Abstract
Somites give rise to the vertebral column and segmented musculature of adult vertebrates. The cell movements that position cells within somites along the anteroposterior and dorsoventral axes are not well understood. Using a fate mapping approach, we show that at the onset of Xenopus laevis gastrulation, mesoderm cells undergo distinct cell movements to form myotome fibers positioned in discrete locations within somites and along the anteroposterior axis. We show that the distribution of presomitic cells along the anteroposterior axis is influenced by convergent and extension movements of the notochord. Heterochronic and heterotopic transplantations between presomitic gastrula and early tailbud stages show that these cells are interchangeable and can form myotome fibers in locations determined by the host embryo. However, additional transplantation experiments revealed differences in the competency of presomitic cells to form myotome fibers, suggesting that maturation within the tailbud presomitic mesoderm is required for myotome fiber differentiation.
Keywords: somite, muscle, morphogenesis, gastrulation, fate mapping, presomitic mesoderm, Xenopus laevis
INTRODUCTION
One of the first major morphogenetic processes during embryogenesis is gastrulation. This process involves an orchestrated series of cell movements that result not only in the reorganization of the embryo to create the three embryonic germ layers (i.e. ectoderm, mesoderm and endoderm), but also a dramatic change in the overall shape of the embryo from a sphere to an elongated tube. These dramatic cell movements lay down the foundation for the overall body plan from which adult organisms emerge.
During gastrulation mesodermal cells positioned along the midline of the embryo give rise to the notochord, while cells lateral to the notochord give rise to the presomitic mesoderm (PSM). The PSM subsequently subdivides into discrete blocks of mesodermal cells called somites. This process begins at the anterior end of the axis and moves posteriorly as the axis elongates. The somites eventually undergo differentiation to give rise to cartilage, bones, dermis, and muscle of adult vertebrates.
In vertebrates, somites give rise to distinct vertebral structures depending on their position along the anteroposterior axis. Studies primarily in the mouse and chick have shown that Hox genes play a role in determining whether a somite gives rise to cervical, thoracic, lumbar, sacral, or caudal vertebrae (Krumlauf, 1994; Wellik, 2007). In the chick embryo, researchers have shown that the anteroposteior patterning of the PSM is controlled by the temporal co-linear expression of specific Hox genes during gastrulation (Iimura and Pourquié, 2006). Similarly, early Hox gene expression during gastrulation has also been observed in the mouse (Forlandi et al., 2003) and frog (Wacker et al., 2004) among cells destined to form the axial mesoderm. Taken together, these results suggest that the mechanism underlying axial mesoderm patterning begins early in the gastrula and is likely conserved across vertebrates.
Although the molecular mechanisms patterning the paraxial mesoderm may be largely conserved across vertebrates, the individual cell behaviors associated with somite formation and differentiation appear quite distinct. For example, in the chick the somite forms as it separates from the PSM in a ventral-to-dorsal direction (Sato and Takahashi, 2005) through a series of cell behaviors referred to as the “ball-and-socket” mode of tissue separation (Kulesa and Fraser, 2002). In Zebrafish, somite formation occurs as a subset of cells in the PSM that are destined to become epithelial border cells undergo very dynamic short-range movements to form a boundary that separates the newly formed somite from the PSM (Henry et al., 2000). In Xenopus laevis, somite formation consists of a series of steps that begins with cells in the PSM that become increasingly elongated in the mediolateral direction followed by changes in cell adhesion and protrusive activity associated with segmental boundary formation (Hildalgo et al., 2009). Once the segment forms, the somite undergoes a 90° rotation in a dorsal-to-ventral direction and consists of elongated myotome fibers aligned parallel to the notochord (Youn and Malancinski, 1981; Afonin et al., 2006).
While the cell behaviors underlying somite formation are becoming clear, the morphogenetic cell movements that originate in the gastrula and lead to the relative positioning of cells within the paraxial mesoderm are not well understood. Fate mapping experiments in the chick reveal that somites are largely comprised of cells that originate from two distinct populations with the medial portion of somites arising from anterior epiblast cells and the lateral portion of somites arising from posterior primitive streak cells (Iimura and Pourquié, 2007). In X. laevis, fate mapping studies have shown that cells lateral to the prospective notochord and surrounding the blastopore lip give rise to axial myotome fibers (Keller, 1991; Lane and Sheets, 2000). In addition, lineage tracing experiments performed at the 32-cell stage showed that a small subset of cells from the C2 and C3 progeny, which populate the circumblastoporal mesoderm, give rise to myotome fibers in discrete locations within somites (Lane and Sheets, 2002). However, the precise trajectory of these cells from the 32-cell stage to myotome formation remain unclear as most of the progeny from the C2 and C3 blastomeres were found in many different mesodermal derivatives ranging from head structures to ventral blood islands.
In this paper, we examine the patterning of the gastrula circumblastoporal mesoderm with respect to the formation and final positioning of myotome fibers within somites, and along the anteroposterior axis in X. laevis. Through a series of fate mapping experiments we show that cells located in precise regions around the gastrula blastopore lip form myotome fibers in discrete locations within somites, as well as among somites restricted to specific regions along the anteroposterior axis. We present a detailed fate map of the gastrula showing areas that are destined to form myotome fibers within specific regions of the tadpole. We further show that although the notochord plays an important role with respect to lengthening the dorsal axis, it does not appear to influence the relative positioning of myotome fibers within anterior and trunk somites. Finally, through a series of heterotopic and heterochronic transplantation experiments we show that gastrula circumblastoporal cells and cells from the tailbud PSM are interchangeable and can form myotome fibers according to their new location. However, only tailbud PSM cells can form myotome fibers when grafted to a mature somite revealing the fact that gastrula circumblastoporal cells are not yet competent to differentiate into aligned myotome fibers and require additional signals.
RESULTS
Fate Mapping of the Circumblastoporal Gastrula Cells
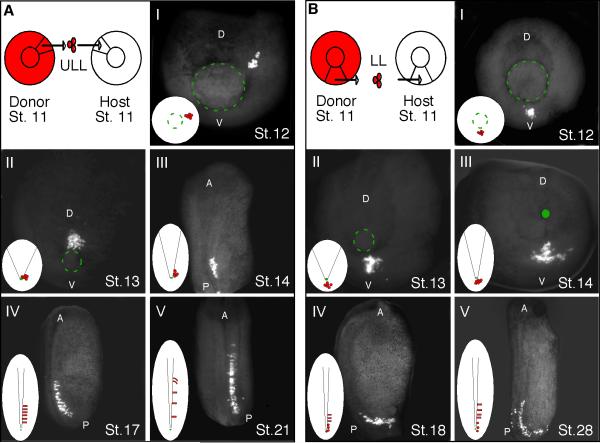
To follow the movement of cells that lead to somite formation along the anteroposterior axis, we performed a series of cell transplantation experiments that allowed us to image a small number of fluorescently labeled mesodermal cells at specific intervals between the onset of gastrulation and tailbud formation (Fig. 1). Homotopic grafts were performed in which rhodamine-dextran amine (RDA)-labeled cells were grafted to the upper lateral lip*1 (ULL; Fig 1A) on either side of the developing notochord and to the lower lip (LL; Fig 1B) regions of gastrulae. Since X.laevis mesodermal cells lie between the ectoderm and endoderm layers, it was necessary to fix and clear the grafted embryos at specific time points to visualize the relative position of the grafted cells at the various stages of development. Cells grafted to the upper lateral lip (Fig. 1A.I) were positioned close to the prospective notochord by the end of gastrulation (Fig. 1A.II). At the onset of neurulation the upper lateral lip cells are found within the forming PSM and by stage 14, cell intercalation behaviors distribute the labeled cells along the anteroposterior axis (Fig 1A.III). As the posterior axis continues to elongate, cells from the upper lateral lip region continue to intercalate among the host PSM cells and adopt a medial to lateral alignment (Fig. 1A.IV), which is characteristic of PSM cells prior to segmentation (Afonin et al., 2006). By stage 21, the anterior-most labeled cells begin to form somites and undergo a 90° rotation (Fig. 1A.V).
Figure 1. The relative position of cells around the gastrula influences their final position along the anteroposterior axis.
