Abstract
Background. An increasing incidence of anal cancer among men suggests a need to better understand anal canal human papillomavirus (HPV) infection among human immunodeficiency virus–negative men.
Methods. Genotyping for HPV was conducted on cells from the anal canal among men who have sex with women (MSW) and men who have sex with men (MSM), aged 18–70 years, from Brazil, Mexico, and the United States. Factors associated with anal HPV infection were assessed using multivariable logistic regression.
Results. The prevalence of any HPV type and oncogenic HPV types did not differ by city. Anal canal HPV prevalence was 12.2% among 1305 MSW and 47.2% among 176 MSM. Among MSW, reporting a lifetime number of ≥10 female sex partners, a primary sexual relationship <1 year in duration, and a prior hepatitis B diagnosis were independently associated with detection of any anal HPV in multivariable analysis. Among MSM, a younger age, reporting ≥2 male anal sex partners in the past 3 months, and never using a condom for anal sex in the past 6 months were independently associated with detection of any anal HPV in multivariable analysis.
Conclusions. Number of sex partners was associated with anal HPV infection in both MSW and MSM. Anal HPV infection in men may be mediated by age, duration of sexual relationship, and condom use.
Anal cancer incidence is increasing in Western countries among both women and men [1, 2]. It is a rare cancer in the general population (1.5 cases/100,000 population) [2] but is more common among men who have sex with men (MSM) [3, 4] and persons with human immunodeficiency virus (HIV) infection [5, 6]. Anal cancer incidence among MSM was estimated to be 12.5–36.9 cases per 100,000 men before the HIV epidemic [7].
Among HIV-negative men, there are few studies of the primary cause of anal cancer, HPV infection, and most studies have been conducted among MSM recruited from gay-identified organizations or HIV projects serving gay men [8–11]. Consequently, there are no comparative studies of anal HPV in which MSM and men who have sex with women (MSW) were recruited from the same source population. Also, studies concerning MSM are equivocal with regard to trends in age-specific prevalence. Understanding age-specific prevalence and other factors leading to differential risk for anal HPV infection may help inform strategies for prevention of anal cancer in men. Our objective was to characterize and compare anal HPV prevalence and risk factors in HIV-negative MSW and MSM.
SUBJECTS, MATERIALS, AND METHODS
Study Design and Questionnaire
Men were recruited in São Paulo, Brazil; Cuernavaca, Mexico; and Tampa, Florida, beginning in June 2005 for the longitudinal HPV in Men (HIM) Study. Among inclusion criteria were an age of 18 to 70 years; no prior anal cancer or genital warts; and no current sexually transmitted disease (STD) diagnosis, including HIV regardless of any other STD status. Additional details of the study design have been previously described [12].
Men were recruited in São Paulo from the general population through advertisements and from a genitourinary clinic that also tests for HIV and sexually transmitted diseases (STDs). Men visiting this clinic for STD symptoms or treatment were excluded. In Cuernavaca, men were recruited through a health plan and from factories and the military. Men in Tampa were recruited from a university campus and the general public. MSM were not targeted for recruitment. Each man received a nominal incentive for participation and consented to the study, which was approved by human subjects committees at each study site.
Procedure
From June 2005 through February 2009, 2589 men enrolled in the HIM Study, and 2048 (79.1%) of these consented to collection of anal canal exfoliated cells. The current analysis assessed the samples provided at the enrollment visit. Men who declined anal sampling were more likely to report an age of 18–30 years, being single and never married, a residence in Tampa; and to be MSW (all P < . 001). Men in Tampa who declined anal sampling were also more likely to report 12 years or fewer of education (P < .0001).
Men completed an 88-item computer-assisted self-interview (CASI) written in the region's primary language (Portuguese, Spanish, or English). The CASI elicited information about participant demographic characteristics, substance use, and sexual behaviors. After the CASI, a clinician examined the participant for signs of STDs before using a saline-wetted Dacron swab to collect exfoliated skin cells from the penis (ie, coronal sulcus, glans, and ventral and dorsal areas of shaft) and scrotum. Then, using a separate swab, cells were collected from between the anal verge and the dentate line, after which the swab was placed into its own standard transport medium and stored at –80°C.
HPV Testing
Samples were analyzed for HPV DNA as described elsewhere [12]. In briefDNA was extracted using the QIAamp Media MDx kit (Qiagen). The polymerase chain reaction (PCR) consensus primer system (PGMY 09/11) was used to amplify a fragment of the HPV L1 gene [13]. HPV genotyping was conducted on all samples using DNA probes labeled with biotin to detect 37 HPV types: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51–56, 58, 59, 61, 62, 64, 66–73, 81–84, IS39, and CP6108 [14]. Accuracy and potential contamination were assessed using nontemplate negative controls and CaSki DNA–positive controls.
Of 2048 men agreeing to the anal swab, funding restrictions limited DNA genotyping to the first 1828 men enrolled in the study, including 624 in São Paulo, 679 in Cuernavaca, and 525 in Tampa. Of these, 14.0% were β-globin negative, which left 1572 men for analysis. β-globin status was different by MSW and MSM status and clinic. We observed 85.2% and 92.7% β-globin positivity among MSW and MSM, respectively (P = .005). We also observed 89.6%, 84.4%, and 83.7% β-globin positivity among men recruited in São Paulo, Cuernavaca, and Tampa, respectively (P = .007). There was no difference among other variables assessed (eg, age, race, marital status, presence of prepuce or warts, history of anal sex, and number of sex partners).
