Abstract
Objectives
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are a unique entity with malignant potential. Histologically, pancreatic ductal adenocarcinoma (PDAC) arising in IPMN (intraductal papillary mucinous carcinoma [IPMC]) appears similar to sporadic PDAC; biologically, however, IPMC seems to have a less aggressive clinical course. Little is known about the genetic signature of IPMC. In this study, we describe a novel xenograft model and cell culture created to biologically and genetically characterize these tumors.
Methods
Xenograft mice and cell lines were created from IPMC. Global genomic changes were evaluated by cytogenetic analysis and array comparative genomic hybridization. Specific mutations and sonic hedgehog (Shh) pathway activity were examined and xenografts evaluated for sensitivity to anti-Shh therapy.
Results
Cytogenetic analysis showed a tetraploid karyotype with multiple aberrations. KRAS and p53 mutations and overexpression of the Shh pathway were identified. Array comparative genomic hybridization revealed multiple chromosomal aberrations comparable with previously published data in IPMNs. Murine xenograft tumors were sensitive to anti-Shh treatment.
Conclusions
Characterization of IPMC cell lines and xenografts reveals similarities to previously published data on IPMN. In comparison to PDAC, moreover, these data reveal shared aberrations and distinct genomic changes. Thus, these xenograft model and cell lines may be useful for future preclinical investigations.
Keywords: intraductal papillary mucinous neoplasms, pancreas, xenograft, cell line, genetic characterization
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a clinically well-described entity of cystic pancreatic tumors.1,2 Intraductal papillary mucinous neoplasms have become a clinically important issue because they are increasingly diagnosed and account for approximately 10% to 20% of all pancreatic resections.3-7 Intraductal papillary mucinous neoplasms are characterized by mucin-producing cells, cystic dilation of the pancreatic duct system, and intraductal papillary growth.8 Histologically, IPMNs may demonstrate a wide spectrum of cellular atypia ranging from mucinous metaplastic epithelium to invasive adenocarcinoma. Similar to conventional pancreatic ductal adenocarcinoma (PDAC), patients with IPMNs often present between the sixth and eighth decades of life, with lesions located predominantly in the head of the pancreas.1,3 However, compared with PDAC, IPMNs have a less aggressive biological behavior.9 Lesions are frequently detected incidentally before malignancy develops,4 and even when the lesion is associated with invasive carcinoma (intraductal papillary mucinous carcinoma [IPMC]), patients have fewer lymph node metastases, relatively limited local tumor spread, and a significantly better prognosis than those with PDAC.6,10-16 Whereas patients with PDAC have 5-year survival rates of less than 25% after curative resection,17-19 patients who undergo surgery for IPMC have a 5-year survival of more than 60%.14,20-23 Consequently, many authors believe that IPMCs have distinct genetic and biological origins and therefore display a different clinical behavior from PDAC.2,24 Although there is evidence for significant genetic differences between IPMC and PDAC,24,25 the pathological and, in particular, genetic backgrounds underlying this hypothesis remain to be defined. To better understand the molecular mechanisms playing a role in neoplastic development, cell culture and xenograft models are widely used.26 However, to our knowledge, there are no established molecular models for IPMNs currently available.
In this paper, we report the creation of IPMC xenograft tumor and cell lines derived from a 57-year-old man who was found to have a pT3 N1 M0 G2 adenocarcinoma arising in a main duct IPMN. These novel IPMC xenograft model and cell line culture may give us new insights into the molecular mechanisms of IPMC and provide a new murine model for future preclinical investigation of malignant IPMNs.