RDA-labeled upper lateral lip-ULL (A) and lower lip - LL (B) cells were grafted from stage 11 donor embryos to the same region of unlabeled host embryos. Images I–V show the location of the grafted cells at specific developmental stages. The green circle highlights the location of the blastopore lip at different developmental time points. In each panel a small diagram highlights the position of the labeled cells with respect to the forming midline. Images AI-II and BI-III are from the perspective of the blastopore. Images AIII and V are from a dorsal perspective and images AIV and BIV-V are from a lateral perspective. (D) dorsal; (V) ventral; (A) anterior; (P) posterior.
Cells grafted to the lower lip region (Fig. 1B.I) undergo involution at the end of gastrulation (Fig. 1B.II) and remain scattered in the lower lip region during the early stages of neurulation (Fig. 1B. II and III). At stage 18 the lower lip cells are still positioned around the lower blastopore lip and are just beginning to join the posterior PSM (Fig 1B.IV). At the late tailbud stage the lower lip cells continue to join the PSM with labeled cells positioned at the anterior end adopting a medial to lateral alignment (Fig 1B.V). Thus, cells positioned in the lower lip region become organized into the PSM at a much later stage than cells positioned in the upper lateral lip region. The relative position of cells around the gastrula greatly influences their final position along the anteroposterior axis, which supports previous observations by Keller (1991).
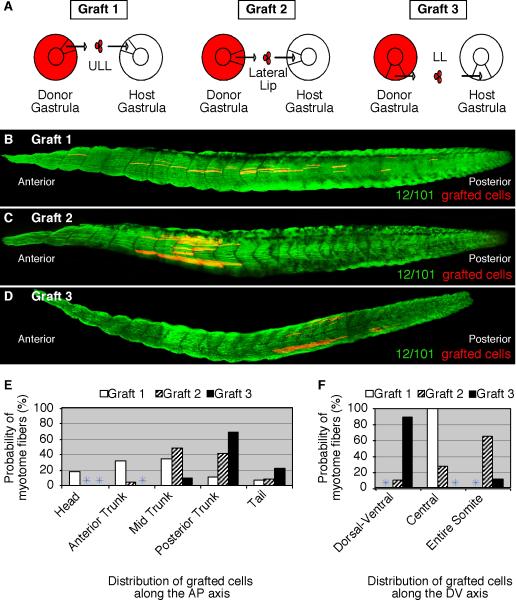
In order to quantify the precise destination of cells that originated from the upper lateral, lateral, and lower lip regions of the gastrula, grafted embryos were allowed to develop to stage 39, at which time axis elongation is almost complete and the tadpole has nearly 45 pairs of somites. Labeled cells grafted to the upper lateral lip region of gastrulae (n=13 embryos) gave rise to myotome fibers in 100% of cases (Table 1). These myotome fibers were positioned along the entire length of the axis (Fig. 2B). In particular, the myotome fibers were most frequently found in the anterior to mid trunk regions (Fig. 2E) as well as located in the central region of the somite, at the level of the notochord (Fig. 2F). Cells grafted to the lateral lip of gastrulae (n=45 embryos) formed myotome fibers in 100% of cases (Table 1) and were found primarily positioned in the mid to posterior trunk regions (Fig. 2C and E) and in the entire2 somite (Fig. 2F). Interestingly, cells grafted to the lower lip region of gastrulae (n=28 embryos) typically formed myotome fibers in 97% of cases (Table 1). These myotome fibers were predominantly located in posterior trunk somites (Fig. 2D and E) and in the dorsal and/or ventral aspects of each somite (Fig. 2F). Together, these results show that the relative position of cells in the gastrula leads to the formation of myotome fibers in discrete locations within a somite and along the anteroposterior axis.
Table 1.
Homotopic grafts of gastrula cells differentiate into myotome fibers.
| Type of graft | No. of samples; clutches | % formed MF +/− Std. Error |
|---|---|---|
| Graft 1 | 13; 3 | 100 +/− 0 |
| Graft 2 | 45; 2 | 100 +/− 0 |
| Graft 3 | 28; 3 | 97 +/− 1 |
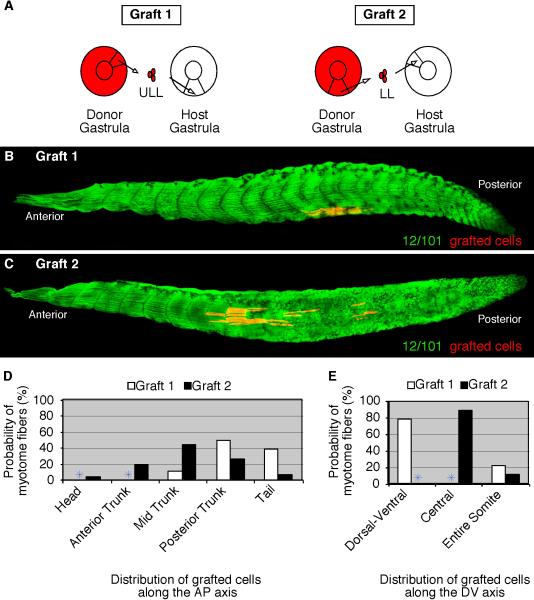
Figure 2. Gastrula cells from different blastopore lip regions form myotome fibers in distinct locations within somites and along the anteroposterior axis.
(A) A diagram depicting homotopic RDA-labeled upper lateral lip-ULL cells (Graft 1), lateral lip cells (Graft 2), and lower lip-LL cells (Graft 3) grafted from stage 11 donor embryos to the same region of unlabeled host embryos. Grafted embryos were allowed to develop to tadpoles (stage 39) and subsequently stained for the myotome-specific marker, 12/101 (green). (B) Grafted ULL cells formed myotome fibers located in the central domain of somites along the entire anteroposterior axis. (C) Grafted lateral lip cells formed myotome fibers found throughout somites position in the trunk whereas grafted lower lip-LL cells formed myotome fibers predominantly in the dorsal and ventral regions of somites in the posterior trunk and tail (D). The probability of ULL (Graft 1), lateral (Graft 2), and LL (Graft 3) cells that give rise to myotome fibers at specific regions along the anteroposterior (E) and dorsoventral (F) axes are shown.
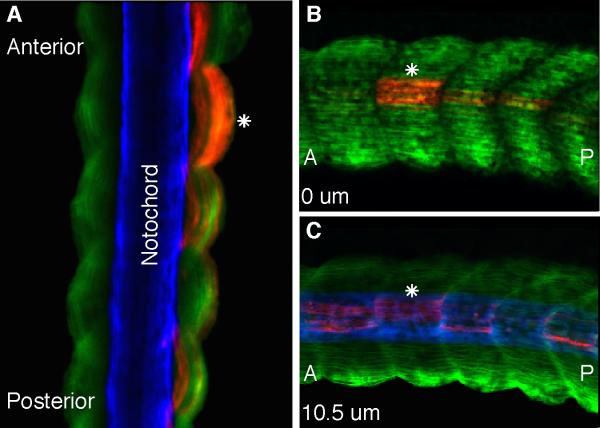
Fate mapping experiments of the paraxial mesoderm in the chick revealed that the medial part of the somite is derived from the primitive streak/tailbud whereas the lateral part is derived from the continuous ingression of epiblast cells (Iimura and Pourquié, 2006). This study suggested that vertebrate somites are derived from two distinct populations of cells that come together to form the lateral and medial aspects of the somite, respectively. Our fate mapping experiments above support the conclusion that somites are derived from different population of cells with the upper lateral lip cells forming primarily the central region of the somite and the lower lip cells forming the dorsal and ventral aspects of the somite. However, to determine whether these gastrula cells are also differentially distributed along the lateral and medial aspect of somites, we performed confocal scans of stage 39 embryos grafted with upper lateral lip cells followed by fixation and immuno staining with the muscle-specific 12/101 and the notochord-specific Tor-70 antibodies. If the X. laevis somites were organized in a similar fashion as observed in the chick then we would predict that cells from the upper lateral lip region would form myotome fibers positioned in the medial aspect of somites, adjacent to the notochord. However, this was not the case. Dorsal scans of somites showed that cells from the upper lateral lip region formed myotome fibers at the level of the notochord that span the entire lateral and medial aspects of somites (Fig. 3A). A lateral to medial scan of the same embryo further confirms this observation with labeled myotome fibers positioned both at the lateral edge of somites (Fig. 3B) as well as more medially, adjacent to the notochord (Fig. 3C). Thus, in X. laevis cells from the upper lateral lip region form myotome fibers that span the entire width of the central domain of somites and are not localized to the medial half of somites. This result suggests that in the case of X. laevis although somites originate from different cell populations they do not appear to segregate into medial and lateral aspects of the somite as observed in the chick (Iimura and Pourquié, 2006).