Statistical Analyses
We classified participants as MSW, MSM, men who have sex with men and women (MSMW), or men who have no sex solely on the basis of their answers to 17 questions about recent and lifetime penetrative sexual behavior (vaginal, anal, and oral sex). Recent sexual behavior was assessed by questions that asked about behavior in the prior either 3 or 6 months. To minimize categories with sparse data, MSM and MSMW were combined. To classify men as MSW or MSM, greater weight was given to recent sexual behavior and lifetime sexual behavior with multiple partners. For example, if a participant acknowledged any anal or oral sex with other men in the past 6 months or lifetime anal sex with ≥3 men, he was classified as MSM. A participant who acknowledged sex with only women in the past 6 months and <3 lifetime male anal sex partners was classified as MSW. A total of 1305 participants (84.1%) were classified as MSW, 176 (11.3%) as MSM, and 70 (4.5%) as men who have no sex, whereas 21 men provided inadequate information for classification.
We considered a specimen to be positive for HPV if it was positive for any of 37 genotypes. We labeled specimens as oncogenic if any of 13 types were detected (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 66 [15]), regardless of the presence of other genotypes. In contrast, specimens with only single or multiple nononcogenic HPV types and no coinfection with oncogenic types were classified as nononcogenic.
We used the Fisher exact test to assess prevalence differences by city. The Cochran-Armitage test assessed linear trends in prevalence.
To assess concordance of anal canal and genital HPV, we selected men with evaluable samples at both the anal canal and genitals (1262 MSW and 173 MSM). Among these, we determined the proportion with the same HPV genotype at both the anal canal and genitals among men who had an HPV genotype detected at the anal canal. Because of sparse data, proportions were determined only for any HPV, oncogenic, nononcogenic, and the most common genotypes.
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) by bivariate and multivariable logistic regression for 3 outcomes: any HPV type, oncogenic types, and nononcogenic types. We used directed acyclic graphs [16, 17] to identify potential confounders (ie, clinic and number of recent female sex partners) and intermediate variables. All multivariable modeling included potential confounders. Intermediate variables were excluded from multivariable modeling [18, 19]. Modeling initially included variables with a P <.20 on a likelihood-ratio test in bivariate analyses (data not shown). We used a backward-elimination method to identify independent risk factors for anal HPV. Variables with a P >.05 on a likelihood-ratio test were individually removed until a final set of risk factors remained. We analyzed data using SAS, version 9.2 (SAS InstituteC).
RESULTS
The majority of men classified as MSM (68.2%) were recruited in São Paulo. Although MSW and MSM did not differ by age, differences were observed for other characteristics, including race, circumcision status, and sexual behaviors (table 1). Study clinicians diagnosed anogenital warts in 6.3% of MSW and 4.0% of MSM. Of these, perianal warts and warts at the anal verge were diagnosed in <1% of both MSW and MSM.
Table 1.
Selected Characteristics of Men Who Have Sex with Women (MSW) and Men Who Have Sex with Men (MSM) in the Human Papillomavirus (HPV) in Men (HIM) Study, 2005–2009
| No. (%) of Men |
|||
| Variable | MSW (n = 1305) | MSM (n = 176) | Pa |
| Clinic | |||
| São Paulo | 406 (31.1) | 120 (68.2) | < .001 |
| Cuernavaca | 508 (38.9) | 29 (16.5) | |
| Tampa | 391 (30.0) | 27 (15.3) | |
| Age | |||
| 18–30 years | 589 (45.1) | 80 (45.5) | .11 |
| 31–44 years | 518 (39.7) | 79 (44.9) | |
| 45–70 years | 198 (15.2) | 17 (9.7) | |
| Raceb | |||
| White | 534 (41.5) | 103 (58.5) | < .001 |
| Black | 181 (14.1) | 36 (20.5) | |
| Mixed/other | 573 (44.5) | 37 (21.0) | |
| Ethnicityb | |||
| Hispanic | 642 (49.7) | 71 (41.5) | .05 |
| Non–Hispanic | 651 (50.4) | 100 (58.5) | |
| Marital statusb | |||
| Single, never married | 501 (38.5) | 112 (64.0) | < .001 |
| Married | 522 (40.2) | 32 (18.3) | |
| Cohabitating | 161 (12.4) | 17 (9.7) | |
| Divorced/separated/widowed | 116 (8.9) | 14 (8.0) | |
| Prepuce present (clinician record) | |||
| Yes | 829 (63.5) | 132 (75.0) | .01 |
| Partially | 38 (2.9) | 2 (1.1) | |
| No | 438 (33.6) | 42 (23.9) | |
| Ever STD diagnosisb | |||
| Yes | 191 (15.0) | 56 (33.9) | < .001 |
