Abstract
Two hundred fifty pediatric (<18 years of age) patients underwent orthotopic liver transplantation because of end-stage liver disease and were given combination therapy with cyclosporine and prednisone. The most common indications for transplantation In decreasing order of frequency were biliary atresia, inborn errors of metabolism, and postnecrotic cirrhosis. The 5-year actuarial survival for the entire group was 69.2%. Age and diagnosis did not influence survival. Infections were the most common cause of death, followed by liver failure and cerebrovascular accident. The impact of retransplantation on survival depends on the indication. The survival is better when retransplantation is carried out after rejection than because of technical complications, and the latter has a better survival than does primary graft nonfunction. The difference in survival among these groups is statistically significant. The quality of life for 164 of 173 survivors is good to excellent; only nine children are currently experiencing medical problems. A persisent problem in pediatric transplantation is the scarcity of small donors.
The clinical manifestations of chronic liver disease in the adult are usually insidious and slow to progress. In contrast, the clinical features of end-stage liver disease in children develop over a short time and are followed by devastating results. A typical example is the patient with cirrhosis secondary to biliary atresia. In these patients, jaundice, hypersplenism, and portal hypertension quickly develop, in addition to the complications of malabsorption such as diarrhea, growth retardation, and bone fractures.
Three years ago, orthotopic liver transplantation left the realm of experimental surgery and became an accepted mode of therapy for patients with irreversible liver disease.1 The first human liver transplant was performed in a child 23 years ago, but progress was slow until cyclosporine was introduced into clinical practice in 1980.2 The immediate impact of cyclosporine on survival was so remarkable that in a few years there has been a proliferation of new transplant centers all over the world. Moreover, the indications for hepatic replacement are constantly expanding to benefit more patients who otherwise would inevitably die of hepatic failure (unpublished data).
We report the results in the first 250 pediatric patients who received liver transplants during the cyclosporine era.
METHODS
From March 1, 1980, to June 30, 1986, 250 children (of 665 liver recipients) underwent hepatic replacement for advanced liver disease. The charts of these 250 patients were reviewed, and the following data recorded: age; sex, indications for primary transplants, indications for retransplantation, and survival. Causes of death were also reviewed, as well as dominant clinical features of liver disease before transplantation.
The techniques of harvesting and of liver replacement have been published in detail elsewhere.3 Many of the patients had previous abdominal operations, such as portoenterostomies (Kasai procedures) in those with biliary atresia, or exploratory laparotomies for diagnostic purposes. Matching was done mainly by size and blood type, although some patients received organs from donors in different blood groups.4 No attempt was made to match human leukocyte antigen (HLA) loci preoperatively.
Immunosuppression
Patients used to receive a dose of cyclosporine orally a few hours before surgery, but this practice has been abandoned. At present, cyclosporine is begun during the operation at a dose of 2 mg/kg intravenously. The same dose is repeated postoperatively every 8 hours until oral feedings are resumed. Oral cyclosporine is then begun at a dose of 17.5 mg/kg/ d, divided in two doses. Intravenously administered cyclosporine is subsequently reduced if adequate levels are maintained by the oral route.5 Cyclosporine dosage is adjusted according to the concentration of the drug in whole blood as determined by high-pressure liquid chroamtography or by radioimmunoassay. RIA is used more frequently in our practice. The dosage is also adjusted based on symptoms and renal function, because the toxicity of the drug does not always correlate with the blood level. Steroids are also started during the operation. A dose of 500 mg prednisolone is given intravenously soon after the allograft liver is revascularized. If the patient’s clinical condition is relatively good, a 5-day course of prednisolone is given, beginning with 100 mg the first day postoperatively, and tapering by 20 mg daily and ending with a maintenance dose of 10 mg daily. Further reductions of the steroid doses are made on an individual basis. In the presence of rejection, patients are given hydrocortisone intravenously. If no response is observed, the 5-day course of steroids is repeated.5 Additional adjuvant therapy has consisted of polyclonal antilymphocyte globulin in the beginning of the series and monoclonal antibody (OKT*3 Orthoclone, Ortho Pharmaceutical Corporation, Raritan, N.J.) recently. The most common indication for OKT*3 therapy has been steroid-resistant rejection.6 In a few patients with latent rejection, low doses of azathioprine have been added to the cyclosporine-steroid regimen.
