Abstract
Elevated serum phosphate has clinically been associated with vascular stiffness and cardiovascular mortality. Mechanistic studies over the past decade looking at phosphate’s local effects on the vessel wall have lent insight into various pathways that culminate in vascular calcification.Smooth muscle cell phenotype change and apoptosis play prominent roles. The sodium-phosphate cotransporter PiT-1 is required for the osteochondrogenic differentiation of smooth muscle cellsin vitro. Less is known about phosphate-driven valve interstitial cell calcification and elastin degradation.In this paper, we review the current knowledge about phosphate-induced changes in the vascular wall.
Keywords: Vascular calcification, phosphate, chronic kidney disease, smooth muscle cell, elastin degradation
1. Introduction
Vascular calcification, the inappropriate deposition of calcium phosphate in the form of hydroxyapatite [Ca10(PO4)6(OH)2] in arteries,is highly prevalent in chronic kidney disease (CKD), atherosclerosis, normal aging and diabetes. Based on its location, vascular calcification can be divided into threedistinct types: intimal, medial and valvular.Intimal calcification is mainly associated with atherosclerotic lesions and isobserved alongside inflammation and lipid deposition. Arterial medial calcification (AMC) orMönckeberg’s sclerosis occurs along the edges of the elastic lamellae in the medial layer of vessels and is typically not associated with inflammation(1-3).Like intimal calcification, AMC incidence is strongly correlated with cardiovascular events and is a strong prognostic marker of cardiovascular mortality in patients with end stage renal disease (ESRD)(4). Calcification of large blood vessels leads to increased stiffness which in turn increases pulse wave velocity and alters arterial wall distensibility(5). Profound increase in vascular stiffness leads to hypertension, left ventricular hypertrophy, compromised coronary perfusion and heart failure contributing to increased cardiovascular and all-cause mortality (4, 6). AMC can also occur in small arterioles, including in skin where it can cause calciphylaxiswith progressive ulcerations due to ischemia, which is associated with an extremely high mortality rate in dialysis patients (7). Valve calcification involves a combination of lipid accumulation, inflammation and neoangiogenesis in early stages, followed by active osteogenic mineral deposition (8). Approximately 30% of persons ≥ 65 years of age have echocardiographic evidence of aortic sclerosis, with 2% exhibiting aortic stenosis (9). In the dialysis population, both mitral and aortic valve calcification occur more frequently and at younger age than in the general population; progression to aortic stenosis occurs at an accelerated rate presumably due to disordered calcium-phosphate metabolism (10). Cardiac valvular abnormalities are associated with increased mortality in dialysis patients, especially when complicated by infective endocarditis (10). Over the past decade our understanding of the mechanisms that cause and regulate vascular calcification has increased significantly. One important emerging risk factor is hyperphosphatemia, which will be the focus of this review.
2. Phosphate and Vascular Calcification
Imbalance of mineral metabolism is a common occurrence in CKD. Elevated serum phosphate has emerged as a non-traditional risk factor for cardiovascular events in CKD(11, 12), as well as in the general community(13). Elevated serum phosphate greater than 5.5–6 mg/dLis strongly correlated with mortality in ESRD patients(14-16).The high risk of mortality is likely mediated, in part, by increased vascular calcification in CKD patients. Although no study has directly shown that lowering serum phosphorus to a specific threshold prevents or ameliorates vascular calcification, the use of non-calcium compared to calcium based phosphate binder attenuated vascular calcification(17-19)and mortality (18)in dialysis patients in some studies, but not others (20). Also, relatively small elevations in serum phosphate in the high normal range (3.5–4.5 mg/dL) have been correlated with increased risk of cardiovascular and all-cause mortality in both CKD patients(21) and the general population without obvious CKD(22). In CKD, hyperphosphatemia occurs as a consequence of diminished phosphate filtration and excretion. In the early stages of CKD (2 to 3), decreased phosphate excretion is compensated by increased secretion ofparathyroid hormone and fibroblast growth factor-23which reduces proximal tubulephosphate reabsorption(23). Phosphate levels are usually maintained within normal range until late stage 4 CKD(24). With disease progression, a combination of inefficient urinary phosphate excretion, disordered bone remodeling and continued intestinal phosphate absorption results in hyperphosphatemia.