| No | 1083 (85.0) | 109 (66.1) | |
| Ever hepatitis B diagnosisb | |||
| Yes | 19 (1.5) | 10 (6.0) | .001 |
| No | 1240 (98.5) | 156 (94.0) | |
| Anogenital warts diagnosed by study clinician | |||
| Yes | 82 (6.3) | 7 (4.0) | .07 |
| No | 1223 (93.7) | 169 (96.0) | |
| Ever had oral or anal sex with a manb | |||
| Yes | 141 (11.0) | 176 (100.0) | < .001 |
| No | 1142 (89.0) | 0 (.0) | |
| Lifetime no. of female sex partnersb | |||
| 0-2 | 213 (17.3) | 85 (52.8) | < .001 |
| 3-9 | 489 (40.4) | 38 (23.6) | |
| ≥10 | 522 (42.3) | 38 (23.6) | |
| Lifetime no. of male anal sex partnersb | |||
| 0–2 | 1293 (100.0) | 28 (17.3) | < .001 |
| 3–9 | 0 (.0) | 68 (42.0) | |
| ≥10 | 0 (.0) | 66 (40.7) | |
| No. of recent female sex partners b, c | |||
| 0 women | 346 (27.4) | 119 (70.8) | < .001 |
| 1 woman | 603 (47.7) | 18 (10.7) | |
| ≥2 women | 315 (24.9) | 31 (18.5) | |
| No. of recent male anal sex partnersb, d | |||
| 0 men | 1293 (100.0) | 61 (36.5) | < .001 |
| 1 man | 0 (.0) | 40 (24.0) | |
| ≥2 men | 0 (.0) | 66 (39.5) | |
| Length of relationship with primary sex partner | |||
| No primary relationshipb | 229 (19.0) | 70 (41.2) | < .001 |
| <1 year | 265 (22.0) | 37 (21.8) | |
| 1–4 years | 189 (15.7) | 23 (13.5) | |
| 5–10 years | 233 (19.3) | 21 (12.4) | |
| >10 years | 291 (24.1) | 19 (11.2) | |
| Frequency of condom use for recent anal sex with women or menb | |||
| Always | 101 (8.3) | 60 (35.1) | < .001 |
| Sometimes | 87 (7.2) | 57 (33.3) | |
| Never | 160 (13.2) | 24 (14.0) | |
| No recent anal sex | 869 (71.4) | 30 (17.5) | |
| Smoking statusb | |||
| Never smoker | 725 (55.6) | 105 (59.7) | .25 |
| Former smoker | 270 (20.7) | 27 (15.3) | |
| Current smoker | 310 (23.8) | 44 (25.0) | |
NOTE. a P values are 2-sided and derived from the Fisher exact test.
Because some observations were missing, category entries do not sum to 100%.
Recent was defined as up to 6 months immediately preceeding the study visit.
Recent was defined as up to 3 months immediately preceding the study visit.
HPV Infection Prevalence
Prevalence of infection due to any HPV type and oncogenic types did not differ by city among MSW (P = .11 for each). Likewise, prevalence of infection due to any HPV type and oncogenic types did not differ significantly by city among MSM (P = .66 and P = .97, respectively).
Approximately 16% of all men had HPV detected at the anal canal; however, anal HPV infection prevalence among MSM was 4–10 times higher than the prevalence among MSW for all HPV groups except unclassified types (table 2). The proportion of MSM and MSW with multiple HPV types was especially disparate (33.0% and 3.2%, respectively). HPV-16 prevalence among MSM was more than double that among MSW (6.3% and 2.2%, respectively).
Table 2.
Prevalence of Anal Canal Human Papillomavirus (HPV) Infection among Men Who Have Sex with Women (MSW) and Men Who Have Sex with Men (MSM) in the HPV in Men (HIM) Study, 2005–2009
| No. (%) (95% CI) |
|||
| Variable | MSW (n=1305) | MSM (n=176) | Total (n=1481) |
| Any HPV | 159 (12.2) (10.5–14.1) | 83 47.2 (39.6–54.8) | 242 (16.3) (14.5–18.3) |
| Oncogenica | 89 (6.8) (5.5–8.3) | 48 (27.3) (20.7–33.9) | 137 (9.3) (7.8–10.8) |
| Nononcogenicb | 70 (5.4) (4.2–6.7) | 35 (19.9) (14.3–26.6) | 105 (7.1) (5.8–8.5) |
| Only unclassifiedc | 162 (12.4) (10.7–14.3) | 21 (11.9) (7.5–17.7) | 183 (12.4) (10.7–14.1) |
| Multiple types | 42 (3.2) (2.3–4.3) | 58 (33.0) (26.1–40.4) | 100 (6.8) (5.5–8.2) |
| Types 6/11 | 23 (1.8) (1.1–2.6) | 17 (9.7) (5.7–15.0) | 40 (2.7) (1.9–3.7) |
| Types 16/18 | 32 (2.5) (1.7–3.4) | 19 (10.8) (6.6–16.3) | 51 (3.4) (2.6–4.5) |
| Types 6/11/16/18 | 52 (4.0) (3.0–5.2) | 34 (19.3) (13.8–25.9) | 86 (5.8) (4.7–7.1) |
| Oncogenic typesd | |||
| 16 | 29 (2.2) (1.5–3.2) | 11 (6.3) (3.2–10.9) | 40 (2.7) (1.9–3.7) |
| 18 | 3 (.2) (.1–.7) | 8 (4.6) (2.0–8.8) | 11 (.7) (.4–1.3) |
| 39 | 5 (.4) (.1–.9) | 8 (4.6) (2.0–8.8) | 13 (.9) (.5–1.5) |
| 51 | 19 (1.5) (.9–2.3) | 8 (4.6) (2.0–8.8) | 27 (1.8) (1.2–2.6) |
| Nononcogenic typese | |||
| 6 | 21 (1.6) (1.0–2.5) | 16 (9.1) (5.3–14.3) | 37 (2.5) (1.8–3.4) |
| 11 | 2 (.2) (.0–.6) | 1 (.6) (.0–3.1) | 3 (.2) (.0–.6) |
| 53 | 10 (.8) (.4–1.4) | 13 7.4 (4.0–12.3) | 23 (1.6) (1.0–2.3) |
| 61 | 11 (.8) (.4–1.5) | 9 5.1 (2.4–9.5) | 20 (1.4) (.8–2.1) |
| 73 | 2 (.2) (.0–.6) | 9 5.1 (2.4–9.5) | 11 (.7) (.4–1.3) |
| 84 | 11 (.8) (.4–1.5) | 16 9.1 (5.3–14.3) | 27 (1.8) (1.2–2.6) |
NOTE. a Proportion of men with at least 1 of 13 oncogenic types, regardless of the presence of any other HPV type.
