Abstract
Background. In 2008, Guinea-Bissau introduced artemether-lumefantrine for treatment of uncomplicated malaria. Previously, 3 times the standard dose of chloroquine, that was probably efficacious against Plasmodium falciparum with the resistance-associated chloroquine-resistance transporter (pfcrt) 76T allele, was routinely used. The present study compared the efficacy and tolerability of a double standard dose of chloroquine with the efficacy and tolerability of artemether-lumefantrine.
Methods. In a randomized open-label clinical trial, artemether-lumefantrine or chloroquine (50 mg/kg) were given as 6 divided doses over 3 days to children aged 6 months - 15 years who had uncomplicated P. falciparum monoinfection. Drug concentrations were measured on day 7. P. falciparum multidrug resistance gene N86Y and pfcrt K76T alleles were identified.
Results. The polymerase chain reaction–adjusted day 28 and 42 treatment efficacies were 162 (97%) of 168 and 155 (97%) of 161, respectively, for artemether-lumefantrine and 150 (95%) of 158 and 138 (94%) of 148, respectively, for chloroquine. When parasites with resistance-associated pfcrt 76T were treated, the day 28 efficacy of chloroquine was 87%. No severe drug-related adverse events were detected. Symptom resolution was similar with both treatments.
Conclusions. Both treatments achieved the World Health Organization–recommended efficacy for antimalarials that will be adopted as policy. High-dose chloroquine treatment regimes should be further evaluated with the aim of assessing chloroquine as a potential partner drug to artemisinin derivatives.
Clinical trials registration. NCT00426439
Resistance of Plasmodium. falciparum to previously used monotherapies, such as sulfadoxine-pyrimethamine and chloroquine is widespread [1]. Most African countries therefore recommend using either artemether-lumefantrine or artesunate-amodiaquine (artemisinin-based combination therapy) for the treatment for uncomplicated P. falciparum malaria, whereas quinine is used for severe malaria [2]. However, tolerance and/or resistance to artesunate [3, 4], lumefantrine [5, 6], and amodiaquine has already been reported, and the effectiveness of a 7-day course of quinine was only 64% in a recent trial [7]. These findings highlight the continuous need for other efficacious antimalarial combinations.
In Guinea-Bissau, West Africa, ∼3 times the standard dose of chloroquine has been routinely used for many years, and chloroquine-resistant P. falciparum strains have not accumulated [8–13]. Furthermore, a double standard dose of chloroquine was more efficacious than was a standard dose of chloroquine (78% vs 38%) for the treatment of uncomplicated malaria caused by P.falciparum with the resistance-associated haplotype [8, 12]. These findings indicate that higher doses of chloroquine can be efficacious against P. falciparum strains resistant to standard-dose chloroquine.
Artemether-lumefantrine has been shown to be highly efficacious in Africa; however, in Guinea-Bissau, alleles associated with increased lumefantrine tolerance are highly prevalent (70%–80%) [9]. Furthermore, chloroquine and lumefantrine appear to select for opposite resistance-associated alleles, suggesting that chloroquine might delay the development of lumefantrine resistance [14–18]. Therefore ,the aim of the present study was to assess and compare the efficacy and tolerability of artemether-lumefantrine with those of a double standard dose of chloroquine in Guinea-Bissau before the introduction of artemether-lumefantrine as first-line therapy. A secondary aim was to determine whether the 2 treatments selected for opposite resistance-associated alleles. We conducted a randomized, comparative clinical trial with artemether-lumefantrine and a double standard dose of chloroquine.
PATIENTS AND METHODS
Participants
From December 2006 through September 2008, children were enrolled at the government health centers that serve the population (∼102,000) that reside in the Projecto de Saúde de Bandim health and demographic surveillance site (Bissau, Guinea-Bissau). P. falciparum has been meso endemic, but the prevalence appears to have decreased [19]. Inclusion criteria were monoinfection with P. falciparum without signs of severe malaria or danger signs, parasitaemia of 800–200.000 trophozoites/μL, axillary temperature >37.5°C or a history of fever during the previous 24 h, age 6 months–15 years, hemoglobin level >50 g/L, no other significant illness, no reported intake of antimalarials during the previous week, and residence in the study area.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained from all parents or legal guardians. Because most of the consenting adults were illiterate, the responsible nurse signed a document indicating that the information had been provided and informed consent had been obtained. The Ministério da Saúde Pública in Guinea-Bissau (064/DGSP/2006) and the regional ethics committee in Stockholm, Sweden (2006/1151-31/1) provided ethical approval. The study was registered at ClinicalTrials.gov (NCT00426439).
Randomization and Treatment
Mothers drew a sealed card with either “chloroquine” or “coartem” (artemether-lumefantrine) written on it from an envelope containing 10 of each card. The study nurses allocated treatment according to the drawn card. Cards and envelopes were prepared by the investigators. Treatment was not blinded. Medication was given and supervised by an experienced nurse. Tablets were crushed for young children unable to swallow whole tablets. Mothers were encouraged to continue breastfeeding.