Vascular calcification is a complex process that is felt to occur as a result of 1) a phenotypic transformation of vascular smooth muscle cells (VSMCs) to osteochondrogenic-like cells, 2) the stimulation of these cells to actively synthesize mineralization-initiating matrix vesicles,3) VSMC apoptosis with release of calcifyingapoptotic vesicles, and 4) animbalance of systemic and locally produced promoters and inhibitors of mineralization. In recent years, direct effects of elevated phosphate on vascular wall function have been clearly established and will be reviewed here.The effects of elevated calcium levels and or increased calcium load may also have direct, procalcifying effects on VSMCs that are independent of phosphate, and the reader is referred to the review by Farzaneh-Far and Shanahan for more information(25).
Direct Effects of Phosphate on Vascular Cell Function
3.1. Regulation of smooth muscle cell phenotype change
There has been a paradigm shift in the understanding of phosphate-induced arterial calcification over the past decade. The pathogenesis is not one of unregulatedphysicochemical deposition of mineral simply due to supersaturation of calcium and phosphate(26).Vascular smooth muscle cell (VSMC) populations within the normal medial wall exhibit a contractile phenotype characterized by expression of cytoskeletal proteins such as smooth muscle (SM)-α-actin, SM22α and SM-myosin heavy chain (27). Strikingly, under elevated phosphate conditions, VSMCs transition to an osteochondrogenic phenotype and lay down matrix in a tightly-regulated process resembling normal bone formation.
In vitro, no matrix calcification occurred in human VSMCs incubated with 1.4 mM phosphate (physiological level), but dose-dependent calcification occurred when phosphate levels wereincreased from 1.6 mM to 3 mM(28). These elevated phosphate concentrations are comparable to those seen in hemodialysis patients with hyperphosphatemia(29). There is loss of smooth muscle markers such as SMα-actin and SM22α, concurrent with upregulation of osteochondrogenic markers including Runx2/Cbfa1, osterix, osteopontin and alkaline phosphatase(28, 30). Runx2/Cbfa1 is the obligate transcription factor in bone differentiation which induces the expression of major bone matrix components including type I collagen, osteocalcin and osteopontin. Rat aorta cultured in 3.8 mM phosphate shows three-fold upregulation of alkaline phosphatase activity(31). Alkaline phosphatase is normally expressed in differentiating osteoblasts, but also disrupts anti-calcification mechanisms in the vessel wall via its effects on pyrophosphate and osteopontin. Pyrophosphate, an important inhibitor of vascular calcification, is hydrolyzed to generate more free phosphate(32). Simultaneously, osteopontin is dephosphorylated, thus removing its ability to chelate calcium and inhibit the growth of apatite crystals(28, 33). Ultrastructural analysis of the surface of VSMCs shows matrix vesicles containing apatite as well as calcifying collagen fibrils(33). The matrix vesicles likely act as nucleation sites for calcification, similar to the vesicles that bud from osteoblasts and hypertrophic chondrocytes in normal bone deposition.
Evidence for in vivo VSMC phenotype change has been found in calcified vascular lesions from animals(34-36) and humans (37-39). Calcified human aortic and carotid samples show upregulation of the transcription factors Runx2/Cbfa1, Msx2 and Sox9, in parallel with expression of the pro-calcification enzyme alkaline phosphatase and matrix proteins such as bone sialoprotein, osteocalcin and collagen type II(39). In dialysis patients, there is increased expression of Runx2/Cbfa1 in areas of both intimal and arterial calcification(40) confirming that this is likely pathogenic in uremic vascular calcification as well. Electron microscopy studies of iliac arteries from dialysis patients have identified 20–500 nm microcalcifications with a core-shell structure that is the calcification nidus; this nidus could represent matrix vesicles or apoptotic bodies(41).The complexity of VSMC phenotype change was demonstrated by a study using whole-genome expression arrays in uremic rats fed a high phosphate diet; the transition from “muscle-related” to “bone-related” gene expression involved dysregulation of 53 genes (42).