MATERIALS AND METHODS
Establishment of the Xenograft Tumor Line
Pathologic diagnosis was of a moderately differentiated (G2) adenocarcinoma arising in the background of IPMN, combined main and branch duct types. Five of the 13 lymph nodes isolated from the specimen contained a metastatic focus. A tissue sample approximately 1 cm in diameter taken from the invasive component was reduced to small pieces with scalpel blades, put into a medium (Roswell Park Memorial Institute [RPMI] 1640 medium, 1×; Mediatech, Inc, Herndon, Va and Matrigel; BD Biosciences, San Jose, Calif), and implanted subcutaneously into 10 immunodeficient mice (Jackson Laboratory, Bar Harbor, Maine) as a first mouse passage (MP1). Tumor growth was monitored, and mice with tumors 350 KLor more were killed; the tumor harvested, reduced to small pieces, and reimplanted into a second generation of mice (MP2). Tumors were then reimplanted several times to increase the number of xenograft tumors from this line. For every tumor passage, part of the tumor tissue was preserved in 10% formalin solution (SF 100-4; Fisher Scientific, Pittsburgh, Pa) for histological examination.
Detection of KRAS and p53 Mutations in Xenografts
A 5- to 10-mg sample DNA was isolated using a standard protocol (Puregene DNA purification kit; Gentra Systems, Minneapolis, Minn). Subsequently, the KRAS loci at codon 12 (exon 2) and p53 sequences (exons 57–9) were amplified using polymerase chain reaction (PCR). Sequences of the primers are listed in Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/MPA/A14). Conditions for the thermocycler were as follows: an initial denaturation step of 95°C for 10 minutes, followed by 33 cycles of 94°C for 30 seconds, 55°C for 45 to 60 seconds (depending on the length of the PCR product), and 72°C for 45 seconds. After amplification, PCR products were purified using the Wizard SV Gel and PCR Clean-Up system (Promega, Madison, Wis). Sequencing in the forward and reverse directions was done by means of an ABI 3730XL Sequencer (Applied Biosystems, Foster City, Calif) in the DNA Core Facility of the Massachusetts General Hospital.
Culture Procedure
Fresh pieces of tissue derived from a harvested xenograft tumor were removed aseptically and transferred to the RPMI medium (RPMI 1640, 1×; Mediatech, Inc). The tissue was minced and transferred to culture dishes. The RPMI 1640 medium containing 2-mmol/L l-glutamine, 10-mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1-mmol/L sodium pyruvate, 4.5-g/L glucose, 1.5-g/L bicarbonate, and 15% fetal bovine serum was used as the culture medium. The cell culture was kept at 37°C and the medium changed twice a week. To calculate the doubling time of the cell culture, a suspension of 5 × 104 cells was plated onto 35-mm plastic dishes in the culture medium described previously. The number of cells was counted in duplicate at 24-hour intervals for 5 days.
To confirm that the cell culture contained tumor cells derived from the IPMC tumor, DNA derived from approximately 3.6 × 106 cells was isolated according to standard procedures. The KRAS locus was amplified by PCR and the purified product sequenced bidirectionally as described previously. In addition, 1 × 106 cultured cells derived from a third culture passage were injected subcutaneously into the flank of a nude mouse to reproduce the IPMN tumor in vivo.
Karyotyping
The cytogenetic study of the cell line was performed in G-banded metaphase cells obtained from a 7-day-old culture and analysis of a total number of 10 cells. Karyotyping was performed at the Dana Farber/Harvard Cancer Center Cytogenetics Core Facility, Brigham and Women’s Hospital, Boston.
Array Comparative Genomic Hybridization
A human IPMC xenograft tumor was harvested and tumor-surrounding murine mesenchyme removed. Fresh-frozen sections were evaluated by hematoxylin and eosin staining to confirm a cellularity of more than 95%. DNA was isolated from 140 mg of tumor tissue by standard procedures (Puregene DNA purification kit). Normal male DNA (Promega, Madison, Wis) was used as reference. Array comparative genomic hybridization (CGH) was performed using Agilent Technologies 244k oligonucleotide arrays (Agilent Control Software, Santa Clara, Calif) according to the recommended protocol as previously described.27 Slides were scanned with an Agilent G2565 micro-array scanner. Sixteen-bit tagged image file format images were captured with GenePix Pro v7.0 (Agilent Control Software; Agilent Technologies, Santa Clara, Calif) and the data extracted (Agilent Feature Extraction Software v9.1; Agilent Technologies) and analyzed (CGH Analytic Software; Agilent Technologies). Copy number alterations were considered significant if the log2 ratio was ±2 SDs from the mean intensity of the entire experiment.28 In addition to tumor tissue derived from the human specimen, xenograft tumors contain a certain percentage of mouse mesenchyme. To exclude artifacts derived from murine tissue, CGH was performed using a sample of normal mouse liver DNA as control.
