Abstract
A new bifunctional chain transfer agent (CTA) containing alkyne end groups was designed, synthesized and used for direct synthesis of clickable telechelic polymers. Good control of reversible addition-fragmentation chain transfer (RAFT) polymerization of N-(2-hydroxypropyl)methacrylamide (HPMA) was achieved by using the new CTA, as indicated by a linear increase of number average molecular weight (Mn) with conversion and low polydispersity (PDI) (<1.1). In particular, enzymatically degradable multiblock HPMA polymers were readily prepared by subsequent reaction with αω, -diazido oligopeptide (GFLG) sequence via CuI catalyzed alkyne-azide cycloaddition. Upon exposure of high molecular weight fractions of multiblock polyHPMA to papain or cathepsin B, the polymer was degraded into segments of molecular weight and narrow polydispersity similar to those of the initial telechelic polyHPMA.
Keywords: biodegradable, telechelic, multiblock, N-(2-hydroxypropyl)methacrylamide, click reaction, RAFT polymerization
Introduction
Water-soluble N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers have been extensively studied as drug and gene delivery systems due to their proven biocompatibility, synthetic flexibility and well-developed conjugation chemistry.1,2 However, one major factor that has hindered the translation of polymer-drug conjugates into the clinic is their nonbiodegradability; hence the molecular weight of the polymer carrier is limited to a range below the renal threshold (45–50 kDa).3,4 Nevertheless, the investigations on the accumulation of polymer-drug conjugates in solid tumors indicate that the higher the molecular weight, the higher the accumulation at target site, with concomitant improvement in efficacy.5,6 To overcome this dilemma, biodegradable high molecular weight HPMA copolymers were designed by connecting synthetic polymeric chains via enzyme-sensitive oligopeptide segments to regulate the molecular weight as well as the biodegradablility of the (linear) multiblock copolymer; eventually the polymer segments would be eliminated from the body without impairing biocompatibility.7,8 Many approaches to the preparation of multiblock copolymers have been reported, consisting of the synthesis of telechelic polymers followed by coupling reactions. 9–12 Controlled/living radical polymerization techniques have been widely used to prepare well-defined polymer precursors. For example, Matyjaszewski’s group synthesized bromo-terminated polystyrene by atom transfer radical polymerization (ATRP) using a bifunctional initiator, dimethyl 2,6-dibromoheptadioate.13 After chain-end-modification, the resulting α,ω-diazido-terminated polymer was coupled with propargyl ether by CuI-catalyzed azide-alkyne cycloaddition (CuAAC), resulting in multi-segmented polymers with moderate to high molecular weights. Gemici et al. synthesized telechelic poly(methyl methacrylate) (PMMA) containing α,ω-thiocarbonylthio groups via RAFT polymerization using a dithiobenzoate chain transfer agent (CTA).11 α,ω-Dithiol telechelic polymers were obtained by transformation of dithiobenzoate end groups into thiols by aminolysis. Multiblock copolymers were produced either by oxidation to form disulfide bridges11 or by thiol-ene click reaction.12 Recently our group has used tetrapeptide (GFLG) modified dithiobenzoates to mediate RAFT polymerization of HPMA.7,8 Following chain end-modification, homo- and hetero-telechelic polyHPMAs were synthesized. Multiblock degradable polyHPMAs were obtained by CuAAC 7 and thiol–ene8 click reactions.
Although RAFT polymerization allows synthesis of a variety of end-functionalized polymers by utilizing functionalized CTA, usually post-polymerization modifications are inevitable to generate telechelic polymers. Few direct syntheses of α,ω-telechelic polymers with controlled structure have been described. Lai et al.14 developed a symmetrical trithiocarbonate for synthesis of telechelic polyacrylates and polyacrylamides in one step. But the limitation of this strategy was the poor control of polymerization of methacrylic monomers. The same problem was observed by Boyer et al.15 where a heterobifunctional CTA bearing α-azide and ω-dithiopyridine groups was used to mediate RAFT polymerization of N-isopropylacrylamide (NIPAAm). Well-defined heterotelechelic poly(NIPAAm) was obtained in one-step. However, when this RAFT agent was used for HPMA and MMA, it failed to control the polymerization process.
In this study, we describe a straightforward approach for direct synthesis of α,ω-dialkyne telechelic polyHPMA with well-controlled structures prepared by RAFT polymerization using a new, efficient bifunctional CTA. To the best of our knowledge, this is the first example of one- step synthesis of a clickable telechelic polymer without post-polymerization modification. Moreover, a diazide-modified enzyme-sensitive oligopeptide, GFLG, was used as linker to connect α,ω-dialkyne polyHPMA via CuI-catalyzed Huisgen 1,3-dipolar cycloaddition, leading to water-soluble and biodegradable multiblock polyHPMA with high molecular weight. The biodegradability was confirmed by incubating the multiblock polyHPMA with papain and lysosomal enzyme cathepsin B.
Experimental Section
Materials
4,4′-Azobis(4-cyanopentanoic acid) (V-501, 99%), N,N-diisopropylethylamine (DIPEA, ≥99%), propargylamine (97%), 5-hexynoic acid (97%), cysteamine, carbon disulfide (99+%), p-toluenesulfonyl chloride (TsCl, 99%), silica gel (60 Å, 60–200 mesh), trifluoroacetic acid (99%), piperidine (99.5+%), copper(I) bromide (99.999%), and L-ascorbic acid were from Aldrich. 2-Chlorotrityl chloride resin, Fmoc-Lys(ivDde)-OH, Fmoc-Gly-OH, Fmoc-Leu-OH, Fmoc-Phe-OH, 1-hydroxybenzotriazole (HOBt) and N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uranium hexafluorophosphate (HBTU) were purchased from Aapptec (Louisville, KY). 4,4′-Azobis(isobutyronitrile) (AIBN, Fluka, 98%) was recrystalled from ethanol. 4-Azidobutyric acid16 and N-(2-hydroxypropyl)methacrylamide (HPMA)17 were prepared as previously described.