Statistical analysis
Patient survival was calculated by the life table method (BMOP statistical software, Los Angeles).
RESULTS
Indications for liver transplantation
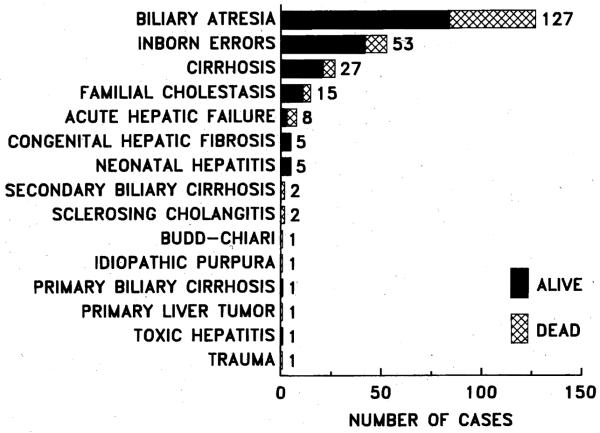
The indications for hepatic replacement are shown in Fig. 1. There were 115 male and 135 female patients, with mean age 5.9 ± 4.92 (SD) years (range 4 months to 18 years). Almost all patients had advanced liver disease, except for an occasional patient who had received a transplant for hepatic malignancy and had liver function that was normal or close to normal.
Fig. 1.
Indications for hepatic transplantation in 250 pediatric patients treated with cyclosporine and prednisone combined.
Biliary atresia
The biliary atretic syndrome was by far the most common indication for liver transplantation. In this group, the majority (84%) of patients had extrahepatic biliary atresia, and the rest had biliary hypoplasia, which in some cases was part of Alagille syndrome.
Almost all of the patients with biliary atresia had undergone a biliary drainage procedure (Kasai), and in several patients the hepatectomy was difficult because of adhesions formed after many previous abdominal operations.
The dominant clinical features in patients with extrahepatic atresia are shown in Table I and are compared with those in other liver transplant recipients who were operated on because of diagnoses other than extrahepatic atresia. The incidence of variceal bleeding and ascites was similar in these two groups of patients; however, cholangitis was a significant problem in patients with extrahepatic biliary atresia and was the cause of numerous hospitalizations. Bone disease was also seen more frequently in patients with extrahepatic biliary atresia. Encephalopathy was observed less often in patients with extrahepatic biliary atresia, because the other group included patients who had received transplants because of acute hepatic failure secondary to viral hepatitis or Wilson disease.
Table I.
Clinical features in 250 pediatric patients before liver transplantation
| Extrahepatic biliary atresia (%) |
Other indications (%) |
|
|---|---|---|
| Ascites | 68 | 75 |
| Bone disease | 82 | 41 |
| Biliary sepsis | 55 | 4 |
| Variceal hemorrhage | 29 | 33 |
| Encephalopathy | 13 | 30 |
| Spontaneous bacterial | 5 | 11 |
| peritonitis |
Inborn errors
Inborn errors of metabolism were the second most common indication for hepatic transplantation; α1-antitrypsin deficiency was the most common diagnosis in this group. Twenty-nine of the 250 pediatric patients underwent hepatic transplantation because of α1-antitrypsin deficiency. The phenotype was ZZ in 22 patients, MZ in two, SZ in two, and was unknown in three patients. The diagnosis of α1-antitrypsin deficiency was made between 1 month and 6 years, with initial symptoms of jaundice in 23 patients, ascites in three patients, and gastrointestinal hemorrhage in three patients. By the time of transplantation, 17 (58.6%) of 29 patients with α1-antitrypsin deficiency had experienced variceal hemorrhage, and the great majority had ascites (Table II). Of the 29 patients, six required retransplantation. The indications were thrombosis of the hepatic artery in four, rejection in one, and thrombosis of the vena cava and portal vein in one patient. Of these six, three with hepatic artery thrombosis died after retransplantation.