Real-Time Quantitative PCR for Sonic Hedgehog Pathway Signaling
RNA was extracted from xenograft tumor tissues of approximately 5- to 10-mg weight (RNAqueous isolation kit; Ambion, Austin, Tex). One-step multiplex TaqMan real-time quantitative reverse transcriptase PCR was performed using an ABI Prism 7700 Sequence Detection system (Applied Biosystems, Foster City, Calif). Expression levels of human sonic hedgehog (Shh), Patched 1 (Ptch1), Patched 2 (Ptch2), Smoothened (Smo), and Gli2 were evaluated, using 18S ribosomal RNA as internal control. Probes and primers were designed to span exon-exon junctions to avoid amplification of contamination genomic DNA (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/MPA/A14). The thermal cycler conditions for these experiments were reverse transcription for 30 minutes at 48°C, initial activation step for 10 minutes at 95°C, followed by 40 cycles of denaturation for 15 seconds at 95°C and annealing/extension for 60 seconds at 60°C. Fluorescence data were collected during the annealing step and analyzed using the Sequence Detection System 1.7 software (Applied Biosystems). Relative gene expression was determined based on corresponding threshold cycle values according to the ABI description in the TaqMan Protocol (TaqMan Cytokine Gene Expression Plate 1 protocol; Applied Biosystems). Variability among runs was controlled by use of a standardized sample in each run. Pooled normal pancreatic tissue was used as control and reference values.
Sensitivity to Anti-Shh Treatment
Intraductal papillary mucinous carcinoma xenograft mice were treated with Shh pathway inhibitors, 300-μg 5E1 sc (anti-Shh antibody), 0.6-mg intraperitoneal cyclopamine (Smoothened inhibitor), and 75-μg intraperitoneal forskolin (Gli antagonist). Xenograft mice carrying tumors approximately 350 μL in size were randomly divided into 3 treatment groups with 5 animals in each group. All therapies were administered by daily injections for 7 days. Control animals received normal saline. Treatment response was determined by tumor volume change, tumor proliferation by immunohistochemistry (Ki-67 staining), and the extension of fibrosis and necrosis by percentage of viable glands (viable gland density).
RESULTS
Intraductal Papillary Mucinous Neoplasm Xenograft Tumors Preserved the Phenotype of the Primary Tumor
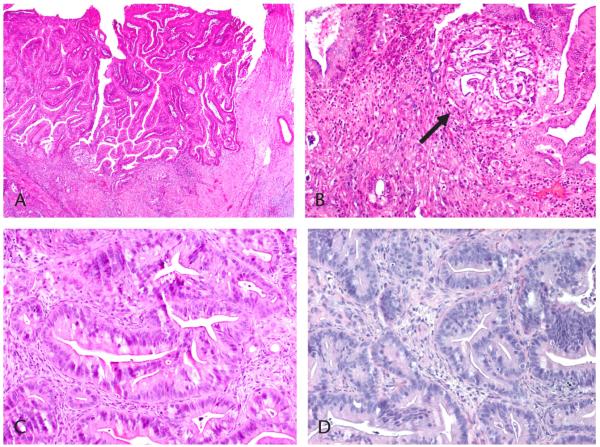
Histological examination of the resected primary tumor revealed a moderately differentiated adenocarcinoma of gastric subtype, arising from a main duct IPMN (Figs. 1A–C). From this primary, a xenograft tumor line was successfully established. Hematoxylin and eosin staining of xenograft tumors revealed that xenografts preserved the characteristic histological attributes of the parent tumor (Fig. 1D). Histological condition remained stable over 4 generations of mice. Thus, xenografts preserved the phenotype of the primary tumor over several mouse passages.