Figure 3. Upper lateral lip cells form myotome fibers in both the medial and lateral aspects of somites.
(A) A dorsal confocal scan of a stage 39 homotopically grafted embryo containing RDA-labeled upper lateral lip cells (red) that have differentiated into myotome fibers as shown by the myotome-specific antibody 12/101 (green). A white asterisk shows labeled myotome fibers are positioned medially, adjacent to the notochord as shown by the Tor-70 antibody (blue) as well as at the lateral edge of the paraxial mesoderm. Lateral to medial confocal scans of the same embryo shows labeled myotome fibers at the lateral (B) and medial (C) edges of somites. (A) anterior; (P) posterior.
The Role of the Notochord in the Formation of Axial Myotome Fibers
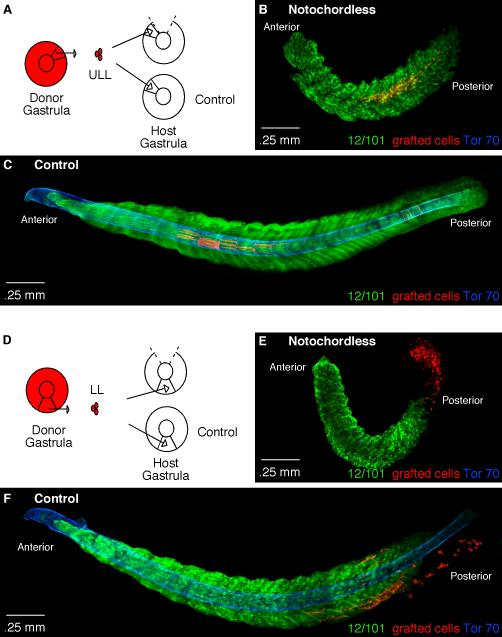
The notochord plays a prominent role during gastrulation with respect to patterning and axis elongation (Harland and Gerhart, 1997). Our transplantation results show that cells from the upper lateral lip region of the gastrula, which lie adjacent to the prospective notochord, form myotome fibers in the tadpole that lie in the center of the somite at the level of the notochord. Given the fact that the upper lateral lip cells lie in close proximity to the notochord, we next investigated whether the notochord plays a role in positioning myotome fibers in the center region of somites and along the anteroposterior axis. In X. laevis the standard approach to obtaining notochordless embryos is to expose the fertilized egg to a specific amount of ultra violet (UV) light, which disrupts cortical rotation and leads to the absence of dorsal tissue formation (Vicent and Gerhart, 1987). However, while exposure to UV light can lead to the formation of notochordless embryos it can also inadvertently disrupt paraxial mesoderm formation. To avoid this problem, we took a microsurgical approach in which we carefully excised the dorsal blastopore lip, which gives rise to the notochord, from embryos at the onset of gastrulation (stage 10). Next, RDA-labeled cells from either the upper lateral or lower lip regions of the gastrula were transplanted to the notochordless embryos (Fig. 4). The grafted embryos were than cultured to stage 25, at a slightly earlier stage than the previous experiments because the notochordless embryos tended to curl, which complicates the imaging process. As expected, in control grafted embryos (n=20 embryos) upper lateral lip cells formed myotome fibers positioned in the central region of the somite, at the level of the notochord as shown by Tor-70 expression, in 90% of cases (Fig. 4C). Interestingly, in notochordless embryos (n=14 embryos) as confirmed by the absence of Tor-70 staining, the upper lateral lip cells continued to form myotome fibers positioned in the center region of somites in 93% of cases (13 out of 14 embryos); however, the axis contained far fewer somites as compared to control embryos (Fig. 4B). Cells grafted to the lower lip region of control embryos are found at the posterior end of the axis and positioned in the dorsal and ventral aspects of the somites (Fig. 4F). In notochordless embryos there is a severe reduction in the length of the axis and as a consequence the grafted cells remain in the PSM and around the blastopore region and are not found within segments (Fig. 4E). These results show that the major role of the notochord is two-fold: first, in axis elongation, as there is a severe reduction in the number of somites in notochordless embryos as compared to control embryos, and second, in providing structural rigidity to the posterior tail as seen by the curvature of the posterior trunk. Interestingly, the notochord did not appear to play a role in maintaining the position of cells destined to form myotome fibers within the central region of somites. Given that notochordless embryos have a truncated axis it was not possible to assess the role of the notochord on the formation of myotome fibers positioned in the dorsal and ventral aspects of posterior somites derived from the lower lip region of the gastrula.
Figure 4. The role of the notochord on the axial positioning of myotome fibers.
(A) A diagram depicting homotopic grafts performed to the upper lateral lip-ULL region in control and notochordless gastrulae. (B) A stage 25 notochordless embryo containing RDA-labeled cells from the ULL (red) that formed myotome fibers in the central region of somites. The notochordless embryo is stained for 12/101 (green) to show myotome formation and Tor-70 (blue) to confirm the absence of the notochord. (C) A control stage 25 embryo containing ULL cells (red) that have formed myotome fibers shown by the muscle-specific antibody 12/101 (green) positioned at the level of the notochord as shown by Tor-70 staining (blue). (D) A diagram depicting homotopic grafts performed to the lower lip-LL region in control and notochordless gastrulae. (E) A stage 25 notochordless embryo with LL-grafted cells (red) stained with the muscle-specific antibody 12/101 (green) and Tor-70 (blue) to confirm the absence of the notochord. (F) A control stage 25 embryo stained with 12/101 (green) and Tor-70 (blue) containing LL-grafted cells (red) that have begun to form myotome fibers mostly in the dorsal and ventral aspects of posterior somites.
The Spatial and Temporal Plasticity of Circumblastoporal Gastrula Cells with respect to Myotome Formation
Results from the fate mapping experiments above reveal that the final placement of myotome fibers along the anteroposterior and dorsoventral axes is strongly influenced by the original position of gastrula cells around the blastopore lip. We next asked whether gastrula cells around the blastopore lip are committed towards forming myotome fibers at specific locations along the anteroposterior or dorsoventral axes. Previous studies have shown that myogenic genes such as Myf5 are differentially expressed at the gastrula stage with expression restricted to the upper lip region (Dosch et al., 1997). Moreover, Hox gene expression at the onset of gastrulation has been shown to influence the final axial position of PSM cells in the chick (reviewed in Iimura et al., 2009). Thus, it is possible that differential gene expression during gastrulation leads to patterning differences that predispose cells towards a precise location within a segment or along the axis. Therefore, to determine whether the final position of myotome fibers is an intrinsic property of these circumblastoporal cells, heterotopic grafts were performed which consisted of transplanting RDA-labeled cells from the upper lateral lip region to the lower lip region of gastrulae as well as the converse experiment of transplanting RDA-labeled cells from the lower lip to the upper lateral lip region (Fig. 5A). Grafted embryos were allowed to develop to stage 39 at which time they were fixed and stained for myotome fibers with 12/101 antibody and the position of grafted cells were assayed. Upper lateral lip cells grafted to the lower lip region of gastrulae (n=40 embryos) gave rise to muscle fibers in 80% of cases (Table 2). These muscle fibers were predominantly found in the dorsal or ventral aspect of somites (Fig 5B and E). In addition, the grafted cells were observed among somites located towards the posterior end of the axis (Fig. 5D). These results are similar to lower lip homotopic grafts in which cells are found in the dorsal and ventral aspects of somites and in the posterior axis (Fig. 2D–F). Thus, the upper lateral lip cells adapted to the lower lip host environment and gave rise to myotome fibers in the location defined by their host environment. Similarly, lower lip cells grafted to the upper lateral lip region of gastrulae (n=38 embryos) gave rise to muscle fibers in 100% of cases (Table 2). The grafted cells were mostly positioned in the central domain of somites (Fig. 5C and E) and along the entire axis (Fig. 5D). These results are similar to the upper lateral lip homotopic grafts in which cells are found in the central region of somites and along the entire axis (Fig. 2B,E–F). Together, these results show that the gastrula cells are not committed to giving rise to myotome fibers in specific locations, but rather can adapt to their new location and form myotome fibers in the pattern defined by the host environment.