Table II.
Dominant clinical features in patients with α1-antitrypsin deficiency before liver transplantation
| n | % | |
|---|---|---|
| Ascites | 23 | 80 |
| Variceal hemorrhage | 17 | 59 |
| Jaundice | 11 | 38 |
| Encephalopathy | 3 | 10 |
Five of the 29 patients with α1-antitrypsin deficiency died. Of the 24 survivors, three patients have slightly abnormal liver function secondary to chronic rejection, and may eventually require retransplantation. Another patient has immunosuppression-induced lymphoproliferative disease. Another patient who had α1-antitrypsin deficiency and biliary atresia has an indwelling percutaneous biliary catheter because of an ischemic obstruction of the bile ducts. This patient had thrombosis of the hepatic artery postoperatively. Twenty of the 24 attend school; the other four are not of school age; but are growing well.
Other metabolic disorders treated with hepatic transplantation were tyrosinemia, Wilson disease, glycogen storage disease, and familial hypercholesterolemia.
Less frequent indications were congenital hepatic fibrosis, familial cholestasis, postnecrotic cirrhosis, secondary biliary cirrhosis, neonatal hepatitis, and malignancy.
Survival
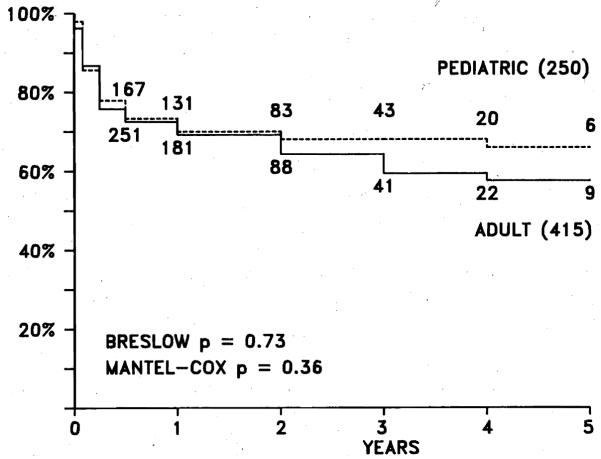
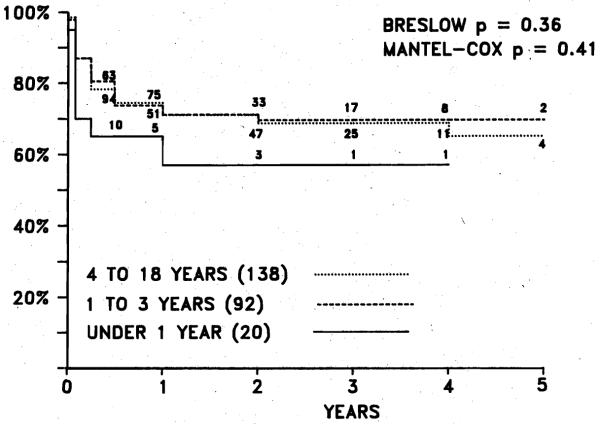
The overall actuarial survival in pediatric patients is 69.2% at 5 years, which is significantly better when compared with a historical group given azathioprine (23%). Compared with that in adults, the survival is identical up to 2 years; the difference in adult survival after 2 years is not statistically significant (Fig. 2). Over the last 2 years there has been an increase in the number of hepatic transplants in infants. The actuarial survival in these small children was not statistically different from that in older patients (Fig. 3); however, the postoperative course in many of these infants was marred by complications.6a
Fig. 2.