Phosphate transport into cells is primarily mediated by sodium-dependent phosphate (NaPi) cotransporters, of which there are 3 typesbased on structure, tissue expression and biochemical characteristics (43, 44). Type I and II NaPicotransporters in the kidney and intestinal epithelium play important roles in whole body phosphate homeostasis(43, 44). The type III NaPicotransporters, PiT-1 and PiT-2, are ubiquitously expressed(45, 46) and have been identified as the major phosphate transporters in VSMCs. Requirement for a sodium gradient was shown in uptake assays with H 323PO4 where phosphate uptake into human VSMCs increased in a time- and dose-dependent manner in the presence of sodium chloride(28). Replacing sodium with choline in the assay medium abolished phosphate uptake by the VSMCs. Different groups have shown that treatment with phosphonoformic acid (PFA, a competitive inhibitor of NaPi transport) inhibits phosphate uptake and VSMC osteochondrogenic differentiation(28, 47, 48).Real-time PCR detects PiT-1 as the predominant NaPicotransporterin human VSMCs with about 8-fold higher expression than PiT-2, and there is no expression of type I or type II NaPicotransporters(49). There is inter-species variation in the expression of PiT-1 and PiT-2; rat VSMCs express similar RNA levels of PiT-1 and PiT-2 (50).
It is well known that PFA, an analogue of inorganic pyrophosphate, prevents calcification by direct inhibition of hydroxyapatite formation (similar to the anti-calcification effect of bisphosphonates)(51-53), therefore mechanistic studies using PFA should be interpreted with caution. The importance of PiT-1 in phosphate-induced VSMC calcification was confirmed by the Giachelli labusing human VSMCs that were stably transduced with PiT-1 specific small hairpin RNA (shRNA)(49). PiT-1 knockdown suppressed phosphate-induced calcification and blocked induction of the osteogenic markers Runx2/Cbfa1 and osteopontin. Neither phosphate loading of matrix vesicles nor apoptosis was mediated by PiT-1. Erk 1/2 activation has been implicated in PiT-1 signaling; treatment of mouse VSMCs with high phosphate induces calcification and VSMC osteochondrogenic differentiation in conjunction with increased phosphorylation of Erk 1/2 (36). Inhibition of Erk phosphorylation by the MEK inhibitor U0126 prevented upregulation of Runx2/Cbfa1 and promoted VSMC lineage markers(36). PiT-1 may also exert effects at the endoplasmic reticulum levelto promote matrix calcification via modification of pro- and anti-calcification proteins that are normally produced by VSMCs (54). While the physiological role of PiT-1 in VSMCs is still unclear, it appears to be critical for embryonic development. PiT-1 knockout embryos arrest between E11.5 and 13.5, and display severe anemia with abnormal yolk sac vasculature(55).
The proteins bone morphogenetic protein 2 (BMP-2) and transglutaminase 2 (TG2) have been linked to phosphate-induced VSMC osteoblastic differentiation and calcification. BMP-2 dose-dependently stimulated phosphate uptake in cultured human VSMCs and upregulated PiT-1 mRNA and protein levels; Runx2/Cbfa1 expression was enhanced (56). BMP-2 levels are increased in the serum of uremic patients; incubation of bovine VSMCs with noggin (an inhibitor of BMP-2) decreased uremic serum-induced Runx2/Cbfa1 expression (57). In a separate study, addition of noggin inhibited mineralization of human VSMCs in high-phosphate media and blocked expression of the osteogenic transcription factor osterix(58). Mouse VSMCs deficient in TG2 show a blunted response to treatment with 2.5 mM phosphate, without upregulation of alkaline phosphatase, PiT-1, Runx2/Cbfa1 and the osteoblastogenesis transcription factor Msx2 (59). TG2 also has a direct role in stabilization of extracellular matrices, by producing protease-resistant isopeptide bonds in substrate proteins such as collagen I, fibronectin and osteopontin(60).