Analytical Techniques
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Mercury-400 spectrometer. Mass spectra were recorded on a Quattro II Triple Quadrupole Mass Spectrometer (Micromass) system using electro-spray ionization (ESI). Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra were measured on a Voyager-DE instrument (STR Biospectrometry Workstation, PerSeptive Biosystems, Farmington, MA). Samples were spotted using dried-droplet method. Fresh solution of saturated α-cyano-4-hydroxy cinnamic acid matrix (CHCA) in a solvent system of 50 : 50 water : acetonitrile 0.1% TFA was prepared by thoroughly mixing the matrix powder with 0.5 mL of solvent in a 1.7 mL Eppendorf tube, and then centrifuged to pellet of un-dissolved matrix. The supernatant of this matrix solution was used for sample preparation for MALDI analysis. Samples (0.5 μL of 1 pmol/μL) were loaded onto a target plate and mixed on the target with 0.5 μL of supernatant of saturated matrix solution. Then the sample spot was air-dried and the mixture allowed to co-crystallize; then the spot was ablated with a pulsed nitrogen (N2) laser (337 nm and 20 Hz) from the plate while the sample was simultaneously desorbed and ionized, then accelerated into a flight tube (typically 20 kV). All MALDI spectra were acquired in reflector mode Polymerization conversion was determined by the measurement of remaining HPMA monomer concentration at different time points using RP-HPLC (Agilent Technologies 1100 series, Zorbax C8 column 4.6×150 mm) with gradient elution from 2 to 90% of Buffer B within 20 min and flow rate 1.0 mL/min (Buffer A: deionized water with 0.1% TFA, Buffer B: acetonitrile containing 0.1% TFA). Number-average molecular weight (Mn), weight-average molecular weight (Mw) and polydispersity (PDI) were measured using size-exclusion chromatography (SEC) on an ÄKTA/FPLC system (GE Healthcare) equipped with three online detectors: three angle light scattering detector miniDAWN TREOS (wavelength 658 nm), RI detector Optilab-rEX (Wyatt Technology, Santa Barbara, CA), UV detector UPC-900 (AKTA) set for 280 nm detection), using GE Healthcare columns: Superose 6 HR10/30 (molecular weight range for hydrophilic neutral polymers 15 kDa - 300 kDa/14 mL separation volume) or Superose 12 HR10/30 column (10 kDa-70 kDa/12 mL separation volume); flow rate 0.4 mL/min. The dn/dc value (0.17 for majority of polymers under this study) was calculated using ASTRA software assuming 100% recovery of sample. The preparative Superose 6 HR 16/60 column was used for the fractionation of click product, separation range 20 kDa - 300 kDa/60 mL eluent); flow rate 1.0 mL/min. Fractions that were collected at 5 min intervals represent 1/12 of the separation volume. UV-vis spectra were measured on a Varian Cary 400 Bio UV-visible spectrophotometer.
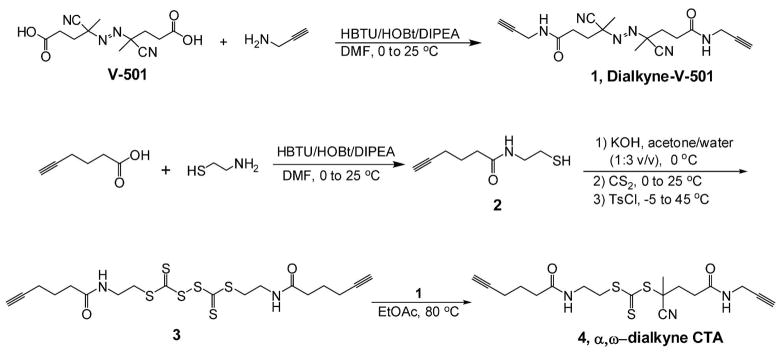
Synthesis of dialkyne functionalized RAFT agent
The synthesis was carried out in four steps (Scheme 1). Step 1: Synthesis of 4,4′-azobis(N,N′-propargyl-4-cyanopentanamide) (dialkyne-V-501, Product 1). Under nitrogen atmosphere, 4,4′-azobis(4-cyanopentanoic acid) (0.5 g, 1.78 mmol), HBTU (1.4 g, 3.7 mmol) and HOBt (0.5 g, 3.7 mmol) were dissolved in anhydrous N,N-dimethylformamide (DMF, 15 mL). The solution was stirred at 0 °C for /5 min followed by addition of DIPEA (646 mg, 5.0 mmol). After addition of propargylamine (201 mg, 3.65 mmol), the solution was stirred at 0 °C for 30 min and at 25 °C for 12 h. Ethyl acetate (EtOAc, 100 mL) and NaHCO3 saturated solution (100 mL) were then added. The aqueous layer was extracted with EtOAc (2 × 25 mL). The organic layer was washed with sat. NaHCO3 (2 × 50 mL), 1 M HCl (2 × 50 mL) and brine (2 × 50 mL). The organic layer was dried over MgSO4, and concentrated under reduced pressure. The final product was obtained by recrystallization from EtOAc/ether (1:1) in 75% yield (475 mg, 1.34 mmol). 1H NMR (400 MHz, CDCl3), δ (ppm from TMS): 1.67 (3H, s, C H3), 1.73 (3H, s, CH3), 2.25 (2H, t, J = 2.4 Hz, C≡CH), 2.45 (8H, m, CNCCH2CH2CO), 4.35 (4H, q, J = 2.4 Hz, NHCH2C), 6.14 (1H, s, NH), 6.19 (1H, s, NH). 13C NMR (100 MHz, CDCl3, δ in ppm): 207.33, 207.28, 155.81, 155.66, 118.46, 110.61, 109.47, 109.40, 70.41, 67.31, 67.20, 65.45, 60.76, 60.45.
Scheme 1.