Actuarial survival (life table method) in 250 pediatric patients compared with that in 415 adult patients during cyclosporine era. Difference not statistically significant.
Fig. 3.
Actuarial survival according to age grouptn 250 pediatric liver transplant recipients. Note that age has not influenced long-term survival thus far. Difference not statistically significant.
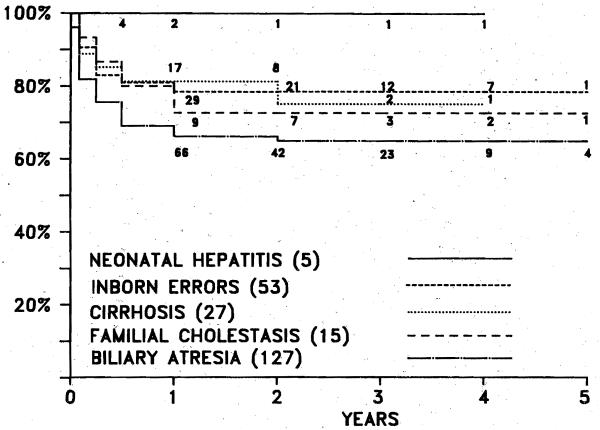
Influence of diagnosis on survival
There is very little difference in survival according to the diagnostic indications for hepatic replacement (Fig. 4). Most of the deaths occur within the first 6 months after transplantation, and the attrition is minimal after 1 year. The 5-year actuarial survival is 77.8% for patients with postnecrotic cirrhosis (n = 27), 79.2% for patients with inborn errors of the metabolism (n = 53), and 66.1% for patients with biliary atretic syndrome (n = 127).
Fig. 4.
Actuarial survival based on diagnostic indications for hepatiC transplantation in 250 pediatric patients. Difference among several groups not statistically significant.
The principal causes of death in 61 consecutive children who died at the University of Pittsburgh, in decreasing order of frequency, were, infection (22 patients, 36%) hepatic necrosis (12 patients, 20%), central nervous system complications (eight patients, 13%), and intraabdominal hemorrhage (seven patients, 11%). Twelve other patients died of miscellaneous causes such as respiratory distress syndrome, myocardial infarction, lymphoproliferative disease, recurrent hepatoma, and multisystem organ failure (Table III). Bacterial infections were seen slightly more often than fungal infections. Deaths caused by viral infections were rare and occurred late. CNS complications consisted of cerebrovascular accident, frequently associated with hypertensive crisis or coagulopathy or both. Fatal intraabdominal hemorrhage was the cause of death in seven patients. Four of these patients had ruptured mycotic aneurysms, and another patient had a fungal infection involving the portal vein. Two other patients had severe coagulopathy secondary to liver failure.
Table III.
Principal causes of death in 61 children who survived at least 24 hours after hepatic replacement
| n | % | |
|---|---|---|
| Infection | 22 | 36 |
| Liver failure | 12 | 20 |
| CNS complications | 8 | 13 |
| Postoperative hemorrhage | 7 | 11 |
| Respiratory distress syndrome | 2 | 3 |
| Myocardial infarction | 2 | 3 |
| Other | 8 | 13 |
Retransplantation
The indications for retraosplantation in 67 (26%) of the 250 patients studied were technical complications in 29 (43.3%) patients, rejection in 29 (43.3%), and primary graft nonfunction in nine (13.4%) (Fig. 5). Technical complications are seen more frequently in children than in adults; hepatic artery thrombosis accounts for nearly all technical problems requiring retransplantation.