While elevated phosphate does play a major role in VSMC phenotype change, there are non-NaPi dependent mechanisms in the uremic setting that accelerate vascular calcification. Moe’s group treated bovine VSMCs with beta-glycerophosphate (a phosphate donor) and noted increased osteopontin and alkaline phosphatase expression, consistent with osteochondrogenic transition (48). This effect was reproduced when bovine VSMCs were incubated with pooled uremic sera from dialysis patients. Addition of PFA (inhibitor of NaPi transport) or levamisole (inhibitor of alkaline phosphatase) only partially inhibited uremic sera-induced osteopontinupregulation(48).In a later study, the cyclic AMP /protein kinase A (cAMP/PKA) signaling pathway was implicated in uremic serum-induced upregulation of Runx2/Cbfa1 and alkaline phosphatase (57). Indeed, other groups have shown that activation of the cAMP/PKA pathway leads to osteoblastic differentiation of cultured murine VSMCs(61, 62).One study reportedthat cAMP downregulated ectonucleotide-pyrophosphatase/phosphodiesterase-1(ENPP1)leading to decreased levels of inorganic pyrophosphate (a potent inhibitor of calcification)(61); however the opposite was noted by Huang et al. with “feedback” upregulation of ENPP1(62). Regardless, production of alkaline phosphatase, which cleaves pyrophosphate, tilts the milieu in favor of calcification.
The non-phosphate dependent mechanisms influencing VSMC phenotype change in uremic serum remain unclear. However, multiple ‘non-traditional’ risk factors that are associated with cardiovascular disease in dialysis patients can induce VSMC calcification in vitro[reviewed in (63)]. Certainly, deficiency of calcification inhibitors is culprit. Fetuin-A normally binds excess calcium and phosphorus molecules to facilitate their clearance from the circulation; levels of fetuin-A go down during inflammation and have been associated with vascular calcification and death in dialysis patients (64). Inflammation is dominant in CKD and creates a pro-fibrotic environment that accelerates kidney and vascular disease[reviewed in (65)].Diabetes is the leading cause of kidney disease in the United States; diabetic serum has been shown to induce osteogenic differentiation and calcification of human VSMCs via activation of the RAGE/p38 MAPK pathway by advanced glycation end products(66). Thus, patients with advanced CKD have multiple risk factors that accelerate phosphate-induced vascular calcification.
3.2. Regulation of smooth muscle cell apoptosis
Human VSMCs cultured in elevated phosphate and calcium conditions have been observed to develop apoptotic bodies with release of plasma membrane-derived vesicles that result in matrix calcification(67). These findings were replicated when vessels from pediatric dialysis patients were cultured in high phosphate conditions; dense medial calcification occurred in association with apoptosis, cell loss and deposition of apatite-containing vesicles(68). The underlying mechanism likely involves downregulation of Growth arrest-specific gene 6 (Gas6). Both Gas6 and its receptor Axl have been shown to be markedly downregulated in phosphate-induced human aortic VSMC calcification(69), and were previously implicated in osteogenic differentiation of vascular pericytes(70). Gas6 exerts anti-apoptotic effects via the Bcl2-mediated PI3K-AKT pathway; phosphorylation inactivates Bcl2 and activates the pro-apoptotic protein BAD, resulting in caspase-3 activation and apoptosis(71).
Apoptosis is accelerated once calcium phosphate crystals are deposited in the matrix. Both synthetic and human atherosclerosis-derived calcium phosphate crystals are phagocytosed by human aortic VSMCs, resulting in a rapid rise in intracellular calcium concentrations and resultant inflammation and cell death(72). These effects were inhibited by the lysosomalproton pump inhibitor, bafilomycin A1(72). Very recently, high-phosphate-induced octacalcium phosphate nanocrystals were shown to induce osteogenic differentiation of mouse VSMCs(73), revealing another pathway whereby crystals can propagate matrix calcification.
There could well be a close interplay between phosphate-induced VSMC phenotype change and apoptosis, as proposed recently by Shanahan’s group(68). VSMCs that adapt to the hostile conditions by undergoing lineage reprogramming from contractile to synthetic bone-forming phenotype are able to secrete matrix vesicles, thus avoiding calcium overload. In contrast, VSMCs that fail to differentiate succumb to apoptosis, which also results in budding of vesicles and matrix mineralization. Both pathways lend to extracellular calcium phosphate deposition with increased threat of apoptosis to surviving VSMCs.
3.3. Regulation of valve interstitial cell calcification
Aortic valve interstitial cells (VICs) are heterogeneous cell populations mainly composed of fibroblasts, myofibroblasts and smooth muscle cells (74). The fibroblast and smooth muscle subpopulations have been found to undergo phosphate-induced calcification, in parallel with increased alkaline phosphatase activity and expression of osteoblastic markers(75), much like the VSMC phenotype change described in the vessel wall. Treatment with levamisole (alkaline phosphatase inhibitor) inhibited calcification as well as osteogenic differentiation(75).