Synthesis of the RAFT agent, S-2-cyano-5-oxo-5-(prop-2-ynylamino)pentan-2-yl S′-(hex-5-ynoyl) aminoethyl trithiocarboate
Step 2: Synthesis of (hex-5-ynoyl)aminoethanethiol (Product 2)
Under nitrogen atmosphere, 5-hexynoic acid (1.0 g, 8.9 mmol), HBTU (3.41 g, 9.0 mmol) and HOBt (1.22 g, 9.0 mmol) were dissolved in anhydrous DMF (20 mL). The solution was stirred at 0 °C for 5 min followed by addition of DIPEA (1.94 g, 15 mmol). After stirring another 5 min, cysteamine (694 mg, 9.0 mmol) was added and the solution was stirred at 25 °C for 5 h. EtOAc (50 mL) and sat. NaHCO3 (100 mL) were added, the layers were separated, and the aqueous layer was extracted with EtOAc (2 × 25 mL). The organic layers were then combined and washed with sat. NaHCO3 (2 × 50 mL), 1 M HCl (2 × 50 mL) and brine (2 × 50 mL). The organic layer was dried over MgSO4, and concentrated to 10 mL. The final product was obtained by recrystallization from EtOAc/hexane (1: 1) in 78.5% yield (1.2 g, 7.0 mmol). 1H NMR (400 MHz, CDCl3, δ in ppm), δ (ppm from TMS): 1.84 (2H, q, J = 7.2 Hz, CCH2CH2CH2CO), 1.95 (2H, s, C≡CH), 2.22 (2H, m, CCH2CH2CH 2CO), 2.34 (2H, m, J = 7.2 Hz, CCH2CH2CH2CO), 2.80 (2H, t, J = 6.4 Hz, SHCH2CH2), 3.56 (2H, q, J = 6.4 Hz, SHCH2CH2), 6.48 (1H, s, NH). 13C NMR (100 MHz, CDCl3, δ in ppm): 172.84, 83.48, 69.17, 38.40, 37.72, 34.88, 24.13, 17.87.
Step 3: Synthesis of bis((hex-5-ynoyl)aminoethylsulfanyl thiocarbonyl)disulfide (Product 3)
Product 2 (514 mg, 3.0 mmol) was dissolved in water/acetone (3/1; 15 mL), kept in ice bath and stirred for 5 min. An aqueous solution of KOH (1 M, 3.6 mmol) was added dropwise over 10 min, then carbon disulfide (228 mg, 3.0 mmol) was added in one portion. The system was stirred at room temperature for 30 min, and cooled to −5 °C in ice/acetone bath. p-Toluenesulfonyl chloride (305 mg, 1.6 mmol) was gradually added over 10 min and stirring was continued at room temperature for one hour, followed 10 min at 45 °C. After cooling down to room temperature, 100 mL DCM was added. The solution was washed with NaCl aq. (2 × 50 mL), and the organic layer was dried over MgSO4. The solvent was removed and the final product was obtained by recrystallization from DCM/ether (1: 1) in 63.1% yield (935 mg, 1.9 mmol). 1H NMR (400 MHz, DMSO-d6, δ in ppm): 1.62 (2H, t, J = 7.2 Hz, CCH2CH2CH 2CO), 2.12 (4H, m, CCH2CH2CH2CO), 2.76 (1H, s, C≡CH), 3.35 (2H, q, J = 6.4 Hz, SCH2CH2), 3.46 (2H, t, J = 6.4 Hz, SCH2CH2), 8.12 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6, δ in ppm): 221.25, 171.78, 84.01, 77.51, 38.88, 37.85, 36.11, 34.04, 24.15, 17.36.
Step 4: Synthesis of S-2-cyano-5-oxo-5-(prop-2-ynylamino)pentan-2-yl S′-(N-hex-5-ynoyl)aminoethyl trithiocarbonate (dialkyne-CTA, Product 4)
Under nitrogen atmosphere, the solution of dialkyne-V501 (Product 1) (231 mg, 0.65 mmol) and product 3 (200 mg, 0.41 mmol) in anhydrous EtOAc/DMF (15 mL, 10 : 1) was stirred at reflux for 20 h. Then, EtOAc was removed under vacuum and the crude product was isolated by column chromatography (silica gel 60 Å, 60–200 mesh) (EtOAc/hexane = 3 : 1). The final compound was obtained as yellow solid in 51% yield (90 mg, 0.22 mmol). 1H NMR (400 MHz, CDCl3, δ in ppm): 1.78 (2H, m, CCH2CH2CH 2CO), 1.82 (3H, s, CH3), 1.96 (1H, s, C≡CH), 2.12 (1H, s, C≡CH), 2.19 (4H, m, CCH2CH2CH2CO), 2.27 (2H, t, J = 7.2 Hz, CNCCH2CH2), 2.44 (2H, t, J = 7.2 Hz, CNCCH2CH2), 3.48 (4H, t, J = 5.2 Hz, SCH2CH2), 3.97 (2H, t, J = 2.4 Hz, NHCH2CCH), 6.64 (1H, s, NH), 7.19 (1H, s, NH). 13C NMR (100 MHz, CDCl3, δ in ppm): 217.27, 173.32, 170.69, 119.35, 83.72, 79.67, 71.81, 69.66, 47.16, 37.82, 36.34, 35.15, 34.31, 31.40, 29.43, 25.04, 24.40, 18.13. Mass spectrum (MALDI-TOF) found [M + H]+: 410.05 m/z (calculated Mw 409); mass spectrum (ESI) found: 410.1 m/z.