Figure 5. Gastrula cells form myotome fibers according to their new host location.
(A) A diagram depicting heterotopic grafts in which RDA-labeled upper lateral lip-ULL cells are grafted to the lower lip region (Graft 1) and RDA-labeled lower-lip-LL cells are grafted to the upper lateral lip region (Graft 2) of stage 11 host embryos. Grafted embryos were allowed to develop to tadpoles (stage 39) and then subsequently stained for the myotome-specific marker, 12/101 (green). (B) ULL-labeled gastrula cells transplanted to the lower lip region form myotome fibers in the ventral region of somites positioned towards the posterior axis of the tadpole. (C) LL-labeled gastrula cells transplanted to the upper lateral lip region form myotome fibers in the central domain of somites positioned along the axis. The probability of ULL (Graft 1) and LL (Graft 2) cells that give rise to myotome fibers at specific regions along the anteroposterior (D) and dorsoventral (E) axes are shown.
Table 2.
Heterotopic grafts of gastrula cells give rise to myotome fibers.
| Type of graft | No. of samples; clutches | % formed MF +/− Std. Error |
|---|---|---|
| Graft 1 | 40; 4 | 80 +/− 2 |
| Graft 2 | 38; 4 | 100 +/− 0 |
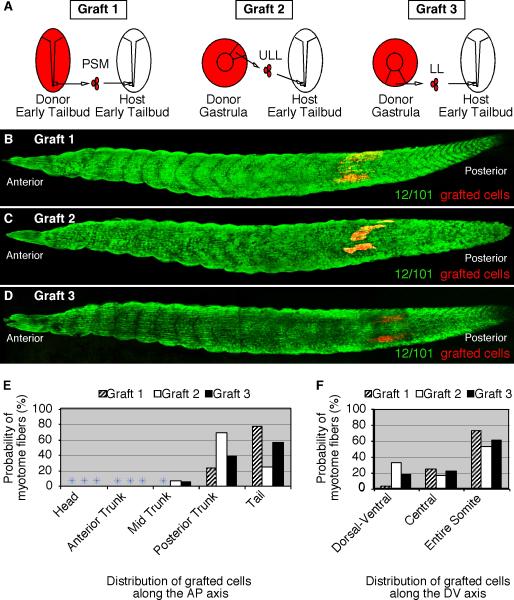
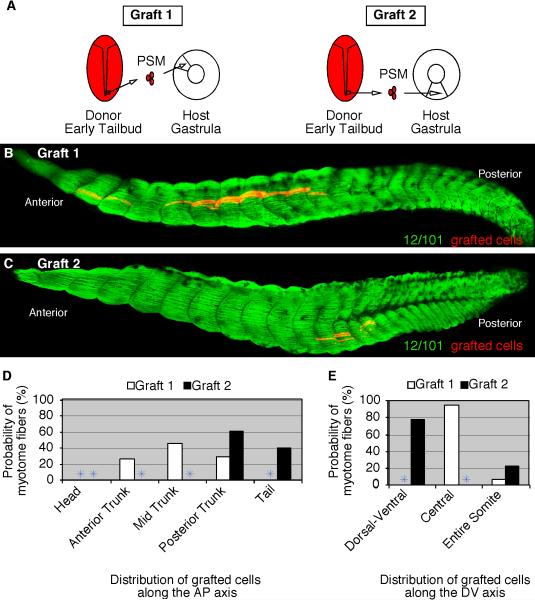
To further examine the plasticity of these gastrula cells, we examined whether they can adapt not only to a change in location, but also a change in the timing of myotome differentiation. Heterotopic and heterochronic grafts were performed between gastrula and early tailbud-stage embryos (Fig. 6A). As a control, we first performed homotopic and homochronic grafts among PSM cells of tailbud-staged embryos (n=33) and cultured them to stage 39 to determine the final axial position of the grafted cells (Fig. 6A, Graft 1). These grafted cells formed myotome fibers in 100% of cases (Table 3) and they were primarily located in the tail (Fig. 6B and E) and throughout the entire somite (Fig. 6F). Similarly, upper lateral lip cells grafted from the gastrula to the posterior PSM of tailbud-staged embryos (n=30) formed myotome fibers in 83% of cases (Table 3). The upper lateral lip cells formed myotome fibers throughout the entire somite (Fig. 6C and F) and in the posterior axis (Fig. 6E). Cells grafted from the lower lip region of the gastrula to the PSM of tailbud embryos (n=30) also formed myotome fibers in the vast majority of cases (93%; Table 3). These grafted cells were also found throughout the somite (Fig. 6F) and restricted towards the posterior end of the axis (Fig. 6D and E). These results show that gastrula cells can adapt to the more advanced tailbud PSM environment to give rise to myotome fibers positioned in relatively similar locations as the host tailbud PSM cells.
Figure 6. Gastrula cells can form myotome fibers when grafted to older tailbud-staged embryos.
(A) A diagram that depicts three different transplantation experiments consisting of RDA-labeled cells grafted from the PSM of tailbud-staged embryos (Graft 1) or from the upper lateral lip-ULL (Graft 2) or the lower lip-LL (Graft 3) regions of gastrulae to the PSM of tailbud-stage embryos (stage 19). Grafted embryos developed to stage 39 and were fixed and stained with the myotome-specific antibody, 12/101. (B) PSM-grafted cells (Graft 1) form myotome fibers throughout the somite and towards the posterior end of the axis. (C) ULL-grafted cells (Graft 2) form myotome fibers also throughout the somite and towards the posterior end of the axis. (D) LL-grafted cells (Graft 3) form myotome fibers towards the posterior end of the axis. The probability of PSM (Graft 1), ULL (Graft 2), and LL (Graft 3) cells that give rise to myotome fibers at specific regions along the anteroposterior (E) and dorsoventral (F) axes are shown.
Table 3.
Gastrula cells grafted to the PSM of tailbud embryos differentiate into myotome fibers.