The fibroblast-like VIC subpopulation has been shown to undergo phosphate-induced calcificationalso in the setting of inflammation(76), thus contributing to calcific valve degeneration.Bovine VICs treated with lipopolysaccharide (100 ng/mL) show a progressive time-dependent increase in alkaline phosphatase activity, with variable expression of osteocalcin and osteopontin among different clonal populations (74). Addition of high 2.4 mM phosphate to the culture medium promoted matrix calcification, with some degree of apoptosis. Interestingly, Mohler et al. previously noted a subpopulation of osteoblast-like VICs in human and canine aortic valves that have the capacity to spontaneously form calcific nodules in cell culture (77).
3.4. Regulation of matrix degradation
VSMCs in the arterial media are surrounded by a complex, highly structured extracellular matrix (ECM) composed of collagen, elastin, fibronectin, heparan sulfate proteoglycans and chondroitin sulfate proteoglycans. Elastin, a major constituent of the ECM in the arterial wall, is secreted from VSMCs as a soluble monomertropoelastin. Tropoelastin interacts with fibrillin or microfibril associated glycoprotein (MAGP) to orient into proper alignment for cross-linking by lysyl oxidase. This cross-linked structure provides elastin with extensive tensile strength and is responsible for the hemodynamic properties of the vessel wall. Therefore, any injury to elastin can lead to decreased vessel compliance. Calcification of elastin is a common occurrence not only in AMC, but also in atherosclerosis and bioprosthetic heart valve calcification(78, 79).
Elevated phosphate can initiate and/or accelerate calcification via its effects on cell populations in the vascular wall, as described in the previous sections 3.1–3.3. Elevated phosphate may also perpetuate matrix mineralization via elastin degradation. Recently Hosaka et al. published an interesting study where α-elastin, a soluble elastin-derived peptide, did not induce mineralization of human VSMCs undernormal extracellular phosphate concentrations(80). However,α-elastin markedly accelerated mineralization when the VSMCs were cultured in high (2.5 mM) phosphate conditions, as confirmed by calcium quantitation and von Kossa staining. Since α-elastin peptide did not induce VSMC mineralization under normal phosphate conditions, it has been suggested that phosphate-induced VSMCosteogenicdifferentiation needs to be present before α-elastin can exert pro-calcification effects(80). In contrast, in a rat aortic ring model of elastocalcinosis, treatment with high phosphate and warfarin resulted in early expression of matrix metalloproteinase MMP-9 (an elastin degrading enzyme), closely followed by TGF-β signaling and VSMC osteogenic lineage reprogramming(81).Once elastin breakdown is initiated, there is increased affinity for calcium (82) which facilitates epitactic growth of apatite along the elastic lamellae. A separate study has verified that peptides derived from elastin degradation bind to elastin laminin receptors (ELRs) on the surface of VSMCs, perpetuatingosteogenicdifferentiation via TGF-β signaling (83) which is known to upregulate Runx2/Cbfa1 (84, 85).Research is ongoing to clarify the role of elastin-derived peptides in vascular calcification.