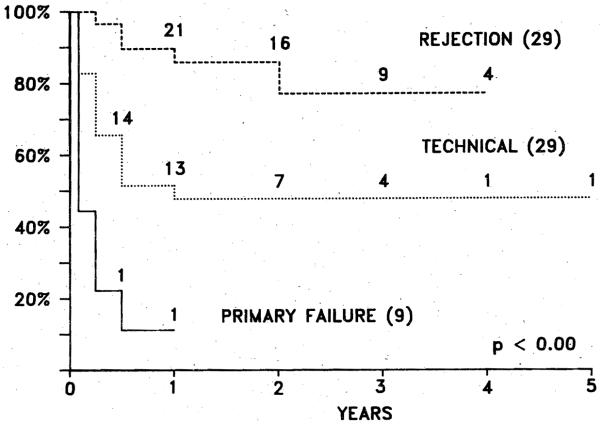
Fig. 5.
Actuarial survival of 67 pediatric patients who lost first graft, based on indication for retransplantation.
The difference in survival after retransplantation for the foregoing three indications is highly significant. Patients requiring a second transplant because of primary graft nonfunction had the poorest survival (approximately 10% at 1 year), but actuarial survival increased to 48% at 4 years when the indication was a technical complication, and to 79% at 4 years when the reason for retransplantation was rejection.
Quality of life
Of the 173 survivors, only nine patients are currently experiencing major medical problems. Two of these patients have biliary strictures secondary to thrombosis of the hepatic artery, and consequent mild hepatic dysfunction. Two other patients, who had transplants before 1 year of age, have indwelling biliary catheters because of necrosis of the major bile ducts after thrombosis of the hepatic artery. Unlike the previous two patients, the hepatic function in these two patients is excellent. Three patients have ongoing chronic rejection and will require retransplantation. One patient who underwent simultaneous liver and kidney transplantation because of cystinosis undergoes hemodialysis for chronic renal failure; he has normal liver function. Last, a 7-year-old child with normal hepatic function has intraabdominal lymphoproliferative disease. This patient recently underwent a debulking procedure and is free of symptoms, although he still has residual tumor; his condition is stable, and immunosuppression therapy has been stopped entirely. The remaining patients live normal lives and have near-normal growth and development. Eight (28%) of 29 patients crossed percentiles in an upward direction and were above the 5th percentile. Thirteen patients were below the 5th percentile before and after surgery; of these, 10 have normal growth velocity. Only four (14%) patients had a reduction in growth to below the 5th percentile. Four (14%) other patients who were normally grown before hepatic replacement continued to grow normally after surgery. Thirteen of these 29 patients had biliary atresia.6b
DISCUSSION
The indications for liver transplantation have expanded since cyclosporine was introduced into clinical practice in 1980. Over a short period of time, a remarkable improvement in survival was observed in patients treated with a combination of cyclosporine and prednisone, in contrast to those given a combination of azathioprine and prednisone in earlier series.2
When liver transplantation was first performed in the 1960s, a frequent indication for hepatic replacement was malignancy, because those patients had no chance for survival otherwise. However, many other patients who would benefit from liver transplantation were not considered because of the experimental nature of the operation. Based on the much improved resuits with cyclosporine, liver transplantation became an accepted therapy for patients with advanced hepatic disease in the 1980s.1, 7 Age limitations are not as relevant as they were before cyclosporine (unpublished data), Nonetheless, a significant problem in pediatric liver transplantation continues to be the scarcity of small pediatric donors.
The appropriate time for transplantation is when the patient begins to show signs of hepatic decompensation. In children, subtle signs are fatigue, failure to grow, and decreased activity level. Liver transplantation should not be delayed until manifestations of end-stage liver disease develop, such as variceal bleeding or encephalopathy. Although it has been difficult to predict which patients will do well after transplantation,8 it is logical to think that patients who still have some reserve would fare better after hepatic transplantation than patients with terminal stages of the disease such as hepatorenal syndrome.