Synthesis of Nα-(4-azidobutanoyl)glycylphenylalanylleucylglycyl-Nε-(4-azidobutanoyl)-lysine (α,ω-diazide-GFLG, diazido peptide linker)
The functionalized peptide with two azido groups at both the N- and C-termini was synthesized using solid phase peptide synthesis (SPPS) methodology and manual Fmoc/t-Bu strategy on 2-chlorotrityl chloride resin. Fmoc-Lys(ivDde)-OH was employed as the first amino acid residue to introduce two amino groups. The protecting groups (Fmoc- and ivDde-) were removed selectively. One amino group was used to introduce azido group into the C-end of the peptide by reaction with 4-azidobutyric acid using HBTU as coupling agent, whereas the other functioned in constructing the peptide sequence. After completion of peptide sequence, the N-terminus was capped with 4-azidobutyric acid to introduce the other azido group. The resulting resin-bound peptide derivatives was washed sequentially with 3 × DMF, 3 × DCM and 3 × MeOH and then dried under vacuum. The peptide was isolated following cleavage from 2-Cl-trityl chloride resin by TFA/TIS/H2O (95: 2.5: 2.5) for 2 h. The solvent was removed and the peptide was washed with dry ether (3 × 10 mL) and then dried under vacuum. Yield 78.2%. 1H NMR (400 MHz, CD3OD), δ (ppm from TMS): 0.62–0.82 (6H, m, 2 × -CH3), 0.99–1.59 (7H, m, CH2CH(CH3)2 and CHCH2CH2CH2CH2NH(CO)), 1.69 (2H, m, CH2CH(CH3)2), 1.76 (4H, m, 2 × COCH2CH2CH2N3), 2.17 (4H, m, 2 × COCH2CH2CH2N3), 2.88 (2H, m, PhCH2), 3.08 (2H, m, CHCH2CH2CH2CH2NHCO), 3.25 (4H, m, 2 × COCH2CH2CH2N3), 3.63–3.86 (4H, m, 2 × NHCH2CO), 4.11 (1H, m, α-H), 4.29 (1H, m, α-H), 4.49 (1H, m, α-H), 7.18(5H, m, Ph-H). 13C NMR (100 MHz, CD3OD, δ in ppm): 174.44, 174.06, 173.72, 172.82, 170.68, 170.25, 136.69, 129.20, 128.45, 126.77, 55.90, 52.42, 50.76, 42.43, 39.76, 38.99, 37.13, 32.82, 32.46, 31.05, 28.68, 25.07, 24.81, 24.75, 24.49, 24.10, 22.91, 22.46, 20.66. MALDI-TOF MS (m/z): 743.25 (cal. [M + H]+: 743.39), 765.25 (cal. [M + Na]+: 765.38), 781.25 (cal. [M + K]+: 781.35. ESI-MS (m/z): 743.4 (cal. [M + H]+: 744.4).
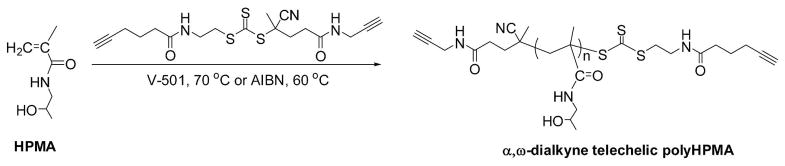
RAFT polymerization of N-(2-hydroxypropyl)methacrylamide (HPMA)
A number of HPMA polymerizations were performed either at 60 °C using 2,2′-azobisisobutyronitrile (AIBN) as initiator or at 70 °C using V-501 as initiator. A typical procedure for kinetic studies was as follows: HPMA (500 mg, 3.5 mmol), dialkyne-CTA (4.9 mg, 12 μmol) and AIBN (0.5 mg in methanol, 3 μmol) were dissolved in 3 mL deionized water (DI H2O). The mixture was bubbledwith N2 for 30 min and then placed in an oil bath preheated to 60 °C. At different time points, the samples were taken out via syringe and quenched in liquid nitrogen. The sample solution (20 μL) was immediately analyzed by HPLC. The monomer conversion was calculated by comparison of the remaining monomer concentration with initial concentration. The remaining fraction of sample solution was precipitated into cold acetone or acetone/ether mixture for molecular weight analysis.
Preparation of multiblock polyHPMA
Telechelic polyHPMA with alkyne end-groups (85 mg, Mn 43 kDa, ~2 μmol) and diazide-GFLG (1.7 mg, 2.2 μmol) were dissolved in 200 μL of degassed L-ascorbic acid (1.7 mg, 10 μmol) solution in DMF. Under nitrogen atmosphere, 150 μL CuBr (2.9 mg, 20 μmol) solution in DMF was added. The reaction mixture kept stirring at room temperature for 24 h. After precipitation into acetone, the polymer was fractionated using a Superose 6 HR/16/60 preparative column. Fractions were collected at 5 min intervals. The fractions were dialyzed (MWCO 6–8,000, Spectra/Por®) against distilled water for 24 h, and freeze-dried.
Enzyme-catalyzed degradation of multiblock polyHPMA
Papain and cathepsin B were used for polymer degradation. The concentration of the enzyme in McIlvaine’s buffer (50 mM citrate/0.1 M phosphate; 2 mM EDTA, pH 6.0) was determined by UV at 280 nm (ABS1%, 1 cm = 25 for papain, 20 for cathepsin B, respectively).18 Glutathione solution in McIlvaine’s buffer (10 mM, 2 mL) was added in equal volume of enzyme stock solution and pre-incubated for 5 min at 37 °C. The degradation of multiblock polyHPMA was performed at 37°C in the presence of papain (8.0 μM) or cathepsin B (5.6 μM) with concentration of polymer 3 mg/mL. At predetermined time points, 0.5 mL sample was withdrawn and analyzed by size-exclusion chromatography on an AKTA FPLC system as described above.
Results and Discussion
Design and synthesis of dialkyne functionalized RAFT chain transfer agent
The approach we proposed relies on the design of a dialkyne-functionalized RAFT CTA. We have chosen trithiocarbonate-based CTA (general formula RS–C(=S)–SR′) because these compounds have been used to polymerize a wide range of monomers.19 In addition, bifunctional trithiocarbonates are more easily designed than bifunctional dithiobenzoates, xanthates, and dithiocarbamates.20 The choice of “R” group is critical in the case of methacrylic monomers. Unlike the previously reported CTAs14,15 where the α-position was substituted with mono-methyl or dimethyl groups, we used a cyano-and a methyl group next to the trithiocarbonate moiety, because electron-withdrawing groups can enhance the activity of the RAFT agent (Scheme 1). Considering a report on “significant loss of azide moiety in polymerization of various monomers”,15 we have selected “alkyne” as our target orthogonal click group; it was successfully used in styrene RAFT polymerization.21 In addition, we attached the alkyne-containing moiety via an amide bond, as it presents a more stable link compared to the ester bond.