Conclusions
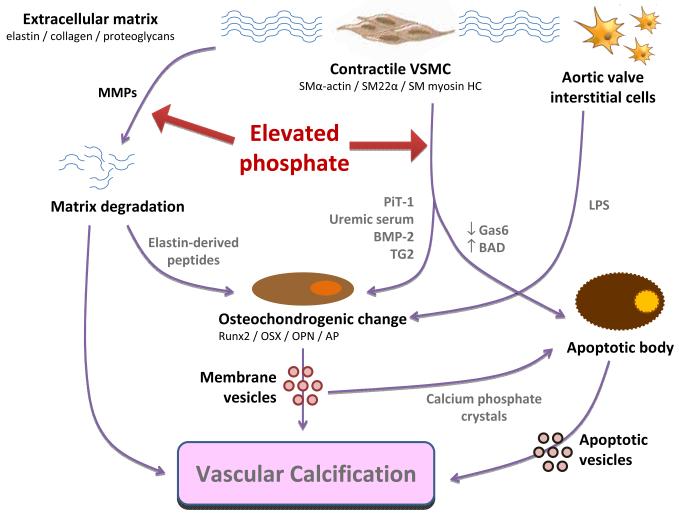
We have discussed various mechanisms whereby hyperphosphatemia disrupts normal vascular wall biology, ultimately resulting in damagingmatrix calcification (summarized in Figure 1). Phosphate is an essential mineral in cellular structures (phospholipids and nucleic acids) and is required for energy production, intracellular signaling and pH buffering; however excess phosphate is toxic and induces VSMC apoptosis. Subpopulations of VSMCs and VICs display a remarkable transdifferentiation andbehave like osteoblasts,secreting hydroxyapatite-laden vesicles to avoid intracellular mineral overload and/or cell death. However this “cell-centric” defense mechanism is maladaptive to the vascular system as a whole, perpetuating ectopic biomineralization with detrimental cardiovascular effects.Effective therapies are currently lacking; the survival benefit from dietary phosphate binders remains controversial(86, 87)likely because, once calcification is clinically apparent, the vascular damage is already advanced.It is now apparent that AMC is a highly-regulated process with interplay of pro- and anti-calcific factors; targeting of these mediatorsoffers the potential for limiting progression or even inducing regression of vascular calcification [see(88) for excellent review by Kapustin and Shanahan]. Until novel therapies emerge, preventative medicine is imperative and calcium-phosphate balance should be an early priority in CKD patients.
Figure 1.
Direct effects of elevated phosphate on the cellular and matrix components of the vessel wall that result in calcification.VSMCs undergo one of two fates. They maytransdifferentiate to a bone-forming phenotype (a process driven byuremic serum and associated with upregulation of PiT-1, BMP-2 and TG2). VSMCs that are unable to adapt to the extracellular mineral imbalance undergo apoptosis. Both routes lead to deposition of membrane-derived vesicles and matrix mineralization, and the extracellular calcium phosphate crystals may thenperpetuate apoptosis.Osteochondrogenic differentiation has also been observed in subpopulations of valve interstitial cells, and has been associated with valvular degeneration in the setting of inflammation.Additionally, elevated phosphate can acceleratedegradation of the elastic lamellae in the vessel wall, in conjunction with activation of MMPs (e.g., with warfarin treatment). In the presence of elevated phosphate, elastin-derived peptides perpetuate VSMC osteochondrogenic differentiation via binding to elastin laminin receptors, thus driving vesicle secretion and matrix calcification. Degradation of elastin also increases its affinity for mineral deposition, further facilitating calcification.AP alkaline phosphatase, BADBcl-2-associated death promoter protein,BMP-2 bone morphogenetic protein 2,Gas6Growth arrest-specific gene 6,HC heavy chain, LPS lipopolysaccharide, MMP matrix metalloproteinase, OPNosteopontin, OSXosterix, PiT-1 type III sodium-phosphate cotransporter 1, SM smooth muscle,TG2transglutaminase 2,VSMC vascular smooth muscle cell.
Acknowledgments
Financial Disclosures: Studies in Dr. Giachelli’s lab are funded by NIH grantsR01 HL62329 and R01 HL081785-01. Dr. Lau is funded by T32 DK007467. Dr. Moe’s lab is funded by the Veteran’s Administration and Dr. Moe is supported by NIH-NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edmonds M, Morrison N, Laws J, Watkins P. Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed) 1982;284:928–930. doi: 10.1136/bmj.284.6320.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giachelli C. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman W, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.London G, Guérin A, Marchais S, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 5.Guerin A, Blacher J, Pannier B, Marchais S, Safar M, London G. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S, O’Neill K, Hood A, Evan A, Moe S. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 8.Shao J, Cai J, Towler D. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 9.Otto C, Lind B, Kitzman D, Gersh B, Siscovick D. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 10.Umana E, Ahmed W, Alpert M. Valvular and perivalvular abnormalities in end-stage renal disease. Am J Med Sci. 2003;325:237–242. doi: 10.1097/00000441-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Hulbert-Shearon T, Levin N, Port F. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 12.Young E, Albert J, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Dhingra R, Sullivan L, Fox C, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 14.Noordzij M, Korevaar J, Bos W, et al. Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant. 2006;21:2513–2520. doi: 10.1093/ndt/gfl257. [DOI] [PubMed] [Google Scholar]