Proportion of men with only nononcogenic types.
Proportion of men positive for HPV DNA by polymerase chain reaction (PCR) but negative for any 1 of 37 types.
Oncogenic types 31, 33, 35, 45, 52, 56, 58, 59 and 66 are not shown; each was detected at the anal canal, but the prevalence was ≤4.0% in MSW and MSM.
Nononcogenic types 26, 40, 42, 54, 55, 62, 67, 68, 69, 70, 71, 72, 81, 82, 83, CP6108, and IS39 are not shown; each was detected at the anal canal, but the prevalence was ≤4.0% in MSW and MSM. Type 64 was not detected.
The most common HPV types in MSW were 16, 6, and 51. In MSM, the most common types were (tie) 6 and 84, 53, and 16. A total of 34 and 36 genotypes were detected in MSW and MSM, respectively.
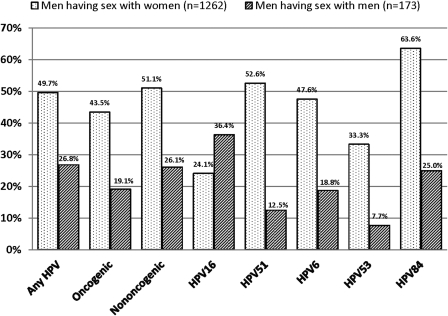
With the exception of HPV-16, a higher proportion of MSW than MSM had type-specific infection at the genitals that mirrored a type detected at the anal canal (Figure 1). For example, among 21 MSW with HPV-6 at the anal canal, 47.6% also had HPV-6 infection at the genitals.
Figure 1.
Proportion of men with type-specific infection at both the anal canal and genitals among men with HPV infection at the anal canal, in the Human Papillomavirus (HPV) in Men (HIM) Study, 2005–2009. Only men with evaluable samples at both the anal canal and genitals were included in analysis; therefore, sample size differs slightly from other tables and figures.
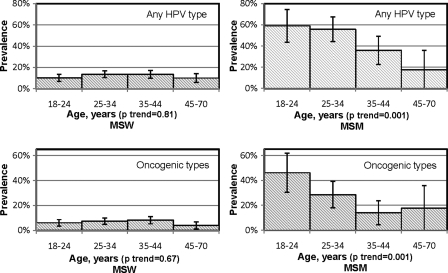
Among MSW, anal HPV infection prevalence was similar across age groups for any HPV and oncogenic types (P for trend = .81 and .67, respectively) (Figure 2). Likewise, age was not associated with any HPV or oncogenic HPV among MSW in bivariate analysis (data not shown).
Figure 2.
Prevalence of anal human papillomavirus (HPV) infection, by age, among 1305 men who have sex with women (MSW) and 176 men who have sex with men (MSM) in the HPV in Men (HIM) Study.
Among MSM, there was a decrease in prevalence with increasing age for both any HPV and oncogenic types (P for trend=.001 for both). Likewise, age was associated with any HPV and oncogenic HPV at the anal canal among MSM in bivariate analysis (for example, in comparison to MSM aged 18–30 years: OR = 40 [95% CI .21–.76] for MSM aged 31–44 years, and OR = 13 [95% CI .04–.51] for MSM aged 45–70 years).
Multivariable Analysis
Multivariable analyses associated 3 factors with any HPV type in MSW (table 3): lifetime number of female sex partners (in comparison to 0–2 women: OR = 2.85 [95% CI 1.44–5.67] for ≥10 women), duration of relationship with a primary sex partner (in comparison to >10 years: OR = 2.00 [95% CI 1.05–3.80] for having a primary relationship of <1 year), and a history of a hepatitis B diagnosis (OR = 4.64 [95% CI 1.60–13.46]). Among MSW, the same 3 variables were also associated with detection of any oncogenic type, in addition to smoking status (in comparison to never smokers: OR = .43 [95% CI .19-.97] for former smokers). No factors were associated with detection of nononcogenic types.
Table 3.