| Type of graft | No. of samples; clutches | % formed MF +/− Std. Error |
|---|---|---|
| Graft 1 | 33; 3 | 100 +/− 0 |
| Graft 2 | 30; 3 | 83 +/− 2 |
| Graft 3 | 30; 3 | 93 +/− 1 |
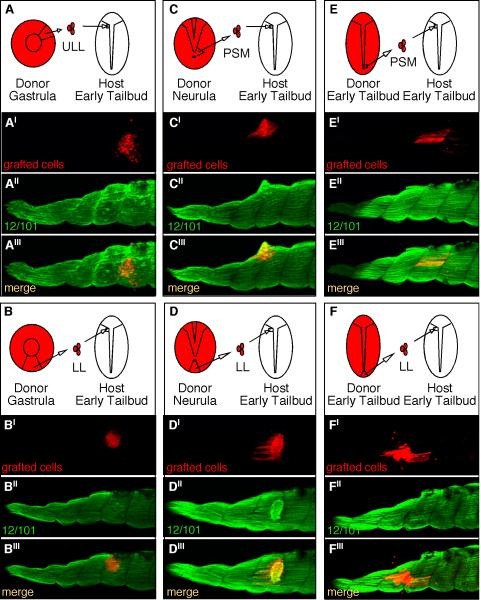
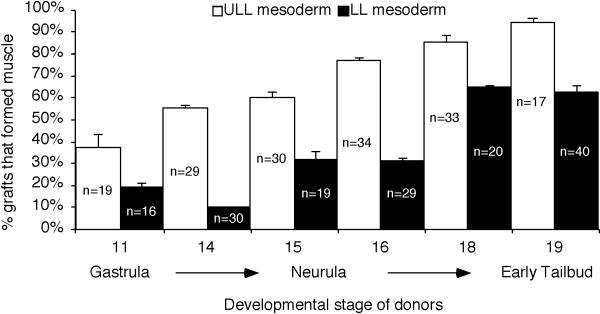
Given that cells from the gastrula can adapt to the tailbud PSM environment and form normal myotome fibers, we next asked whether gastrula cells could differentiate directly into myotome fibers when grafted to mature somites thus bypassing the normal maturation process associated with myogenesis. To address this question, labeled cells were grafted from either the upper lateral (Fig. 7A) or lower lip (Fig. 7B) regions of gastrulae to the anterior paraxial mesoderm of early tailbud embryos where the anterior-most somites have begun to form. Grafted embryos were allowed to develop to stage 39 at which time they were fixed, stained with the myotome-marker 12/101 and the fate and morphology of the grafted cells were determined. Our results show that upper lateral lip cells grafted to the anterior end of early tailbud embryos (n=19) differentiated into myotome fibers in 37% of cases while cells grafted from the lower lip region (n=16 embryos) give rise to myotome fibers in only 16% of cases (Fig. 8). The majority of cells grafted from the gastrula, regardless of whether they came from the upper lateral or lower lip regions of the blastopore, formed clusters of cells that did not integrate well into the somite and did not form normal elongated myotome fibers (Fig. 7A′ and B′). In fact, the lower lip cells were generally not positive for 12/101 (Fig. 7B″′), and in some rare cases a couple of cells from the upper lateral lip region appeared to be positive for 12/101 (Fig. 7A′′′). Thus, the majority of gastrula cells did not appear competent to differentiate into myotome fibers when directly transplanted to a mature somite. We then performed additional transplantation experiments to determine the stage in which PSM cells become competent to differentiate into myotome fibers. Cells were grafted from embryos ranging in age between stages 11 and 19 (early tailbud) to the anterior paraxial mesoderm of early tailbud embryos. Collectively, we found that PSM cells gradually become more competent to differentiate into myotome fibers (Fig. 8). For example, cells from the upper lateral PSM and lower lip region of neurula embryos (stage 15) grafted to the anterior paraxial mesoderm of tailbud embryos tended to develop into an abnormal clump of cells; however these clumps were more likely to be positive for 12/101 (Fig. 7C and D). In addition, among the thirty embryos that received grafts from the neurula PSM (Fig. 7C) we found that in 60% of cases the grafted embryos contained labeled cells that had differentiated into myotome fibers. In contrast, among the nineteen embryos transplanted with lower lip cells from neurulae, only 32% of them contained labeled cells that had differentiated into myotome fibers (Fig. 7D). Interestingly, although the clumps appear positive for 12/101, the majority of the cells did not adopt normal myotome morphology. In embryos that received grafts from tailbud PSM (n=17 embryos) we found that in 94% of cases the grafted PSM cells differentiated into myotome fibers (Fig. 7E). Interestingly, cells grafted from the lower lip region of tailbud embryos (n=40) gave rise to myotome fibers in only 63% of cases with a significant population of cells that were 12/101 positive, but did not have a clear myotome morphology (Fig. 7F). Together, these experiments reveal that PSM cells first become competent to differentiate into muscle as indicated by their ability to express 12/101 (Fig. 7B). This is then followed by their ability to become competent to undergo proper morphogenesis to form elongated and aligned myotome fibers (Fig. 7E). Cells from the upper lateral lip region that become part of the PSM acquire competency to form myotome fibers prior to cells located in the lower lip region of the blastopore in progressively older embryos (Fig. 8). These results suggest that additional signals encountered within the PSM during maturation are important for instructing cells to become properly elongated myotome fibers.
Figure 7. Mesodermal cells gradually become competent to form myotome fibers when grafted to the anterior paraxial mesoderm of early tailbud-staged embryos.
RDA-labeled cells grafted from the gastrula upper lateral lip-ULL (A), gastrula lower lip-LL (B), neurula PSM of a stage 15 embryo (C), neurula tailbud lower-lip of a stage 15 embryo (D), tailbud PSM of a stage 19 embryo (E), and the lower lip of a stage 19 embryo (F) were all grafted to the anterior paraxial mesoderm of stage 19 tailbud embryos where somites have begun to form. Grafted embryos were cultured to stage 39 and then stained with the myotome marker, 12/101 (A″–F″) to determine whether the RDA-labeled cells (A′–F′) had adopted a myotome fate and morphology as shown by the merged images (A″′–F″′).
Figure 8. Cells gradually become competent to form myotome fibers.
RDA-labeled cells were grafted from the upper lateral (ULL) and lower lip (LL) regions of embryos between stages 11 and 19 to the anterior paraxial mesoderm of early tailbud hosts (stage 19) where somites have begun to form. The percentage of embryos that contained grafted cells that formed myotome fibers was determined. The total number of embryos used for each grafting experiment is shown. Error bars indicate standard error deviations.
Tailbud PSM Cells Can Adopt the Local Gastrula Cell Behaviors and Delay Differentiation
Our transplantation results reveal that gastrula cells can form myotome fibers when transplanted to the PSM of tailbud stage embryos. Interestingly, transplantation experiments to mature somites of tailbud embryos revealed differences between the competency of gastrula and tailbud PSM cells to give rise to myotome fibers. Given these differences, we asked whether tailbud PSM cells can adapt to the younger gastrula environment and form myotome fibers according to their new location and developmental timing. To address this question, RDA-labeled cells from the tailbud PSM were grafted either to the upper lateral (graft 1) or lower lip (graft 2) regions of gastrulae (Fig. 9A). The grafted embryos were allowed to develop to stage 39 at which time they were fixed and then assayed for myotome formation with the muscle-specific antibody 12/101. Tailbud PSM cells grafted to the upper lateral lip region of gastrulae (n=18 embryos) differentiated into myotome fibers in 94% of cases (Table 4). These grafted cells were located in the central domain of each segment (Fig. 9B and E) and found throughout the trunk (Fig. 9D). The distribution of the grafted PSM cells at stage 39 was similar to that observed among homotopic upper lateral lip cell transplantations (Fig. 2 B). Tailbud PSM cells grafted to the lower lip region of the gastrula (n=19 embryos) formed myotome fibers in 58% of cases, which is significantly less than that observed among PSM cells grafted to the upper lateral lip region (Table 4). The grafted tailbud PSM cells that did form myotome fibers were found in the posterior trunk and tail regions (Fig. 9C and D) and restricted primarily to the dorsal and ventral aspects of the somite (Fig. 9E). This distribution is similar to that observed in the lower lip fate mapping experiments (Fig. 2 D). In the cases that grafted cells did not form myotome fibers, they were found scattered in the posterior axis with morphology characteristic of cells undergoing cell death. Since lower lip gastrula cells enter the PSM at much later stages of development compared to upper lateral lip cells (Fig. 1) it may be the case that tailbud PSM cells grafted to the lower lip underwent apoptosis due to a longer delay in progressing through the myogenic program in comparison to tailbud PSM cells grafted to the upper lateral lip of gastrulae (Table 4). These results suggest that there is likely a limited window of temporal flexibility where older PSM cells can successfully delay entering the myogenic program. If this delay is extended beyond this window then cells lose their ability to form myotome fibers and undergo cell death.
Figure 9. Tailbud-staged PSM cells can form myotome fibers when grafted to younger gastrulae.
Grafts were performed in which RDA-labeled PSM cells were grafted to the upper lateral lip (Graft 1) and lower-lip (Graft 2) regions of stage 11 host embryos and allowed to develop to tadpoles (stage 39). Grafted embryos were fixed and stained for the myotome marker, 12/101. (B) PSM cells grafted to the upper lateral lip region (Graft 1) formed myotome fibers in the central region of somites along most of the anteroposterior axis. (C) PSM cells grafted to the lower lip region (Graft 2) formed myotome fibers in the ventral aspect of somites positioned towards the posterior end of the embryo. The probability of tailbud PSM cells grafted to the upper lateral lip (Graft 1) and lower lip (Graft 2) gastrulae that give rise to myotome fibers at specific regions along the anteroposterior (D) and dorsoventral (E) axes are shown.
Table 4.