FIGURE 1.
Histological diagnosis of IPMC. Hematoxylin and eosin stain. The primary tumor shows intraductal papillary growth (A). The arrow indicates malignant features (B) and invasive ducts (C) (original magnifications ×40, ×100, and ×200, respectively). The IPMC xenograft tumor reveals features similar to those of a moderately differentiated adenocarcinoma (D; original magnification ×100).
Mutational Analysis of KRAS and p53
Pancreatic ductal adenocarcinoma is known for its signature progression model, with an early dependence on KRAS mutations and a high incidence of p53 mutations.29 However, little is known about the significance of these genetic aberrations in IPMC. To determine whether xenografted murine IPMCs bear similar signaling mutations, we performed sequence analysis. Direct sequencing of isolated xenograft tumor DNA showed the presence of a mutation in codon 12 of exon 2 of the KRAS gene (GGT>GTT) changing the amino acid sequence from glycine to valine; codon 13 remained unchanged. Furthermore, p53 evaluation showed a mutation involving codon 273 (CGT>TGT) of exon 8, which changed the sequence from arginine to cysteine. In contrast, the exons 5, 6, 7, and 9 were unchanged and showed wild-type sequences. These same genetic changes were identified in later mouse passages, suggesting minimal genetic drift. Thus, invasive adenocarcinoma arising in IPMN shares some mutational similarities with PDAC. Histologically and genetically, furthermore, the xenografts seem to faithfully recapitulate the human tumor and preserve these characteristics over the course of several mouse passages.
Cell Culture
A cell line was established that was derived from an IPMC specimen (Supplementary Fig. 1, for the IPMC cell culture, Supplemental Digital Content 2, http://links.lww.com/MPA/A15). Tumor growth occurred after a few days. The doubling time in cell culture was 32 hours in the phase of exponential growth. Molecular analysis of DNA isolated from the cell culture showed the presence of the mutation in codon 12 of the KRAS gene (GGT>GTT; Gly>Val; Supplementary Fig. 2, for the KRAS mutation at codon 12, Supplemental Digital Content 3, http://links.lww.com/MPA/A16), which was present in the DNA of the xenograft tumors and the parent tumor. To confirm that these cells preserve tumorigenic potential, approximately 1 × 106 cultured cells were injected subcutaneously into the flank of an immunodeficient nude mouse, resulting in tumor growth. The resulting xenograft tumor was harvested and histological examination result showed the features of the parent IPMC specimen.
Karyotyping
Karyotyping of the novel IPMC cell culture was performed. GTG-banding of 14 cells revealed an essentially tetraploid karyotype with multiple chromosomal aberrations. Recurrent numerical changes and aberrations were observed in addition to nonclonal changes. Karyotypes of 5 representative cells were assessed. Some of the chromosomal aberrations were recurrent, whereas others were single changes. Results of one representative cell were as follows (Fig. 2): X,−X,−Y,−Y,−3,−4,+5,−6,−6,−8,−9,+10,−11,−12,idic(12)(p13),−13,−13,add(13)(p13),−14,−15,add(15),−17,−17,−17,−17,−18,−18,−19,−19,+20, +del(20)(q11.2)×2,−22,+mar1,+mar2. In summary, all karyotypes examined shared the following abnormalities: −4, −6, −8, −17, and −18.
FIGURE 2.
Karyotype of IPMC cell culture. A typical G-banded karyotype of the IPMC cell culture shows basically tetraploid features with multiple chromosomal abnormalities. mar indicates marker chromosomes.