Factors Associated with Anal Canal Human Papillomavirus (HPV) Infection among Men Who Have Sex with Women (MSW) and Men Who Have Sex with Men (MSM) in the HPV in Men (HIM) Study, 2005–2009: multivariable analyses
| MSW: OR (95% CI) |
MSM: OR (95% CI) |
|||||
| Factor | Any HPVa | Oncogenicb | Nononcogenicc | Any HPVd | Oncogenice | Nononcogenice |
| Age, years | ||||||
| 18–30 | Reference | Reference | Reference | Reference | Reference | Reference |
| 31–44 | 1.03 (.66–1.60) | .96 (.54–1.69) | 1.21 (.64–2.30) | .51 (.23–1.09) | .35 (.16–.79) | 1.34 (.59–3.04) |
| 45–70 | .81 (.41–1.62) | .74 (.28–1.99) | 1.15 (.45–2.94) | .20 (.04–.94) | .52 (.12–2.19) | -f |
| Lifetime no. of female sex partners | ||||||
| 0–2 women | Reference | Reference | Reference | Reference | Reference | Reference |
| 3–9 women | 1.69 (.87–2.30) | 2.21 (.89–5.51) | 1.13 (.47–2.74) | 1.40 (.58–3.41) | 1.50 (.62–3.65) | .91 (.33–2.51) |
| ≥10 women | 2.85 (1.44–5.67) | 3.18 (1.24–8.20) | 2.26 (.94–5.44) | 1.00 (.28–3.52) | .63 (.14–2.79) | 1.73 (.33–8.93) |
| Lifetime no. of male anal sex partners | ||||||
| 0–2 men | n/a | n/a | n/a | Reference | Reference | Reference |
| 3–9 men | n/a | n/a | n/a | 3.73 (1.00–13.97) | 3.21 (.81–12.66) | 5.70 (.67–48.84) |
| ≥10 men | n/a | n/a | n/a | 2.39 (.57–10.07) | 3.78 (.92–15.48) | 4.83 (.57–40.81) |
| No. of recent male anal sex partners | ||||||
| 0 men | n/a | n/a | n/a | Reference | Reference | Reference |
| 1 man | n/a | n/a | n/a | 1.78 (.48–6.56) | 1.20 (.41–3.52) | 5.94 (1.53–23.10) |
| ≥2 men | n/a | n/a | n/a | 4.99 (1.46–16.97) | 3.19 (1.24–8.25) | 3.68 (1.08–12.51) |
| Length of relationship with primary sex partner | ||||||
| No primary relationship | 1.97 (1.00–3.88) | 2.94 (1.18–7.32) | 1.45 (.58–3.64) | .90 (.23–3.46) | .90 (.20–4.14) | 1.00 (.16–6.22) |
| <1 year | 2.00 (1.05–3.80) | 2.28 (.95–5.48) | 1.80 (.77–4.20) | 1.55 (.37–6.40) | 1.64 (.34–7.92) | 1.03 (.15–6.96) |
| 1–4 years | 1.39 (.69–2.78) | 1.41 (.53–3.76) | 1.70 (.70–4.17) | 4.35 (.87–21.72) | 1.93 (.37–10.13) | 2.33 (.34–15.81) |
| 5–10 years | 1.60 (.85–3.00) | 1.91 (.80–4.56) | 1.30 (.55–3.04) | .63 (.12–3.25) | .87 (.15–5.24) | .41 (.03–5.82) |
| >10 years | Reference | Reference | Reference | Reference | Reference | Reference |
| Frequency of condom use for recent anal sex with women or men | ||||||
| Always | Reference | Reference | Reference | Reference | Reference | Reference |
| Sometimes | 1.41 (.60–3.31) | 1.05 (.37–2.96) | 2.20 (.53–9.14) | 2.14 (.82–5.58) | 1.83 (.77–4.35) | 1.13 (.42–3.04) |
| Never | .83 (.36–1.92) | .50 (.17–1.45) | 1.78 (.45–6.97) | 6.07 (1.47–24.97) | 1.81 (.58–5.68) | 1.95 (.51–7.50) |
| No recent anal sex | 1.15 (.58–2.28) | 0.87 (.39–1.93) | 1.85 (.55–6.26) | .76 (.16–3.62) | .36 (.09–1.45) | .29 (.06–1.52) |
| Ever hepatitis B diagnosis | ||||||
| Yes | 4.64 (1.60–13.46) | 5.39 (1.58–18.40) | 2.03 (.45–9.13) | 1.25 (.28–5.59) | .27 (.03–2.35) | 3.43 (.70–16.76) |
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Smoking status | ||||||
| Never | Reference | Reference | Reference | Reference | Reference | Reference |
| Former | .78 (.48–1.27) | .43 (.19–.97) | 1.13 (.59–2.16) | .60 (.22–1.67) | .81 (.27–2.38) | .48 (.10–2.38) |
| Current | 1.34 (.90–2.00) | 1.05 (.60–1.85) | 1.34 (.75–2.40) | 1.19 (.53–2.65) | .77 (.33–1.84) | 1.66 (.67–4.09) |
NOTE. Any HPV cases are positive for at least 1 of 37 HPV types. Oncogenic type cases are positive for at least 1 of 13 oncogenic HPV types, regardless of the presence of nononcogenic types. Nononcogenic cases are positive for at least 1 of 24 nononcogenic types and have no coinfection with oncogenic types. Each model is adjusted by confounders, which are clinic and number of recent female sex partners. CI, confidence interval; OR, odds ratio; n/a, not applicable.
Variables remaining in the model were lifetime number of female sex partners, length of relationship with primary sex partner, and any hepatitis B diagnosis. Their ORs were adjusted by confounders and variables remaining in the model. Smoking status and frequency of condom use for recent anal sex are included only for comparison purposes and are adjusted only by confounders.
Variables remaining in the model were lifetime number of female sex partners, length of relationship with primary sex partner, ever hepatitis B diagnosis, and smoking status. Their ORs are adjusted by confounders and variables remaining in the model. Frequency of condom use for recent anal sex is included only for comparison purposes and is adjusted only by confounders.
No variables were significant in the final model. Each estimate is included only for comparison purposes and is adjusted only by confounders.
Variables remaining in the model were number of recent male anal sex partners and frequency of condom use for recent anal sex. Their ORs are adjusted by confounders and variables remaining in the model. Other variables are included only for comparison purposes and are adjusted only by confounders.