From the viewpoint of statistical significance, the underlying disease does not influence the outcome. Cirrhosis secondary to biliary atresia, hepatitis, α1-antitrypsin deficiency, and Wilson disease is a clear-cut indication for, hepatic replacement. Tyrosinemia, on the other hand, is an inborn error of metabolism associated with a high incidence of hepatoma. These patients should undergo hepatic replacement before malignancy occurs. Likewise, other metabolic disorders that have their origin in the liver, such as glycogen storage disease, hemochromatosis, and possibly hemophilia, are treated more frequently with liver transplantation. The presence of malignancy is not an absolute contraindication, although the risk of recurrence exists. In a previous investigation, patients who had incidental neoplasms in the liver and underwent transplantation for other reasons have shown no evidence of recurrence thus far.9
Most of the deaths in pediatric liver transplantation occur within the first 6 months. Infections are the most common cause of death, and the type of infection varies according to the time of onset after transplantation and the precipitating cause. Early infections are usually bacterial or fungal and are precipitated by complications related to the operation, such as arterial thrombosis, bile leaks, or intestinal perforations, Viral complications are seen late, and usually follow unwise therapy for rejection.
The majority of the children will have an almost normal life except for the need of medication. This is a small price, considering that death would be their fate without liver transplantation. The majority of these children grow normally after hepatic replacement and are able to attend school and participate in daily activities.
Patients with primary graft nonfunction deteriorate rapidly, and retransplantation is urgent. Thrombosis of the hepatic artery may lead to regional necrosis of the liver and abscess formation. Although a few patients have survived without arterial supply, these patients should also undergo retransplantation immediately.10 Retransplantation because of rejection is associated with the best survival, probably because these patients are usually free of infection and are in better condition than those with primary graft nonfunction or thrombosis of the hepatic artery. Retransplantation has played an important role in improving survival.11
We conclude that liver transplantation is the treatment of choice for several conditions leading to end-stage liver disease. The most important problem in pediatric liver transplantation is the unavailability of small pediatric donors.
Acknowledgments
Supported by Research Grants from the Veterans Administration and by Project Grant AM-29961 from the National Institutes of Health.
REFERENCES
- 1.National Institutes of Health Consensus Development Conference Statement Liver transplantation: Leure 20-23, 1983. Hepatology. 1984;4(suppl 1):1075. [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel CO, Todo S, Kam I, Lynch S. Factors in the development of liver transplantation. Transplant Proc. 1985;17:107–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Esquivel CO, et al. Refinements in the surgical technique of liver transplantation. Semin Liver Dis. 1985;5:349–56. doi: 10.1055/s-2008-1040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986;100:342–8. [PubMed] [Google Scholar]
- 5.Starzl TE, Iwatsuki S, Van Thiel DR, et al. Evolution of liver transplantation. Hepatology. 1982;2(5):614–36. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquivel CO, Fung JJ, Markus B, et al. OKT, in the reversal of acute hepatic allograft rejection. Trans Proc. 1987;19:2443–6. [PMC free article] [PubMed] [Google Scholar]
- 6a.Esquivel CO, Koneru B, Karrer F, et al. Liver transplantation under one year of age. J Pediatr. 1987;110:545–8. doi: 10.1016/s0022-3476(87)80545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b.Urbach AH, Gartner JC, Jr, Malatack J, et al. Linear growth following pediatric liver transplantation. Am J Dis Child. 1987;141:547–9. doi: 10.1001/archpedi.1987.04460050089037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel C. Immunosuppression and other non-surgical factors in the improved results of liver transplantation. Semin Liver Dis. 1985;5:334–43. doi: 10.1055/s-2008-1040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw BW, Jr, Wood RP, Gordon RD, Iwatsuki S, Gillquist WP, Starzl TE. Influence of selected patient variables and operative blood loss on six-month survival following liver transplantation. Semin Liver Dis. 1985;5:385–93. doi: 10.1055/s-2008-1040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwatsuki S, Gordon RD, Shaw BW, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–7. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzakis AG, Gordon RD, Shaw BW, Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1986;40:667–71. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw BW, Jr, Gordon RD, Iwatsuki S, Starzl TE. Hepatic transplantation. Transplant Proc. 1985;17:264–71. [PMC free article] [PubMed] [Google Scholar]