Array CGH Reveals Multiple Copy Number Changes
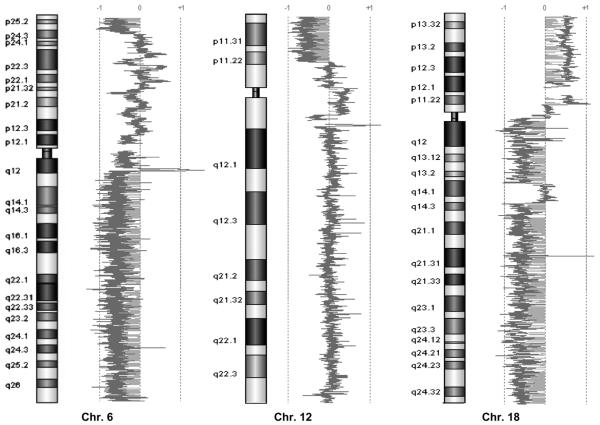
To evaluate copy number changes below the detection limit of karyotyping, we performed array CGH on IPMC xenografts (Fig. 3). Array CGH has become a powerful tool to examine chromosomal abnormalities in human tissues with high resolution. This technique allows us to detect chromosomal gains and losses and identify potential tumor suppressor genes or oncogenes that might play a role in the development of neoplastic changes in malignant tumors. To exclude potential artifacts derived from murine mesenchyme, which might hybridize to the array, normal mouse liver DNA was used as control. Hybridizing mouse DNA to the (human) array platform, no losses or gains of larger chromosomal regions were found, indicating that the copy number changes detected in xenografts were real and not attributable to mouse mesenchyme contamination.
FIGURE 3.
Array CGH. Array CGH results for chromosome 6, 12, and 18, showing the following copy number changes: loss of distal 6p, loss of 6q, loss of 12p13.1–13.33, loss of 18q, and gain of 18p.
Using array CGH in xenograft DNA, we found chromosomal gains at 3q, 5p, 9p, 18p, and 19q, whereas single-copy losses involved chromosomes 4, 5q, 6q, distal 6p, 8p, 9q, 12p13.1–13.33, 13q, 14q, 15q, 17p, 18q, 19p, and 21. Chromosomal losses included regions of several known tumor suppressor genes such as APC (chromosome 5q21–q22), Pth (chromosome 9q22.3), BRCA2 (chromosome 13q12.3), RB1 (chromosome 13q14.1–q14.2), p53 (chromosome 17p13.1), SMAD 4 (chromosome 18q21.1), and DCC (chromosome 18q21.3), suggesting that these genes play a role in neoplastic changes in IPMNs. No homozygous deletions were found with array CGH. The abnormalities we found for this cell line using array CGH were similar to those found using karyotyping but involved more specific regions (−4, −6q, −6p distal, −8p, −17p, and −18q).
Real-Time Quantitative PCR for Shh Pathway Signaling
In addition to mutations of tumor suppressor genes, Shh misexpression and subsequent hedgehog (Hh) pathway activation are believed to play an important role in the initiation and maintenance of PDAC.30 However, to date, the role of Shh in the development of adenocarcinoma arising in IPMNs is poorly understood. To determine whether the Hh pathway eventually plays a role in malignant changes in IPMNs, we investigated whether it is overexpressed in IPMC xenografts.
Compared with normal pancreatic tissue, Shh pathway members were found by reverse transcriptase PCR to be over-expressed in the IPMC xenograft tumors examined (Fig. 4). The Shh ligand was overexpressed with an average of 634.3-fold. Furthermore, there was overexpression of hedgehog pathway members: receptors Ptch1, Ptch2, and Smo were elevated an average of 436.7-, 56-, and 93.3-fold, respectively. In addition, the Hh target gene Gli2 was overexpressed 2606-fold compared with a pool of normal pancreatic control DNA. Thus, Shh overexpression and subsequent Hh pathway activation were observed in xenografts derived from IPMC.
FIGURE 4.