Variable remaining in the model was number of recent male anal sex partners. Its ORs are adjusted by confounders. Other variables are included only for comparison purposes and are adjusted only by confounders.
Sparse data for the nononcogenic outcome resulted in an unstable estimate.
In multivariable analysis with MSM, age was inversely associated with any HPV type (in comparison to 18–30 years of age: OR = .20 [95% CI .04–.94] for ages 45–70 years) and oncogenic types (in comparison to 18–30 years of age: OR = .35 [95% CI .16–.79] for 31–44 year olds). Also, a larger number of recent male anal sex partners (in comparison to 0 men: OR, 4.99 [95% CI 1.46–16.97] for ≥2 men) and never using condoms for recent anal sex (in comparison to always using condoms: OR = 6.07 [95% CI 1.47–24.97]) were independently associated with detection of any HPV (table 3). A larger number of recent male anal sex partners was also associated with detection of oncogenic (in comparison to 0 men: OR = 3.19 [95% CI 1.24–8.25] for ≥2 men) and nononcogenic types (in comparison to 0 men: OR = 5.94 [95% CI 1.53–23.10] for 1 man and OR = 3.68 [95% CI 1.08–12.51] for ≥2 men).
Among a subset of MSM who were asked about receptive anal sex (n = 63), any HPV prevalence was 3 times higher among MSM who acknowledged receptive anal sex than among MSM who denied receptive anal sex (P = .005) (data not shown).
DISCUSSION
Our observation of high prevalence of anal HPV infection among 176 HIV-negative MSM from Latin America and the United States is consistent with past estimates for HIV-negative MSM in Western Europe, Australia, and the United States [8–11]. For all HPV outcomes, prevalence among MSM was at least twice as high as among MSW who were recruited from the same source population.
We are aware of 4 studies of anal HPV infection among HIV-negative MSM that have used genotyping assays that detected a comparable number of HPV types as in the current study [8–11]. Estimates from these studies for oncogenic HPV prevalence were 26%–73%, whereas HPV-16 prevalence was 9%–27%. Our prevalence estimates in MSM are somewhat lower (27.3% for oncogenic and 6.3% for HPV-16). The MSM in this study were not recruited from gay-identified organizations or events or from HIV-prevention projects, as in other studies in which MSM with increased risk for STDs may have been enrolled; however, one-third of all men enrolled in São Paulo where recruited at an STD clinic that tests for STDs and HIV. It is also possible that different anal sample collection methods, such as different sampling devices, could account for the varying prevalence estimates among studies.
Our estimates among MSW are consistent with our prior research [20, 21]. However, the estimates are somewhat higher than in 2 other studies [22, 23], possibly because of the current study's genotyping assays that detect a larger number of genotypes.
Because anal HPV infection is often observed in men who deny receptive anal sex [24, 25], the mechanism of transport to the anal canal is unclear. Multiple studies have detected mucosal/genital HPV genotypes on fingers [26, 27], and 1 investigation offered evidence of HPV transmission between hands and anogenital sites [28]. In the current study, almost 50% of MSW with any anal HPV genotype had dual type-specific infection, at both the anal canal and the genitals, a pattern that also occurred with all common genotypes except HPV-16. We did not ask questions regarding nonpenetrative sexual behaviors, which may have been helpful in understanding anal HPV infection in MSW.
We observed a stable age-specific prevalence of anal HPV in MSW aged 18–70 years, reflecting our previous results in a smaller group of MSW from the same cohort [29] but in contrast to our prior study of 222 MSW aged 18–40 years [20].
In contrast, the current study observed a decreasing age-specific prevalence among MSM, which has been reported elsewhere [30]; however, these results differ from 2 studies of anal HPV infection in HIV-negative MSM that reported a similar prevalence of anal HPV infection across age groups [8, 9]. Different source populations may explain the different estimates. For example, Chin-Hong's large study among urban MSM in the United States selected men on the basis of sexual behavior that put them at high risk for HIV infection [9], whereas MSM in the current study were not targeted for recruitment and, therefore, may have a different pattern of risk for anal HPV infection throughout their lifetimes.
These age-specific HPV infection prevalence trends may result from age-specific sexual behavior. For example, MSM aged 45–70 years reported a mean of .9 new anal sex partners in the prior 3 months, whereas MSM aged <45 years reported 2.7 new partners. Conversely, MSW <45 years of age and >45 years of age reported the same mean of .5 new partners. In addition, when MSM were stratified by number of male anal sex partners (ie, 0 vs. 1 vs. ≥2 partners) in the prior 3 months and by age group, the prevalence of any HPV type at the anal canal decreased with age in every category of number of male anal sex partners (data not shown).
In addition to sexual behavior, immunological processes may mediate age-specific anal HPV infection prevalence in men. Multiple studies have reported increasing age-specific antibody prevalence against HPV type 6, 11, 16, and/or 18 among men [31–33] and a positive association between sex with men and HPV seroprevalence in men [32, 34, 35]. Nevertheless, even if immune responses control some anal infections in older MSM, higher initial prevalence may still leave a substantial burden of infection in older age.
In addition to age, condom use for anal sex and number of sexual partners were associated with anal HPV in MSM. Compared with MSM who reported always using condoms for anal sex, MSM who never used condoms were 6 times more likely to harbor anal canal HPV. In contrast, a previous study reported that risk for anal HPV increased among women who reported condoms being used by male partners; however, that investigation did not ask if condoms were actually used during anal sex [36]. Our finding that, among MSM, the number of partners was associated with anal HPV is consistent with prior reports [8, 9].