The successful synthesis of the new RAFT agent, dialkyne-CTA, was verified by 1H NMR, 13C NMR, and ESI-MS (Figures S1–3 in the Supporting Information) that confirmed the expected structure of dialkyne-CTA. The purity was validated by a single peak in HPLC (Figure S4 in the Supporting Information).
RAFT polymerization
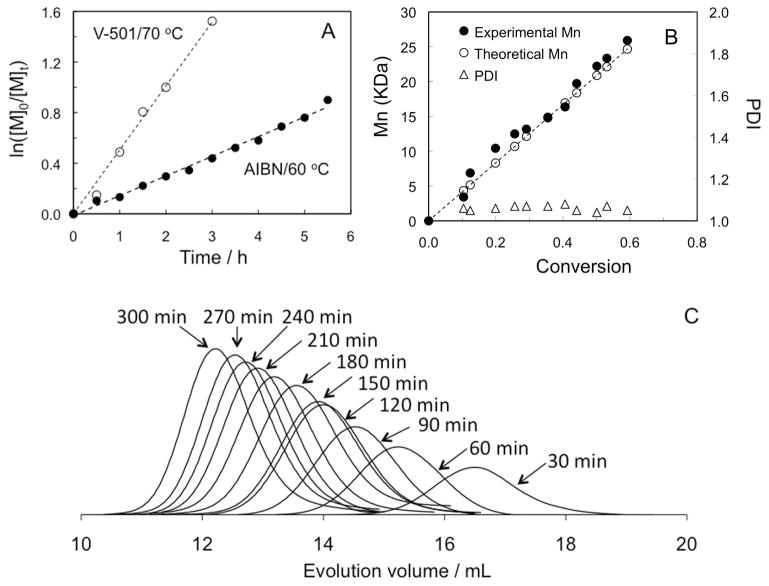
To assess the efficiency of dialkyne-CTA, RAFT polymerizations of HPMA were carried out as depicted in Scheme 2. The results were shown in Figure 1, Table 1 and Table S1 (Supporting Information). Both conversion and molecular weight increased with reaction time. Linear relationship between polymerization time and ln([M]0/[M]t) (where [M]0 is the initial monomer concentration and [M]t is the monomer concentration at time t) indicate that the pseudo-first-order kinetic character of these RAFT polymerizations and the steady concentration of active radicals during the polymerization, as shown in Figure 1A. The controlled nature of HPMA polymerization was demonstrated by the linear increase of Mn with monomer conversion (Figure 1B). The agreement between theoretical and experimental molecular weights over the course of polymerization and the narrow molecular weight distribution indicate that well-defined polymer structures were achieved (Figure 1B). The unimodal and symmetrical chromatograms coupled with the clear shift to lower elution volumes with time further demonstrate the controlled nature of the polymerization of HPMA (Figure 1C).
Scheme 2.
RAFT polymerization of HPMA mediated by dialkyne-CTA
Figure 1.
RAFT polymerization of HPMA in DI H2O mediated by α,ω-dialkyne-CTA. A) Pseudo-first-order kinetic plots at 60 °C ([HPMA]0/[CTA]0/[AIBN] = 290:1:0.25); and 70 °C ([HPMA]0/[CTA]0/[V501] = 90:1:0.2), respectively; B) Evolution of molecular weight (Mn) and polydispersity (PDI) vs HPMA conversion at 60 °C; C) SEC traces at varying polymerization time on an AKTA FPLC system using a Superose 12 HR/10/30 column with PBS (pH 7.3) as mobile phase.
Table 1.
HPMA RAFT polymerization in water mediated by α,ω-dialkyne CTAa
| Entry | [HPMA]0/[CTA]0/[I]0 | Time | Conv.b | Mn, calc. | Mn, SEC | PDIc |
|---|---|---|---|---|---|---|
| h | % | kDa | kDa c | |||
| 1 | 84/1/0.2 | 3 | 80 | 10.0 | 9.9 | 1.07 |
| 2 | 395/1/0.2 | 3 | 24 | 14.0 | 14.4 | 1.12 |
| 3 | 395/1/0.2 | 5 | 41 | 23.6 | 29.8 | 1.05 |
| 4 | 395/1/0.2 | 10 | 70 | 40.0 | 40.6 | 1.05 |
[HPMA]0 = 1 M, polymerization was carried out at 70 °C with V-501 as the initiator.
Monomer conversion determined by HPLC.
Determined by FPLC using a Superose 12 HR/10/30 column with PBS (pH 7.3) as mobile phase.
In particular, by comparison of Entry 1 and Entry 2 in Table 1, it can be concluded that the ratio of [HPMA]/[CTA] controls the molecular weight; this suggests that dialkyne-CTA indeed mediated the polymerization process.
