.; Pridgen E.; Molnar L. K.; Farokhzad O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S.; Liu W.; Misra P.; Tanaka E.; Zimmer J. P.; Ipe B. I.; Bawendi M. G.; Frangioni J. V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh L.; Nigavekar S. S.; Nair B. M.; Lesniak W.; Zhang C.; Sung L. Y.; Kariapper M. S. T.; El-Jawahri A.; Llanes M.; Bolton B.; Mamou F.; Tan W.; Hutson A.; Minc L.; Khan M. K. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomed.-Nanotechnol. Biol. Med. 2007, 3, 281–296. [DOI] [PubMed] [Google Scholar]
- Owens D. E.; Peppas N. A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [DOI] [PubMed] [Google Scholar]
- Longmire M.; Choyke P. L.; Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime S.; Caravan P. Biodistribution of Gadolinium-Based Contrast Agents, Including Gadolinium Deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimbo L. M.; Sarparanta M.; Santos H. A.; Airaksinen A. J.; Makila E.; Laaksonen T.; Peltonen L.; Lehto V. P.; Hirvonen J.; Salonen J. Biocompatibility of Thermally Hydrocarbonized Porous Silicon Nanoparticles and their Biodistribution in Rats. ACS Nano 2010, 4, 3023–3032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.