Among MSW, number of lifetime sex partners was also associated with anal HPV, which is consistent with our prior 2 reports [20, 29]. Among MSW we also observed an inverse association between length of sexual relationship and anal HPV. While bivariate analyses among MSM also indicated that longer relationships were protective (data not shown), the association did not remain significant in multivariable analyses. Finally, a history of hepatitis B was strongly associated with both any HPV and oncogenic HPV in MSW. We are not aware of other such reports, although hepatitis has been associated with anal cancer [37].
Because we used self–reported data and limited our definition of recent sex to behavior within the prior 3 or 6 months, it is possible that some men were misclassified as either MSW or MSM; however, we have found the CASI instrument to be highly reliable with men in these 3 cities [38]. Our definition of MSW required that a man had shown a pattern of sexual behavior with women, had reported no recent anal sex with a male, and additionally had reported ≤ 2 male anal partners in his lifetime. We classified a man as MSW even if he acknowledged 1 or 2 male sex partners in his lifetime because sexual experimentation, especially during adolescence, is common in these 3 countries [39–41]. To test our definition, we assessed the prevalence of groups of HPV and individual types in MSW who acknowledged no sex with men in their lifetimes. There was no difference in anal HPV prevalence between MSW with 0 lifetime male anal sex partners and MSW with 1 or 2 lifetime male partners (data not shown).
Although all men reported being HIV-negative, we did not test for HIV; therefore, we cannot be certain that all were HIV-negative.
The number of HIV-negative MSM in this study was limited to 176 largely urban men, which, although comparable in size to a number of anal HPV studies in MSM [8, 10, 11], limits generalization. It possibly also limited our ability to identify statistically significant associations in multivariable analyses. However, a strength of the current study is that both MSM and MSW were recruited from the same source populations which provides a more valid context for comparisons. Finally, although clinicians were trained to deliver the swab directly into the anal canal, it is possible that the swab may have sometimes touched the perianal skin before entry into the anal canal; thus, our estimates may include HPV detected at the perianal region.
Why MSM would have higher β-globin status than MSW is unclear. One idea is that the practice of receptive anal sex might abrade the mucosal epithelium of the anal canal, allowing better collection of exfoliated cells. However, a subset of MSM were asked about receptive anal sex practices (n = 83). Of these there was no difference in β-globin status among men acknowledging lifetime receptive anal intercourse (β-globin positivity: 84.5%) and those who said they never had receptive anal intercourse (92.0%, P = .35). With regard to different β-globin status by clinic site, it is possible that clinicians collecting samples in São Paulo used more pressure on the Dacron swab as it swabbed the anal canal than when the procedure was performed in Cuernavaca or Tampa. While clinicians were all highly trained by an infectious disease physician in this procedure, differential pressure on the Dacron swab by city would be difficult to detect. Nevertheless, since there was little difference in the prevalence of anal HPV by city, we believe different β-globin results by city should not significantly impact our results.
There were differences in the proportion of men at each clinic site who agreed to an anal sample. For example, more MSW rejected the anal sampling and therefore were excluded the study. If these men also have less risk for anal HPV, then our MSW sample may have been biased toward a population with higher anal HPV prevalence.
Our observation of a higher prevalence of anal HPV in MSM may be due to higher incidence of infection, longer duration, or a combination of these. Further studies are needed to estimate these parameters so that we may better understand how the natural history of anal HPV infection has led to a much higher incidence of anal cancer in MSM.
We believe the current study provides important data for practitioners seeking to lower risk for anal HPV infection in their patients at a time when anal cancer incidence is increasing. Specifically, our data suggest a lower risk for anal HPV infection in MSM who limit their number of male anal sex partners and who always use condoms for anal sex. Secondly, MSW who restrict their number of female sex partners and who remain in relationships for more than 10 years also may lower their risk for anal HPV infection.
Funding
This work was supported by National Cancer Institute, National Institutes of Health 1R03CA134204-01 and 3R03CA134204-02S1 [AGN] and NIH RO1 CA098803 01–A1 [ARG].
Acknowledgments
We thank the men who provided personal information and biological samples for the study; the HIM Study Team in São Paulo, Cuernavaca, and Tampa, including Lenice Galan, Elisa Brito, Filomena Cernicchiaro, Rubens Matsuo, Vera Souza, Ricardo Cunha, Birgit Fietzek, Raquel Hessel, Viviane Relvas, Fernanda Silva, Juliana Antunes, Graças Ribeiro, Roberta Bocalon, Rosária Otero, Rossana Terreri, Sandra Araujo, Meire Ishibashi, the CRT– DST/AIDS Nursing team, Jorge Salmeron, Aurelio Cruz, Pilar Hernandez, Carlos Hernandez, Griselda Diaz Garcia, Oscar Rojas Juarez, Manuel Quiterio Trenado, Alejandrina Alvarez Martinez, Isabel Conde Cruz, Christine Gage, Kathy Eyring, Nadia Lambermont, Emily Jolles, Kayoko Kay, Kim Isaacs, Andrea Leto, Kyle Wolf, Anthony Bilotto, Abidemi Ajidahun, Michael Blackmer, Michael O'Keefe, Bradley Sirak, and Ray Viscidi; and Digene, for donations of supplies.