Tailbud PSM cells grafted to gastrulae give rise to myotome fibers.
| Type of graft | No. of samples; clutches | % formed MF +/− Std. Error |
|---|---|---|
| Graft 1 | 18; 2 | 94 +/− 2 |
| Graft 2 | 19; 2 | 58 +/− 1 |
DISCUSSION
While many molecules that play a role in somitogenesis have been identified and appear to be well conserved among vertebrates, the precise cell behaviors that originate in the gastrula and lead to the formation of somite-derivatives remain unclear. In this paper we used a cell transplantation approach to determine the movement of cells originating from the circumblastoporal region of the gastrula to their final destination as myotome fibers with respect to both the anteroposterior and dorsoventral axes. We further examined the temporal and spatial plasticity of these cells as they undergo myotome differentiation. We show that cells surrounding the circumblastoporal region of the gastrula as well as PSM cells of the tailbud retain some temporal and spatial flexibility with regards to their final axial position. We further show that although these gastrula cells have some spatial and temporal flexibility with regards to myotome formation, they do require a certain amount of maturation within the PSM before they can fully differentiate into aligned myotome fibers.
Final Position of Myotome Fibers is an Outcome of Gastrulation Movements
Although several groups have constructed fate maps of the X. laevis gastrula (Dale and Slack, 1987; Keller, 1991; Lane and Smith, 1999), the precise fate mapping of cells destined to form myotome fibers along the anteroposterior and dorsoventral axes has not been done. In this paper we fate mapped the circumblastopore lip of the gastrula to determined the precise location of their progeny with respect to myotome formation in the tadpole (Fig. 10A; see asterisks). Our results show that cells originating from the gastrula upper lateral lip region form myotome fibers in the anterior most somites as well as myotome fibers located within the central region of somites, at the level of the notochord. Moreover, these cells form myotome fibers positioned along the entire axis with fewer myotome fibers observed in the posterior trunk and tail regions (Fig. 10A, green). This finding is consistent with that observed by Lane and Sheets (2002), as the progeny of the C2 blastomere are likely to populate the upper lateral lip region of the gastrula and are also found in the central region of somites. Work by Shook et al., (2004) showed that centrally-located myotome fibers also arise from the ingression of superficial mesoderm cells also positioned in the upper lateral lip region of the gastrula. Cells originating from the lateral region of the blastopore lip (shown in yellow, Fig. 10A) form myotome fibers that are distributed throughout the entire somite. These lateral cells give rise to myotome fibers positioned throughout the trunk. In contrast, cells from the lower blastoporal lip region (shown in pink, Fig. 10A) form myotome fibers primarily restricted to the dorsal and ventral domains of somites in the trunk and tail regions. Together, our data shows that somites are comprised of multiple mesodermal populations that originate from distinct regions within the gastrula.
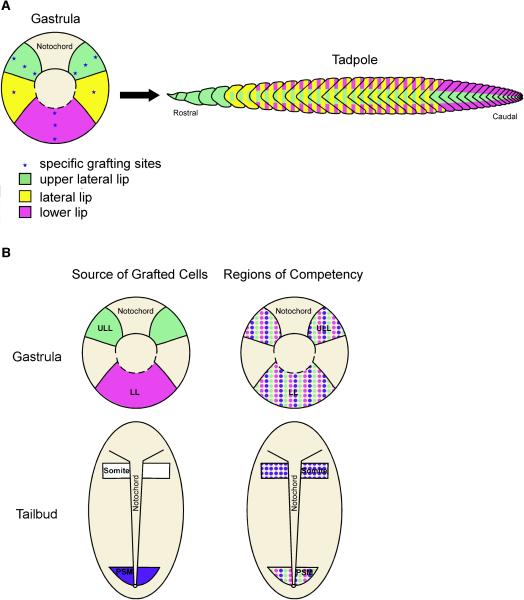
Figure 10. A mesodermal fate map and competence map of gastrula and PSM tailbud cells.
(A) A fate map of the tadpole musculture projected onto the gastrula shows the following: upper lateral lip cells (green) give rise to the anterior-most somites as well as myotome fibers positioned in the central region of somites along the entire axis; cells positioned in the lateral circumblastoporal region (yellow) give rise to myotome fibers found both in the central and dorsoventral regions of somites throughout the trunk region; and lower lip cells (purple) form myotome fibers found in the dorsal and ventral domains of somites located in the trunk and tail regions of the tadpole. (B) A diagram summarizing the competency of gastrula and tailbud PSM cells to give rise to myotome fibers when grafted to different locations. Gastrula cells from the upper lateral (green) and lower (purple) lip regions are competent to give rise to myotome fibers when grafted to any region of the gastrula and to the PSM of tailbud-staged embryos (Fig. 10B; Regions of Competence). Similarly, cells from the PSM of tailbud embryos (blue) are competent to form myotome fibers when transplanted to the gastrula and tailbud PSM. However, PSM cells are also competent to form myotome fibers when transplanted to the anterior end of the paraxial mesoderm whereas gastrula cells are not.
The differences in the distribution of myotome fibers within somites and along the anteroposterior axis may reflect important regional characteristics. For example, in the chick embryo the formation of the paraxial mesoderm appears to involve two distinct sources of cells: an anterior-most primitive streak population of cells that behave in a stem cell-like fashion, which give rise to the medial portion of the somite; and a non-stem cell population of cells from the lateral epiblast that form the lateral portion of the somite (Iimura et al., 2007). Results from our transplantation experiments showed that cells grafted from the upper lateral lip region gave rise to myotome fibers found along the entire trunk and tail axis. Although these upper lateral lip cells tend to be more dispersed along the anteroposterior axis in comparison to cells from the lower lip region, we did not observe differences in clonal expansion which would indicate the possibility of a stem-cell like behavior. This observation is also supported by a previous study examining mitotic patterns in X. laevis which found that cells in the paraxial mesoderm are not mitotically active (Saka and Smith, 2001). Instead, our data supports the hypothesis that regional differences in the distribution of myotome fibers is due to the relative differences in convergent and extension movements experienced by cells at different positions around the gastrula blastopore lip. More specifically, cells positioned in the upper lateral lip region participate in more significant convergent and extension movements than cells located in the lower lip region. This can be clearly seen in the grafted embryos that were imaged at different developmental stages (Fig. 1). Cells from the upper lateral lip region are found more extensively distributed along the anteroposterior axis by stage 21 in comparison to lower lip cells, which are confined to the posterior trunk and tail regions (Fig. 1). The differences in the anteroposterior position of these circumblastoporal cells are likely influenced by the early and rapid elongation of the notochord (Keller et al., 1989). It is clear from the tri-labeled images that show myotome (12/101-green), notochord (Tor 70-blue) and grafted cells (red) that the notochord extends further posteriorly in comparison to the segmented musculature (Fig. 4F). This dramatic elongation by the notochord may drag cells from the upper lateral lip region posteriorly, shearing past the lateral paraxial mesoderm. In fact, cells grafted to the upper lateral lip region are often found spanning many more segments (Fig. 2B) than cells that originated from the lower lip region of the blastopore (Fig. 2D). In addition, excision of the prospective notochord led to a significant reduction in axis elongation as well as a subsequent reduction in the number of segments containing labeled cells from the upper lateral lip region (Fig. 4B). Interestingly, the position of cells within the central domain of somites was not influenced by the absence of the notochord (Fig. 4B). Thus, the role of the notochord is not to regulate the relative position of myotome fibers within a somite, but rather in influencing the extent to which the posterior axis elongates. Therefore, we propose that the differences in these cell movements lead to the formation of somites comprised of a mixture of cells from distinct regions of the gastrula.