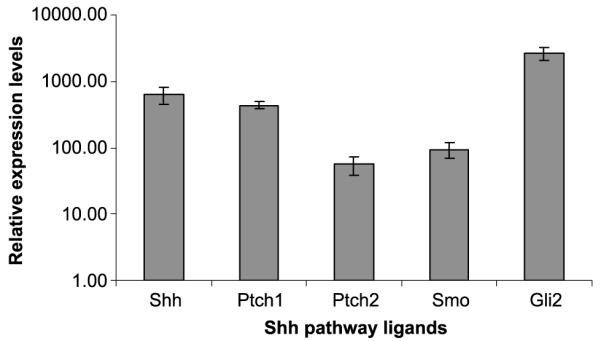
Sonic hedgehog pathway misexpression. Real-time quantitative PCR results for relative expression of Shh pathway members compared with normal pancreas. The graph shows fold overexpression of sonic hedgehog (Shh), Patched1 (Pch1), Patched2 (Ptch2), Gli2, and Smoothened (Smo).
Sensitivity to Anti-Shh Treatment
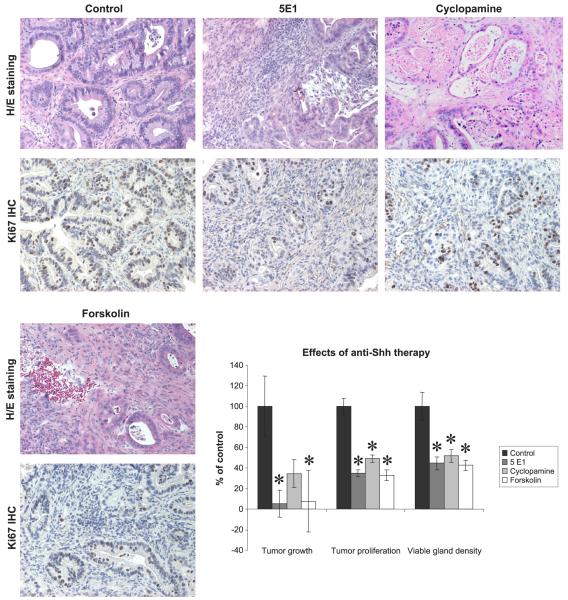
To determine whether the Shh pathway is important for maintenance and growth of IPMCs, xenografted tumors were treated with targeted anti-Shh therapy. The Shh pathway was attacked on 3 different levels: with 5E1 (against the ligand), cyclopamine (against Smo), and forskolin (against Gli). Treatment with 5E1 and forskolin resulted in a significant (P = 0.007 and P = 0.049, respectively) reduction in tumor growth compared with controls (Fig. 5). Treatment with cyclopamine also led to marked tumor growth reduction; however, this reduction did not reach statistical significance (P = 0.17). Besides the reduction in tumor growth, all treatments (5E1, cyclopamine, and forskolin) resulted into a highly significant (P < 0.001) reduction in proliferative cells and viable gland density (Fig. 5). In summary, all tumors derived from the IPMC xenograft tumor line were highly sensitive to Shh pathway inhibition. Thus, Shh is an important factor for tumor growth and survival in IPMC that is similar to its role in PDAC.
FIGURE 5.
Histological examination results. Anti-Shh treatment effects. Hematoxylin and eosin and immunohistochemistry Ki-67 staining (all images in original magnification ×100). Compared with controls, xenografts treated with 5E1, cyclopamine, or forskolin show a reduction of viable glands and proliferative cells. Treatment of xenograft mice with 5E1 and forskolin significantly reduces tumor growth (P = 0.007 and P = 0.049, respectively), proliferation (Ki-67–positive cells), and viable gland density compared with controls (P < 0.001).
DISCUSSION
Compared with PDAC,17-19 IPMC seems to have a less aggressive biological behavior, with subsequent improved survival. On the molecular level, significant differences between IPMC and PDAC have been reported.24,25 However, in contrast to the great number of models available for the more common PDAC,26,31,32 to our knowledge, no cell cultures or xenograft lines have been created from IPMC.