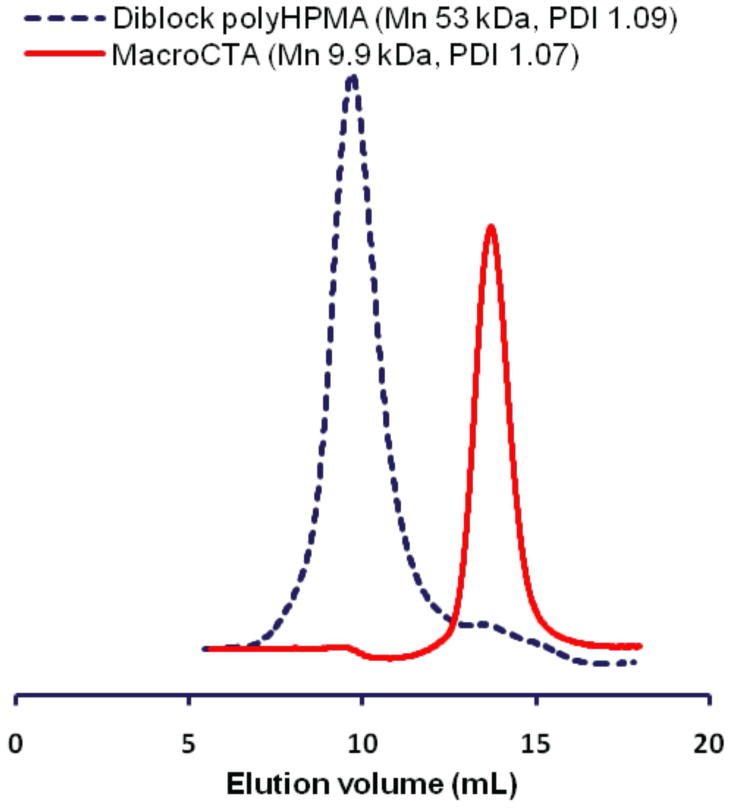
To further confirm the living behavior of the α,ω-dialkyne polyHPMA generated by the new RAFT agent, polyHPMA (Mn 9.9 kDa, PDI 1.07, Entry 1, Table 1) was used as macroCTA to mediate a new polymerization of HPMA. The SEC trace of the diblock polyHPMA showed a unimodal peak and a clear shift from the macroCTA (Figure 2). The observed molecular weight of diblock polyHPMA (53 kDa, PDI 1.09) was very close to the expected value (50 kDa), indicating the retention of the trithiocarbonate moiety and near-quantitative mediating efficacy
Figure 2.
Size-exclusion chromatograms of polyHPMA macroCTA (Mn 9.9 kDa, PDI 1.07) and the diblock polyHPMA (Mn 53 kDa, PDI 1.09). The diblock polyHPMA was obtained by RAFT polymerization in DI H2O at 70 °C using V-501 as the initiator and [HPMA]/[macroCTA] = 400. The SEC traces were obtained using an AKTA FPLC system on a Superose 12 HR/10/30 column with PBS (pH 7.3) as mobile phase.
Stability of trithiocarbonate moiety and alkyne-end group analysis
A series of dialkyne-CTA solutions in methanol with concentration range from 5.28 μM to 0.1 mM were prepared and scanned using UV–vis spectroscopy from 200 nm to 350 nm. A maximum absorbance around 305 nm (feature absorbance for SC=SS) was recorded. From the slope of the line A=εCl, the extinction coefficient of dialkyne-CTA was obtained as 12130 M−1cm−1. A series of polymer solutions in methanol were then analyzed. The average molecular weight of the polymers calculated based on εCTA agrees well with the theoretical values and the Mn measured by SEC (Table 2). These results indicate that the majority of the trithiocarbonate moieties were retained after polymerization.
Table 2.
Comparison of different characterization resultsa
| Time | Conv.b | Mth | MnUVc | MnSEC | PDI by SEC |
|---|---|---|---|---|---|
| h | % | kDa | kDa | kDa | |
| 1 | 25.6 | 4.2 | 5.4 | 4.1 | 1.06 |
| 2 | 55.4 | 8.1 | 8.1 | 7.6 | 1.08 |
| 4 | 70.9 | 10.1 | 11.0 | 10.1 | 1.02 |
The polymerization was conducted in D2O at 70 °C with [HPMA]/[CTA]/[I]0 = 90:1:0.33 and V-501 as the initiator.
The monomer conversion was measured by HPLC.
Using ε300 = 12130 M−1cm−1 in methanol.
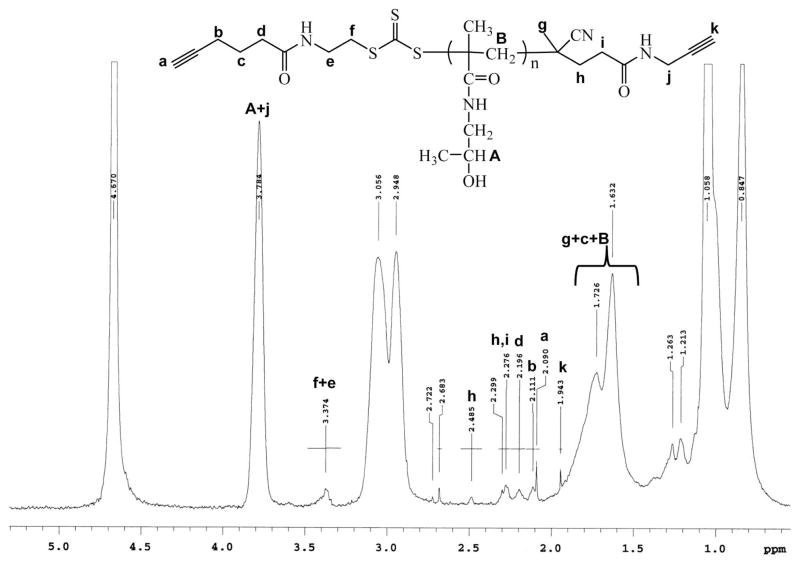
The presence of the dialkyne end groups on the polyHPMA chain is a prerequisite for efficient chain extension to multiblock polymers. The polymer structure was further evaluated by 1H NMR (Figure 3). The two single peaks, 2.09 ppm and 1.94 ppm, reflect the signals of alkynyl groups, suggesting a telechelic α,ω-dialkyne polyHPMA.
Figure 3.
1H NMR spectrum of α,ω-dialkyne polyHPMA (recorded in D2O).