Temporal and Spatial Plasticity of PSM Cells
While the fate mapping experiments show that gastrula stage cells are destined to become myotome fibers at relatively specific regions within a somite and along the anteroposterior axis, their axial regionalization does not appear to be an intrinsic property. Our heterotopic grafts show that cells from the gastrula can adopt the cell behaviors of the host to give rise to myotome fibers in axial positions characteristic of their new location (Fig. 10B). This plasticity extends to cells grafted between the gastrula and the PSM of tailbud-staged embryos. Recent studies in the chick have shown that the collinear activation of Hox genes influences the timing of mesodermal cell ingression and ultimately the position of paraxial cells along the anteroposterior axis (Iimura and Pourquie, 2006). A similar collinear activation of Hox genes has also been observed in the X. laevis gastrula (Wacker et al., 2004). These studies suggest that the axial patterning of mesodermal cells is activated during gastrulation by specific Hox genes. In the context of our transplantation experiments, it is likely that despite the initial Hox genes expressed in the gastrula and tailbud PSM cells, pre-myotome cells are still able to adapt to new axial positions. This is consistent with the plasticity in Hox gene expression observed among PSM cells of the chick (McGrew et al., 2008). Thus, although cells may express a specific Hox gene that predisposes them to an anteroposterior location, the new environment can redirect cells to a different location. This may be due to physical forces driven by the collection of cells undergoing myogenesis and/or by active signaling cues found in the new environment.
Mesodermal Cell Maturation is Important for Myotome Formation
Mesodermal cells from the gastrula appear to be interchangeable with PSM cells of the tailbud. However, transplantation experiments to the anterior paraxial mesoderm where mature somites have formed identified a striking difference in which tailbud PSM cells could differentiate into aligned myotome fibers whereas gastrula mesodermal cells could not (Fig. 10B). These experiments revealed that cells within the tailbud PSM are clearly more advanced in their ability to form elongated and properly aligned myotome fibers than gastrula cells. A series of transplantation experiments reveal that cells gradually become more competent to form myotome fibers and that cells from the upper lateral lip region acquire this competency prior to cells positioned in the lower lip region (Fig. 8). Interestingly, grafted PSM cells are able to express the muscle-specific antigen, 12/101, prior to acquiring the ability to form elongated and aligned myotome fibers. Previous studies have shown that integrins, heterodimeric transmembrane receptors that mediate cell adhesion, are important for proper somite segmentation and myotome alignment in X. laevis (Marsden and DeSimone, 2003; Hildalgo et al., 2009) as well as during somitogenesis in other vertebrates (Drake et al., 1992; Yang et al., 1993; Zagris et al., 2004; Julich et al., 2005; Koshida et al., 2005). In X. laevis α-5 integrin mRNA is first detected from the midgastrula stage onward (Joos et al., 1995). Knockdown of α-5 integrin has been shown to disrupt somite rotation and segment boundary formation (Kragtorp and Miller, 2007). Thus, it may be the case that gastrula and tailbud PSM cells express different levels or types of integrins which influence their ability to make the necessary attachments to the intersomitic boundaries and complete the process of myogenesis.
Conclusions
In this paper we show that somites in X. laevis are comprised of cells that originate from different regions of the gastrula circumblastoporal lip. We propose that different amounts of convergent and extension movements lead to the mixture of these populations of cells within somites along the axis. The origin of somite precursor cells is different from that proposed in other vertebrates which is associated with differences in stem cell-like properties (Iimura et al., 2007). We also show through heterochronic and heterotopic cell transplantation experiments that mesodermal cells in the gastrula and PSM cells of the tailbud are interchangeable despite significant differences in age. However, cells in the tailbud are competent to form elongated and aligned myotome fibers when placed in the anterior paraxial mesoderm, whereas gastrula cells are not. These results highlight the important maturation process that mesodermal cells must experience within the PSM in order to form integrated and properly aligned myotome fibers.
EXPERIMENTAL PROCEDURES
Embryo Culture and RDA Injections
To induce ovulation X. laevis females were injected with 0.4ml human chorionic gonadotropin (HCG) hormone. Eggs were collected 18 to 22 hours post injection and artificially fertilized with X. laevis male sperm in 1/3× MBS at room temperature (Danilchick et al., 1991).
After fertilization, future donor embryos were transferred to Petri dishes coated with 2% agar and filled with 1× MBS. At the one cell stage, embryos were injected with 9.2nl of 10,000 MW rhodamine dextran amine (RDA) using a Drummond Nanoject II Automatic Oocyte Injector. Injected embryos were transferred to 1/3× MBS containing 2% Ficol and after 24 hours they were moved to 1/3× MBS. Embryos were allowed to develop to the desired stage and then screened for fluorescence using an Olympus SZX12 fluorescence-dissecting microscope.
Cell Transplantations and Extirpations
RDA-labeled donor and unlabeled host embryos were allowed to develop to various developmental stages (Nieuwkoop and Faber, 1994). At the appropriate stage, the protective vitelline envelope was removed from both host and donor embryos using forceps. Eyebrow and eyelash tools were used to remove approximately 10–50 RDA - labeled cells from donor embryos. Cell transplantations were performed in 2% agar coated Petri dishes containing 1× MBS and then transferred to 2% agar coated Petri dishes containing 1/3× MBS for long-term culture. At stage 39 embryos were fixed in 1× MEMFA (100mM MOPS pH 7.4; 2mM EGTA, 1mM MgSO4; 3.7% formaldehyde) for one hour at room temperature or overnight at 4°C. Fixed embryos were transferred to 100% methanol for long-term storage.
To perform notochord extirpations, the gastrula fate map (Keller, 1991) along with the position of dorsal bottle cells was used to predict the position of the prospective notochord at the onset of gastrulation (stage 10). Eyebrow and eyelash tools were then used to excise the prospective notochord region from stage 10 embryos. Extirpated embryos were then used in cell transplantation experiments as host embryos and cultured to the appropriate stage in 1/3× MBS.
Immunohistochemistry
To confirm whether the grafted cells adopted a myotome fate, the grafted embryos were subjected to immunohistochemistry using 12/101, a muscle-specific antibody (Kintner and Brockes, 1984). To confirm the absence of the notochord in the extirpation experiments, embryos were stained with the notochord-specific antibody Tor-70, diluted to 1:5 (Bolce et al., 1992). The immunostaining procedure followed previously published methods (Hemmati-Brivanlou and Melton, 1994). Embryos subjected to 12/101 were then incubated with a 1:1000 dilution of Alexa Fluor® 488 goat anti-mouse IgG secondary antibody (Molecular Probes), whereas embryos subjected to Tor- 70 were incubated with a 1:1000 dilution of Alexa Fluor® 647 goat anti-mouse IgM secondary antibody (Molecular Probes). Stained embryos were then cleared with 2:1 benzyl benzoate: benzyl alcohol and mounted onto microscope slides for imaging on a Nikon C1 confocal microscope and a Nikon E600 Physioscope Fluorescence microscope. Confocal images were taken with a 10× objective and then montages of the entire paraxial mesoderm were put together using Photoshop CS2.
Image Analysis and Quantification of Grafted Cells Along the Anteroposterior and Dorsoventral Axes
Transplanted embryos were divided into 5 regions: head (smaller cranial somites 1-4), anterior trunk (somites 5-9), mid trunk (somites 10-14), posterior trunk (somites 15-20), and tail (somites 21-45) as shown in the supplemental figure 1. The position and morphology of the grafted cells were determined. If the grafted cells had an elongated cell shape typical of a muscle fiber they were scored as positive (+). The position of the grafted cell with regards to their location within the five areas was noted. In addition, we determined the position of grafted cells within somites with respect to the dorsoventral axis. The location of the grafted cells were scored according to the following: (1) “central” which is the region of the somite that is parallel to the notochord; (2) “dorsal and/or ventral” which encompasses the regions above and below the central domain of the somite; and (3) “entire” somite which were cells positioned in the central, dorsal and/or ventral regions of the somite. The probability in which transplanted cells formed muscle fibers along the anteroposterior axis and within the somite for each type of transplantation experiment was determined and compiled into graphs.
Supplementary Material
Grafted embryos were divided into five regions consisting of the head (smaller cranial somites 1–4), anterior trunk (somites 5–9), mid trunk (somites 10–14), posterior trunk (somites 15–20), and tail (somites 21–45). For any given transplantation experiment, which typically consisted of 30 embryos, the specific regions containing labeled cells were noted. The frequency in which cells were found in each specific region was used as the numerator and the denominator consisted of the sum for all regions containing labeled cells within any given experiment. This fraction was then used to calculate the probability that grafted cells would give rise to myotome fibers in a specific region along the anteroposterior axis. The frequency in which labeled cells were observed in each region was calculated rather than the precise number of labeled cells that differentiated into myotome fibers because the labeled cells were often positioned very close to one another making it challenging to discern the exact number of labeled cells (B). The percentages were then used to generate the data for each graph (C).