- 15.Tentori F, Blayney M, Albert J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Klassen P, Lazarus J, Ofsthun N, Lowrie E, Chertow G. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 17.Chertow G, Burke S, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Raggi P, Bellasi A, Kooienga L, Spiegel D. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 19.Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 20.Qunibi W, Moustafa M, Muenz L, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 21.Kestenbaum B, Sampson J, Rudser K, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 23.Hruska K, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin A, Bakris G, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 25.Farzaneh-Far A, Shanahan C. Biology of vascular calcification in renal disease. Nephron Exp Nephrol. 2005;101:e134–138. doi: 10.1159/000087578. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill W. The fallacy of the calcium-phosphorus product. Kidney Int. 2007;72:792–796. doi: 10.1038/sj.ki.5002412. [DOI] [PubMed] [Google Scholar]
- 27.Shanahan C, Weissberg P, Metcalfe J. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993;73:193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- 28.Jono S, McKee M, Murry C, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 29.Block G. Prevalence and clinical consequences of elevated Ca × P product in hemodialysis patients. Clin Nephrol. 2000;54:318–324. [PubMed] [Google Scholar]
- 30.Steitz S, Speer M, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 31.Lomashvili K, Garg P, O’Neill W. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006;69:1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- 32.Lomashvili K, Cobbs S, Hennigar R, Hardcastle K, O’Neill W. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 33.Wada T, McKee M, Steitz S, Giachelli C. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 34.El-Abbadi M, Pai A, Leaf E, et al. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75:1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graciolli F, Neves K, dos Reis L, et al. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol Dial Transplant. 2009;24:1416–1421. doi: 10.1093/ndt/gfn686. [DOI] [PubMed] [Google Scholar]
- 36.Speer M, Yang H, Brabb T, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shroff R, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 38.Kaden J, Bickelhaupt S, Grobholz R, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13:560–566. [PubMed] [Google Scholar]
- 39.Tyson K, Reynolds J, McNair R, Zhang Q, Weissberg P, Shanahan C. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 40.Moe S, Duan D, Doehle B, O’Neill K, Chen N. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 41.Schlieper G, Aretz A, Verberckmoes S, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Román-García P, Carrillo-López N, Fernández-Martín J, Naves-Díaz M, Ruiz-Torres M, Cannata-Andía J. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46:121–128. doi: 10.1016/j.bone.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Takeda E, Taketani Y, Morita K, Miyamoto K. Sodium-dependent phosphate co-transporters. Int J Biochem Cell Biol. 1999;31:377–381. doi: 10.1016/s1357-2725(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 44.Werner A, Dehmelt L, Nalbant P. Na+-dependent phosphate cotransporters: the NaPi protein families. J Exp Biol. 1998;201:3135–3142. doi: 10.1242/jeb.201.23.3135. [DOI] [PubMed] [Google Scholar]
- 45.Boyer C, Baines A, Beaulieu E, Béliveau R. Immunodetection of a type III sodium-dependent phosphate cotransporter in tissues and OK cells. Biochim Biophys Acta. 1998;1368:73–83. doi: 10.1016/s0005-2736(97)00159-4. [DOI] [PubMed] [Google Scholar]
- 46.Kavanaugh M, Miller D, Zhang W, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugitani H, Wachi H, Murata H, Sato F, Mecham R, Seyama Y. Characterization of an in vitro model of calcification in retinal pigmented epithelial cells. J Atheroscler Thromb. 2003;10:48–56. doi: 10.5551/jat.10.48. [DOI] [PubMed] [Google Scholar]
- 48.Chen N, O’Neill K, Duan D, Moe S. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Yang H, Giachelli C. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 50.Villa-Bellosta R, Bogaert Y, Levi M, Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. 2007;27:1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 51.Williams G, Sallis J. Structure--activity relationship of inhibitors of hydroxyapatite formation. Biochem J. 1979;184:181–184. doi: 10.1042/bj1840181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleisch H, Russell R, Bisaz S, Mühlbauer R, Williams D. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Eur J Clin Invest. 1970;1:12–18. doi: 10.1111/j.1365-2362.1970.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 53.Villa-Bellosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol. 2009;29:761–766. doi: 10.1161/ATVBAHA.108.183384. [DOI] [PubMed] [Google Scholar]