Formation of multiblock HPMA copolymers by chain extension via CuAAC
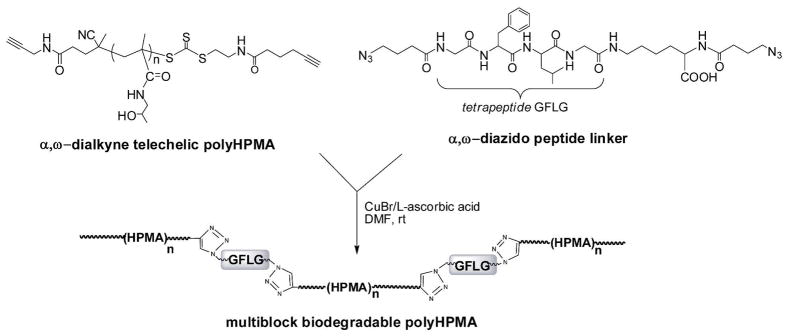
To validate our hypothesis and to prepare biodegradable linear multiblock polyHPMA with high molecular weight (≫ 50 kDa), an enzyme-sensitive oligopeptide flanked with azido groups at both N- and C-termini, Nα-(4-azidobutanoyl)glycylphenylalanylleucylglycyl-Nε-(4-azidobutanoyl)-lysine (α,ω-diazide-GFLG), was synthesized (Figures S5–6 in the Supporting Information) and used as a linker. The α,ω-dialkyne telechelic polyHPMA, generated by the new RAFT agent, was reacted with α,ω-diazide-GFLG to fabricate multiblock polyHPMA via click reaction (Scheme 3). PolyHPMA (Mn 43 kDa, PDI 1.05) was chosen as the initial polymer for chain extension and CuBr/L-ascorbic acid as catalyst system as previously reported.22 SEC was used to evaluate the click reaction process. The clicked products were fractionated using preparative size exclusion column.
Scheme 3.
Preparation of multiblock biodegradable polyHPMA via click chemistry
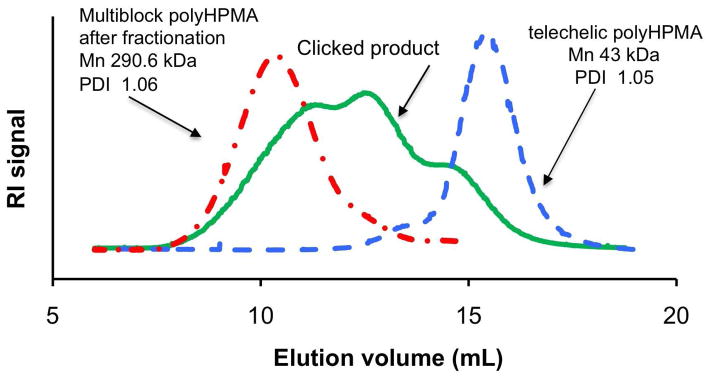
As shown in Figure 4, the highest Mn of the multiblock copolymer was about 291 kDa. However, the reaction mixture still contained oligomers and the initial polymer. This result may be attributed to the difficulty to achieve 1:1 stoichiometry with polymers.23 Optimization of reaction conditions should provide a higher molecular weight shift during the click reaction.
Figure 4.
SEC profiles showing click reaction of α,ω-dialkyne polyHPMA (Mn = 43 kDa, PDI = 1.05) with α,ω-diazido GFLG using CuBr/L-ascorbic acid as catalyst in DMF; and the fractionated clicked polyHPMA (Mn = 290.6 kDa, PDI = 1.06). Superose 6 HR/10/30 column was used.
Enzyme catalyzed degradation of HPMA copolymers
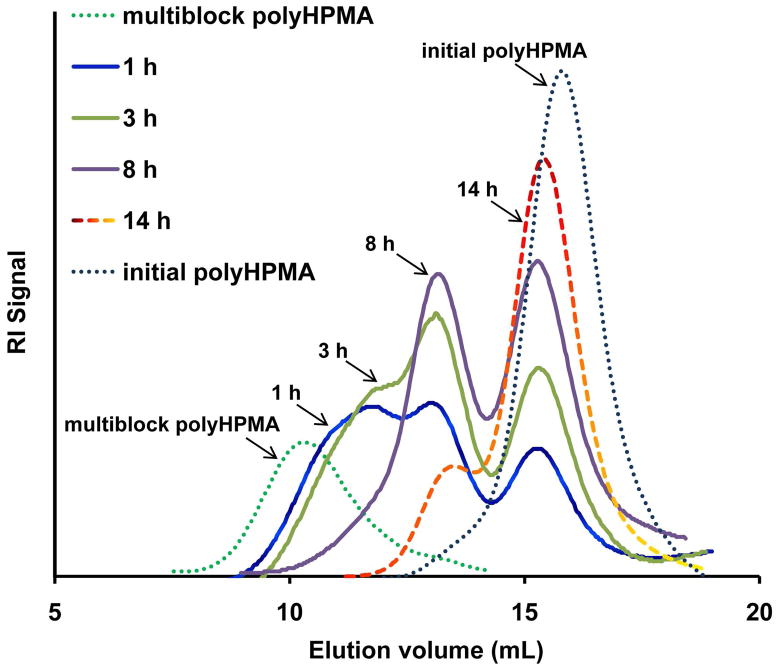
One fraction of multiblock polyHPMA with molecular weight of 290.6 kDa was incubated with papain (an enzyme having similar activity as lysosomal cathepsin B) at pH 6.0, 37 °C. The SEC trace of the polymer moved to lower molecular weights with the increase in incubation time (Figure 5). After 14 h of incubation, the polyHPMA degraded into fragments (Mn 49 kDa, PDI 1.04) with similar characteristics as the original polymer. When different fraction of multiblock polyHPMA (Mn = 182 kDa, PDI = 1.07) was degraded using papain (8.0 μM) and (lysosomal) cathepsin B (5.6 μM), similar results were obtained (Figure S7). A small shoulder in the cleavage product at 14 h is noticeable in Figure 5. However, after second addition of papain at 8 h the shoulder almost disappeared (Figure S7c). The RAFT group (SC=SS) in the polymer backbone was stable in the degradation solution, which was confirmed by the UV–vis spectra (Figure S8). The identical degradation products from different fractions clearly indicate that the degradation of the multiblock polyHPMA was due to the degradability of the enzyme-sensitive peptide sequence GFLG.