ACKNOWLEDGEMENTS
We would like to thank Laura Burrus, David Shook, Paul Skoglund, and David Weisblat for helpful comments on the manuscript, Marie Sabillo for assistance with the composite images, and Richard Harland for the Tor 70 antibody. The 12/101 antibody, developed by J. Brockes, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences at the University of Iowa, Iowa City, IA 52242.
Grant sponsors: NIH MBRS SCORE: 1SC3GM081165-01A1 to C.R.D.; NSF GK-12 Grant DGE-0337949 to V.K.S.; Beckman Scholars Program Award to A.S., and NIH P20MD000544 from the National Center on Minority Health and Health Disparities.
Footnotes
In this paper, we use a synonymous nomenclature for identifying the sub-domains of the blastopore lip, using “upper lip” in place of “dorsal lip” and “lower lip” in place of “ventral lip” (Wunderlich et al., 2005). The goal is to avoid confusion arising from referencing ventral lip progeny as dorsal axial myotome fibers as well as providing a more accurate framework for discussing non-ventral progeny that arise from this lower lip region.
Labeled cells that were found in all three regions of the somite (central, dorsal, and ventral) were categorized as being found in the “entire” somite (i.e. in a non-restricted pattern within the somite).
REFERENCES
- Afonin B, Ho M, Gustin JK, Meloty-Kapella C, Domingo CR. Cell behaviors associated with somite segmentation and rotation in Xenopus laevis. Developmental Dynamics. 2006:3268–3279. doi: 10.1002/dvdy.20979. [DOI] [PubMed] [Google Scholar]
- Bolce ME, Hemmati-Brivanlou A, Kushner PD, Harland RM. Ventral ectoderm of Xenopus forms nerual tissue, including hindbrain, in response to activin. Development. 1992;115 doi: 10.1242/dev.115.3.681. [DOI] [PubMed] [Google Scholar]
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–278. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JM. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987;100:279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Danilchick M, Peng HB, Kay BK. Xenopus laevis: Practical uses in cell and molecular biology. Pictorial collage of embryonic stages. Methods Cell Biol. 1991;36:679–681. [PubMed] [Google Scholar]
- Dosch R, Gawantka V, Deius H, Blumenstock C, Niehrs C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterining in Xenopus laevis early tailbud embryos. Cells Tissues Organs. 1997:1–12. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Davis LA, Hungerford JE, Little CD. Perturbation of beta 1 integrin-mediated adhesions results in altered somite cell shape and behavior. Dev Biol. 1992;149:327–338. doi: 10.1016/0012-1606(92)90288-r. [DOI] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhard J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Henry CA, Hall LA, Hille MB, Solnica-Krezel L, Cooper MS. Somites in zebrafish doubly mutant for knypek and trilobite form without internal mesenchymal cells or compaction. Curr Biol. 2000;10:1063–1066. doi: 10.1016/s0960-9822(00)00677-1. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Sirour C, Bello V, Moreau N, Beaudry M, Darribère T. In vivo analyzes of dystroglycan function during somitogenesis in Xenopus laevis. Dev Dyn. 2009;238(6):1332–45. doi: 10.1002/dvdy.21814. [DOI] [PubMed] [Google Scholar]
- Iimura T, Denans N, Pourquie O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr Top Dev Biol. 2009:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442 doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- Iimura T, Yang X, Weijer C, Pourquie O. Dual mode of paraxial mesoderm formation during chick gastrulation. Proc Natl Acad Sci USA. 2007;104:2744–2749. doi: 10.1073/pnas.0610997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos TO, Whittaker CA, Meng F, DeSimone DW, Gnau V, Hausen P. Integrin alpha 5 during early development of Xenopus laevis. Mech Dev. 1995;50:187–199. doi: 10.1016/0925-4773(94)00335-k. [DOI] [PubMed] [Google Scholar]
- Keller R, Cooper MS, Danilchick M, Tibbetts P, Wilson P. Cell intercalation during notochord development in Xenopus laevis. J. Exp Zoo. 1989;251:134–154. doi: 10.1002/jez.1402510204. [DOI] [PubMed] [Google Scholar]
- Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-dependent fibronectin accumulation for maintenance fo somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR. Integrin α5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn. 2007;236:2713–2720. doi: 10.1002/dvdy.21280. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Cell dynamics during somite boundary formation revealed by time-lapse analysis. Science. 2002;289:991–995. doi: 10.1126/science.1075544. [DOI] [PubMed] [Google Scholar]
- Lane MC, Sheets MD. Designation of the anterior/posterior axis in pregastrula Xenopus laevis. Dev Biol. 2000;225:37–58. doi: 10.1006/dbio.2000.9803. [DOI] [PubMed] [Google Scholar]
- Lane MC, Sheets MD. Primitive and definitive blood share a common origin in Xenopus: a comparison of lineage techniques used to construct fate maps. Dev Biol. 2002;248:52–67. doi: 10.1006/dbio.2002.0717. [DOI] [PubMed] [Google Scholar]
- Lane MC, Smith WC. The orgins of primite blood in Xenopus: Implications for axial patterning. Development. 1999:423–434. doi: 10.1242/dev.126.3.423. [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion an are required for convergent extension in Xenopus. Curr Biol. 2003:1182–1191. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- McGrew MJ, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Gilhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal table of Xenopus laevis development (Daudin) Garland Publishing Inc.; New York and London: 1994. [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Bio. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yasuda K, Takahashi Y. Morphological boundary forms by a novel inductive event mediated by Lunatic finge and Notch during somitic sementation. Development. 2002;129:3633–3644. doi: 10.1242/dev.129.15.3633. [DOI] [PubMed] [Google Scholar]
- Sato Y, Takahashi Y. A novel signal induces a segmentation fissure by acting in a ventralto-dorsal direction in the presomitic mesoderm. Dev Biol. 2005;282:183–191. doi: 10.1016/j.ydbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Shook DR, Majer C, Keller R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol. 2004;270:163–185. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Gerhart JC. Subcortical rotation in Xenopus eggs: an early step in embryonic axis specification. Dev Biol. 1987;123(2):526–39. doi: 10.1016/0012-1606(87)90411-8. [DOI] [PubMed] [Google Scholar]
- Wacker SA, Jansen HJ, McNulty CL, Houtzager E, Durston AJ. Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev Biol. 2004;268:207–219. doi: 10.1016/j.ydbio.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev. Dyn. 2007;236:2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Gustin J, Domingo C. Muscle specification in the Xenopus laevisgastrulation stage embryo. Dev Dyn. 2005;233:1348–1358. doi: 10.1002/dvdy.20451. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Youn BW, Malancinski GM. Somitogenesis in the amphibian Xenopus laevis: scanning electron microscopic analysis of intrasomitic cellular rearrangements during somite rotation. J Embryol Exp Morphol. 1981;64:23–43. [PubMed] [Google Scholar]
- Zagris N, Christopoulos M, Giakoumaki A. Developmentally regulated expression and functional role of alpha 7 integrin in the chick embryo. Dev Growth Differ. 2004;46:299–307. doi: 10.1111/j.1440-169X.2004.00747.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Grafted embryos were divided into five regions consisting of the head (smaller cranial somites 1–4), anterior trunk (somites 5–9), mid trunk (somites 10–14), posterior trunk (somites 15–20), and tail (somites 21–45). For any given transplantation experiment, which typically consisted of 30 embryos, the specific regions containing labeled cells were noted. The frequency in which cells were found in each specific region was used as the numerator and the denominator consisted of the sum for all regions containing labeled cells within any given experiment. This fraction was then used to calculate the probability that grafted cells would give rise to myotome fibers in a specific region along the anteroposterior axis. The frequency in which labeled cells were observed in each region was calculated rather than the precise number of labeled cells that differentiated into myotome fibers because the labeled cells were often positioned very close to one another making it challenging to discern the exact number of labeled cells (B). The percentages were then used to generate the data for each graph (C).