- 54.Villa-Bellosta R, Levi M, Sorribas V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-alpha, and Pi. Pflugers Arch. 2009;458:1151–1161. doi: 10.1007/s00424-009-0688-5. [DOI] [PubMed] [Google Scholar]
- 55.Festing M, Speer M, Yang H, Giachelli C. Generation of mouse conditional and null alleles of the type III sodium-dependent phosphate cotransporter PiT-1. Genesis. 2009;47:858–863. doi: 10.1002/dvg.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Yang H, Giachelli C. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen N, Duan D, O’Neill K, et al. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 58.Mathew S, Tustison K, Sugatani T, Chaudhary L, Rifas L, Hruska K. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson K, Polewski M, Terkeltaub R. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorand L, Graham R. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 61.Prosdocimo D, Wyler S, Romani A, O’Neill W, Dubyak G. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: synergistic modulation by cyclic AMP and hyperphosphatemia. Am J Physiol Cell Physiol. 2010;298:C702–713. doi: 10.1152/ajpcell.00419.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang M, Sage A, Lu J, Demer L, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun. 2008;374:553–558. doi: 10.1016/j.bbrc.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moe S, Chen N. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 64.Ketteler M. Fetuin-A and extraosseous calcification in uremia. Curr Opin Nephrol Hypertens. 2005;14:337–342. doi: 10.1097/01.mnh.0000172719.26606.6f. [DOI] [PubMed] [Google Scholar]
- 65.Silverstein D. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–1452. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 66.Tanikawa T, Okada Y, Tanikawa R, Tanaka Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46:572–580. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds J, Joannides A, Skepper J, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 68.Shroff R, McNair R, Skepper J, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Son B, Kozaki K, Iijima K, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 70.Collett G, Wood A, Alexander M, et al. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- 71.Son B, Kozaki K, Iijima K, et al. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol. 2007;556:1–8. doi: 10.1016/j.ejphar.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 72.Ewence A, Bootman M, Roderick H, et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 73.Sage A, Lu J, Tintut Y, Demer L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2010 doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertipaglia B, Ortolani F, Petrelli L, et al. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur) Ann Thorac Surg. 2003;75:1274–1282. doi: 10.1016/s0003-4975(02)04706-9. [DOI] [PubMed] [Google Scholar]
- 75.Mathieu P, Voisine P, Pépin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–357. [PubMed] [Google Scholar]
- 76.Rattazzi M, Iop L, Faggin E, et al. Clones of interstitial cells from bovine aortic valve exhibit different calcifying potential when exposed to endotoxin and phosphate. Arterioscler Thromb Vasc Biol. 2008;28:2165–2172. doi: 10.1161/ATVBAHA.108.174342. [DOI] [PubMed] [Google Scholar]
- 77.Mohler Er, Chawla M, Chang A, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- 78.Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension. 1997;29:999–1006. doi: 10.1161/01.hyp.29.4.999. [DOI] [PubMed] [Google Scholar]
- 79.Bobryshev Y, Lord R, Warren B. Calcified deposit formation in intimal thickenings of the human aorta. Atherosclerosis. 1995;118:9–21. doi: 10.1016/0021-9150(95)05588-n. [DOI] [PubMed] [Google Scholar]
- 80.Hosaka N, Mizobuchi M, Ogata H, et al. Elastin Degradation Accelerates Phosphate-Induced Mineralization of Vascular Smooth Muscle Cells. Calcif Tissue Int. 2009 doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 81.Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28:856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 82.Rucker R. Calcium binding to elastin. Adv Exp Med Biol. 1974;48:185–209. doi: 10.1007/978-1-4684-0943-7_10. [DOI] [PubMed] [Google Scholar]
- 83.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 84.Heldin C, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 85.Lee K, Kim H, Li Q, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Navaneethan S, Palmer S, Craig J, Elder G, Strippoli G. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54:619–637. doi: 10.1053/j.ajkd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–1324. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 88.Kapustin A, Shanahan C. Targeting vascular calcification: softening-up a hard target. Curr Opin Pharmacol. 2009;9:84–89. doi: 10.1016/j.coph.2008.12.004. [DOI] [PubMed] [Google Scholar]