References
- 1.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101:281–8. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 2.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113:2892–900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cress RD, Holly EA. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973-1999. Prev Med. 2003;36:555–60. doi: 10.1016/s0091-7435(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 4.Frisch M, Smith E, Grulich A, Johansen C. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol. 2003;157:966–72. doi: 10.1093/aje/kwg067. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond C, Taylor TH, Aboumrad T, Bringman D, Anton-Culver H. Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis. 2005;32:314–20. doi: 10.1097/01.olq.0000162366.60245.02. [DOI] [PubMed] [Google Scholar]
- 7.Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247:1988–90. [PubMed] [Google Scholar]
- 8.Vajdic CM, van Leeuwen MT, Jin F, et al. Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sex Transm Infect. 2009;85:330–5. doi: 10.1136/sti.2008.034744. [DOI] [PubMed] [Google Scholar]
- 9.Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. J Infect Dis. 2004;190:2070–6. doi: 10.1086/425906. [DOI] [PubMed] [Google Scholar]
- 10.van der Snoek EM, Niesters HG, Mulder PG, van Doornum GJ, Osterhaus AD, van der Meijden WI. Human papillomavirus infection in men who have sex with men participating in a Dutch gay-cohort study. Sex Transm Dis. 2003;30:639–44. doi: 10.1097/01.OLQ.0000079520.04451.59. [DOI] [PubMed] [Google Scholar]
- 11.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177:361–7. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomark Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Infdisogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 16.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 18.Rothman KJ, Greenland S, editors. Modern epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 19.Greenland S, Neutra R. Control of confounding in the assessment of medical technology. Int J Epidemiol. 1980;9:361–7. doi: 10.1093/ije/9.4.361. [DOI] [PubMed] [Google Scholar]
- 20.Nyitray A, Nielson CM, Harris RB, et al. Prevalence of and risk factors for anal human papillomavirus infection in heterosexual men. J Infect Dis. 2008;197:1676–84. doi: 10.1086/588145. [DOI] [PubMed] [Google Scholar]
- 21.Nyitray AG, Smith D, Villa L, et al. Prevalence of and risk factors for anal human papillomavirus infection in men who have sex with women: a cross-national study. J Infect Dis. 2010;201:1498–508. doi: 10.1086/652187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolau SM, Camargo CG, Stávale JN, et al. Human papillomavirus DNA detection in male sexual partners of women with genital human papillomavirus infection. Urology. 2005;65:251–5. doi: 10.1016/j.urology.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Van Doornum GJ, Prins M, Juffermans LH, et al. Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genitourin Med. 1994;70:240–6. doi: 10.1136/sti.70.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piketty C, Darragh TM, Da Costa M, et al. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003;138:453–9. doi: 10.7326/0003-4819-138-6-200303180-00008. [DOI] [PubMed] [Google Scholar]
- 25.Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004;190:1685–91. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 26.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–36. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 27.Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect. 1999;75:317–9. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyitray AG, Smith D, Villa L, et al. Prevalence of and risk factors for anal human papillomavirus in men having sex with women: a cross-national study. J Infect Dis. 2010;201 doi: 10.1086/652187. 1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breese PL, Judson FN, Penley KA, Douglas JM., Jr. Anal human papillomavirus infection among homosexual and bisexual men: prevalence of type-specific infection and association with human immunodeficiency virus. Sex Transm Dis. 1995;22:7–14. doi: 10.1097/00007435-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: national health and nutrition examination survey 2003-2004. J Infect Dis. 2009;200:1059–67. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 32.Lu B, Hagensee ME, Lee JH, et al. Epidemiologic factors associated with seropositivity to human papillomavirus type 16 and 18 virus-like particles and risk of subsequent infection in men. Cancer Epidemiol Biomarkers Prev. 2010;19:511–6. doi: 10.1158/1055-9965.EPI-09-0790. [DOI] [PubMed] [Google Scholar]
- 33.Hagensee ME, Kiviat N, Critchlow CW, et al. Seroprevalence of human papillomavirus types 6 and 16 capsid antibodies in homosexual men. J Infect Dis. 1997;176:625–31. doi: 10.1086/514082. [DOI] [PubMed] [Google Scholar]
- 34.Stone KM, Karem KL, Sternberg MR, et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186:1396–402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- 35.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, -18, and -33 seroprevalence. Sex Transm Dis. 2004;31:247–56. doi: 10.1097/01.olq.0000118425.49522.2c. [DOI] [PubMed] [Google Scholar]
- 36.Goodman MT, Shvetsov YB, McDuffie K, et al. Acquisition of anal human papillomavirus (HPV) infection in women: the Hawaii HPV cohort study. J Infect Dis. 2008;197:957–66. doi: 10.1086/529207. [DOI] [PubMed] [Google Scholar]
- 37.Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–8. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 38.Nyitray AG, Kim J, Hsu CH, et al. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in men (HIM) study. Am J Epidemiol. 2009;170:965–74. doi: 10.1093/aje/kwp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cáceres C, Konda K, Pecheny M, Chatterjee A, Lyerla R. Estimating the number of men who have sex with men in low and middle income countries. Sex Trans Infect. 2006;82:iii3–9. doi: 10.1136/sti.2005.019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidman SN, Rieder RO. A review of sexual behavior in the United States. Am J Psychiatry. 1994;151:330–41. doi: 10.1176/ajp.151.3.330. [DOI] [PubMed] [Google Scholar]
- 41.Carrier J. Mexican male bisexuality. In: Klein F, Wolf T, editors. Bisexualities: Theory and research. New York, NY: Haworth Press; 1985. pp. 75–85. [Google Scholar]