In the present study, we investigate a novel cellular model for IPMC and evaluate the role of the Shh pathway in tumor maintenance and growth. Using array CGH, we identify a number of chromosomal losses, including known tumor suppressor genes such as APC, BRCA2, RB1, p53, and SMAD4. It is likely that these genes play a role in the neoplastic changes of IPMNs. Furthermore, xenograft tumors were found to be dependent on the Shh pathway for survival and proliferation.30 Xenografts up-regulate this tumor pathway, and inhibition results in significant reduction of tumor growth, proliferation, and viable gland density. To our knowledge, this is the first study to use xenografts to investigate chemotherapies in IPMCs. The present molecular model of IPMC allows a better understanding of specific cellular characteristics in this type of tumor. Furthermore, it permits investigation of molecular and genetic features in changeable environments in vitro and in vivo.
Although patients with IPMC show better survival than patients with its counterpart, PDAC,6,10-16 the latter remains a potentially deadly disease; approximately 30% of patients undergoing curative surgery die within a 5-year period,2,13,14,21-23 mainly from metastatic disease. Before entering clinical development, chemotherapeutics are usually tested in commercially available cell lines followed by xenograft models established from these lines. Xenografted tumors are considered to maintain their fundamental genotypic features despite serial passages and to represent the genetic heterogeneity of the primary tumor.26 Thus, the novel IPMC xenograft model and cell line model presented here not only provide insight into molecular mechanisms involved in neoplastic changes in IPMCs but may also serve as a platform for testing cancer drug treatments in vivo.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr V. Petkova of the Harvard Institutes of Medicine for providing the TaqMan technical assistance and the Cytogenetics Core Laboratory of the Dana Farber/Harvard Cancer Center for karyotyping the novel IPMC cell culture.
This work was supported by funding from the Karin Grunebaum Cancer Research Foundation (S.F.), the German Research Foundation (S.F.), the Marshall K. Bartlett Fellowship (J.L.), the American College of Surgeons Clowes Award, and the National Institutes of Health (S.P.T.) and by research grant P30CA00651 from the National Cancer Institute/National Institute of Health.
Footnotes
Supplemental digital contents are available for this article. Direct URL citations appear in printed text and are provided in the HTML and PDF versions of this article on the Journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. discussion 321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlon KC. Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol. 2005;23:4518–4523. doi: 10.1200/JCO.2005.22.517. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–433. doi: 10.1001/archsurg.138.4.427. discussion 433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417–428. doi: 10.1016/s1091-255x(02)00163-4. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 8.Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007;20(suppl 1):S71–S93. doi: 10.1038/modpathol.3800706. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Goggins M. Epigenetic alterations in intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2006;13:280–285. doi: 10.1007/s00534-005-1056-2. [DOI] [PubMed] [Google Scholar]
- 10.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 12.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 14.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 15.D’Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–18. doi: 10.1016/S1091-255X(02)00152-X. discussion 18–19. [DOI] [PubMed] [Google Scholar]
- 17.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 19.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 20.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 21.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal”pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima N, Sakamoto M, Mukai K, et al. Intraductal papillary components in invasive ductal carcinoma of the pancreas are associated with long-term survival of patients. Hum Pathol. 2001;32:834–841. doi: 10.1053/hupa.2001.26473. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 24.Fujii H, Inagaki M, Kasai S, et al. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151:1447–1454. [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 27.Waldman FM, DeVries S, Chew KL, et al. Chromosomal alterations in ductal carcinomas in situ and their in situ recurrences. J Natl Cancer Inst. 2000;92:313–320. doi: 10.1093/jnci/92.4.313. [DOI] [PubMed] [Google Scholar]
- 28.Iafrate AJ, Feuk L, Rivera MN, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 29.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 30.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M, Shimada Y, Tanaka H, et al. Characterization of six cell lines established from human pancreatic adenocarcinomas. Cancer. 1999;85:832–840. doi: 10.1002/(sici)1097-0142(19990215)85:4<832::aid-cncr10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Sorio C, Bonora A, Orlandini S, et al. Successful xenografting of cryopreserved primary pancreatic cancers. Virchows Arch. 2001;438:154–158. doi: 10.1007/s004280000343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.