Figure 5.
SEC traces monitoring degradation of multiblock polyHPMA fraction (Mn = 290.6 kDa, PDI = 1.06) in the presence of papain (8.0 μM, citrate-phosphate buffer, pH 6.0, 37 °C) at different time points and comparison with initial telechelic polyHPMA (Mn = 43 kDa, PDI = 1.05).
Compared with previously reported biodegradable polyHPMA system24 where the architecture of the polymer was branched and partially crosslinked with enzyme-degradable peptides, the current design is based on linear, high molecular weight polyHPMA. The latter imposes considerably less steric hindrance on the formation of the enzyme-substrate complex, which results in higher degradation rates. The results also indicate that the biodegradability of the multiblock polyHPMA guarantees its clearance from the circulation after it completes its task as a drug carrier.
Conclusions
In this study, we have described a facile and straightforward approach to create water-soluble and biodegradable multiblock polyHPMAs via RAFT polymerization and click chemistry. A new α,ω-dialkyne trithiocarbonate-based RAFT CTA bearing α,ω-alkynyl groups was designed, synthesized and employed to generate telechelic α,ω-dialkyne polymers in one step. The RAFT polymerization in water demonstrated high control efficiency of the new CTA for polymerization of HPMA, resulting in well-defined telechelic α,ω-dialkyne polyHPMAs with narrow polydispersities. In addition, an enzymatically degradable α,ω-diazide-GFLG was synthesized by SPPS methodology. Its reaction with α,ω-dialkyne polyHPMA led to multiblock polyHPMAs with high molecular weight. The degradation of fraction of the multiblock polyHPMAs in the presence of papain or lysosomal cathepsin B validated the strategy used to prepare biodegradable multiblock polymers. This new approach provides a platform for the design and preparation of biodegradable polyHPMA-based drug carriers. The evaluation of biological properties of biodegradable multiblock polyHPMA-based drug carriers is in progress.
Supplementary Material
Acknowledgments
The research was supported in part by NIH grants GM69847, CA51578, and CA132831, and by the University of Utah Research Foundation.
Footnotes
Supporting Information Available: Characterization of dialkyne-CTA and α,ω-diazido-GFLGK, details of RAFT polymerization of HPMA, fractions of multiblock polyHPMA and the refractive index (RI) SEC traces of degradation of multiblock polyHPMA at different time points. This material is available free of charge via the Internet at http://pubs.acs.org/.
References and Notes
- 1.Kopeček J. Biomaterials. 1984;5:19–25. doi: 10.1016/0142-9612(84)90062-0. [DOI] [PubMed] [Google Scholar]
- 2.Kopeček J, Kopečková P. Adv Drug Deliv Rev. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopeček J. Mol Pharm. 2010;7:922–925. doi: 10.1021/mp1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan R, Vicent MJ. Adv Drug Deliv Rev. 2010;62:272–282. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Maeda H. Bioconjug Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 6.Shiah JG, Dvořák M, Kopečková P, Sun Y, Peterson CM, Kopeček J. Eur J Cancer. 2001;37:131–139. doi: 10.1016/s0959-8049(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. React Funct Polym. 2011;71:294–301. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan H, Yang J, Kopeček J, Kopečková P. Biomacromolecules. 2011;12:247–252. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsarevsky N, Sumerlin B, Matyjaszewski K. Macromolecules. 2005;38:3558–3561. [Google Scholar]
- 10.Golas P, Tsarevsky N, Sumerlin B, Walker L, Matyjaszewski K. Austr J Chem. 2007;60:400–404. [Google Scholar]
- 11.Gemici H, Legge T, Whittaker M, Monteiro M, Perrier S. J Polym Sci Part A: Polym Chem. 2007;45:2334–2340. [Google Scholar]
- 12.Gondi S, Vogt A, Sumerlin B. Macromolecules. 2007;40:474–481. [Google Scholar]
- 13.Golas PL, Tsarevsky NV, Sumerlin BS, Walker LM, Matyjaszewski K. Austr J Chem. 2007;60:400–404. [Google Scholar]
- 14.Lai JT, Filla D, Shea R. Macromolecules. 2002;35:6754–6756. [Google Scholar]
- 15.Boyer C, Liu J, Bulmus V, Davis T, Barner-Kowollik C, Stenzel M. Macromolecules. 2008;41:5641–5650. [Google Scholar]
- 16.von Maltzahn G, Ren Y, Park J, Min D, Kotamraju V, Jayakumar J, Fogal V, Sailor M, Ruoslahti E, Bhatia S. Bioconjugate Chem. 2008;19:1570–1578. doi: 10.1021/bc800077y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopeček J, Bažilová H. Europ Polym J. 1973;9:7–14. [Google Scholar]
- 18.Glazer AN, Smith EL. J Biol Chem. 1961;236:2948–2951. [PubMed] [Google Scholar]
- 19.O’Reilly R, Hansell C. Polymers. 2009;1:3–15. [Google Scholar]
- 20.Aoyagi N, Endo T. J Polym Sci, Part A: Polym Chem. 2009;47:3702–3709. [Google Scholar]
- 21.Ranjan R, Brittain W. Macromol Rapid Commun. 2008;29:1104–1110. [Google Scholar]
- 22.Yang J, Jacobsen MT, Pan H, Kopeček J. Macromol Biosci. 2010;10:445–454. doi: 10.1002/mabi.200900295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quémener D, Davis TP, Barner-Kowollik C, Stenzel MH. Chem Commun. 2006:5051–5053. doi: 10.1039/b611224b. [DOI] [PubMed] [Google Scholar]
- 24.Dvořák M, Kopečková P, Kopeček J. J Controlled Release. 1999;60:321–332. doi: 10.1016/s0168-3659(99)00087-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.