Artemether-lumefantrine tablets (Coartem; Novartis) contained 20 mg of artemether and 120 mg of lumefantrine. Tablets were initially provided by the World Health Organization (WHO). After 1 year, the tablets expired and new tablets were purchased from Novartis. One to 4 tablets per dose were prescribed according to weight and were given at 0 h, 8 h, 24 h, 36 h, 48 h, and 60 h. Approximately 200 mL of milk was given with the drug.
Chloroquine phosphate tablets containing 100 mg of chloroquine base were provided by Recip AB. Fifty milligrams per kilogram of chloroquine base was given as 2 daily doses of ∼10 mg/kg on days 0 and 1 and 2 doses of 5 mg/kg on day 2.
The dose was given again to children who vomited within 30 min after treatment. If a child vomited again during the next 30 min, the child was given quinine intramuscularly and withdrawn. Concomitantly prescribed drugs were administered and recorded. P. falciparum infections detected after day 7 were treated with the other study drug (ie, artemether-lumefantrine if treatment at enrollment was chloroquine).
Procedures
Children were seen twice daily on days 0, 1, and 2 and once on day 3 and then were visited weekly at home on days 7–70. Each time, clinical condition was assessed, temperature was measured, and mothers were questioned about their child's general condition and the occurrence of convulsions, fever, other symptoms, or hospital admission. On days 0–3, children and mothers were asked daily about tinnitus, impaired hearing, headache, sleep disturbance, dizziness, vomiting, diarrhea, stomach pain, fluid intake, anorexia, rash, itch, cough, joint pain, and palpitations. If a child was not seen during a weekly visit, a health care worker revisited the house on the subsequent 2 days. Children were given free medication and were encouraged to return at any time if their health deteriorated. If a child was admitted to the hospital, the diagnosis and treatment was recorded, and he or she was withdrawn. Each day that a child was seen, including unscheduled visits to the health centers, Giemsa-stained thick and thin smears were evaluated to quantify asexual parasitemia (per 200 white blood cells). A slide was considered to show negative results after examination of 100 high-power fields. A blood sample was placed on filter paper (Whatman 3 MM) for later genotyping whenever a slide was made, except for on days 2 and 3 and when no parasites were found during unscheduled visits. Hemoglobin level was measured using a HaemoCue (Ängelholm) on days 0, 42, and 70. On day 7, exactly 100 μL of capillary blood was placed on filter paper for analysis of chloroquine or lumefantrine concentration. Pretreated filter papers (not available for the first 31 children randomized to artemether-lumefantrine) for analyses of lumefantrine concentrations were stored at −20 °C. Filter papers were placed in separate sealed plastic bags to avoid contamination. Drug concentrations were determined by high-performance liquid chromatography [20, 21]. Minimum detectable lumefantrine and chloroquine concentrations were 100 nmol/L and 50 nmol/L, respectively.
DNA was extracted from filter papers with use of an ABI Prism 6100 Nucleic Acid Prepstation (Applied Biosystems). DNA was frozen in aliquots at −20 °C until amplification by polymerase chain reaction (PCR). Reinfections and recrudescences were distinguished using a nested PCR protocol according to WHO recommendations [22]. P falciparum glutamate-rich protein, followed by merozoite surface protein 2 and, then, merozoite surface protein 1 were analyzed sequentially in reappearing parasitemias and matching day-0 samples. The chloroquine resistance transporter (pfcrt) 76K and the multidrug resistance (pfmdr1) 86N alleles associated with chloroquine susceptibility and in vivo and in vitro tolerance to lumefantrine [15–17] and the 76T and 86Y alleles associated with chloroquine resistance [23] were determined on day 0 and on the day of reappearing parasitemia. We used PCR-based methods that are described elsewhere [22, 24]. PCR and restriction products were resolved on agarose gels (Amresco), stained with ethidium bromide, and visualized under UV transillumination (GelDoc; Biorad).
Objectives and Outcomes
The primary end point was PCR-adjusted adequate clinical and parasitological response (ACPR) on day 42. Our secondary end points were PCR-adjusted ACPR on days 28 and 70; PCR-unadjusted ACPR on days 28, 42, and 70; hemoglobin recovery; fever and parasite clearance; protection from reinfection; drug tolerability; and selection of resistance-associated alleles.
We used the WHO criteria to identify early treatment failure, late clinical failure, and late parasitological failure. The follow-up period was extended to 70 days to enable follow-up of retreated children and to determine the duration that the drugs would exert a selective pressure on P. falciparum genotypes.
Statistics
We aimed to detect a cure rate difference of 10% with a power of 90%. We assumed a type I error of 5%, a power of 90%, and that 20% of the children would be lost to follow-up by day 70. Sample size was calculated to be 496. For 80% power, 382 children would be needed.
Data analysis was performed using Stata, version 9.0 (Stata). ACPR was assessed using survival analysis. Hazard ratio was calculated using Cox regression, and hazard assumption was tested. Quantile regression with 100 repetitions was used to compare body temperatures, parasite densities, hemoglobin levels, and drug concentrations. Symptoms and genotypes were analyzed using a χ2 test and logistic regression.
RESULTS
Trial Profile
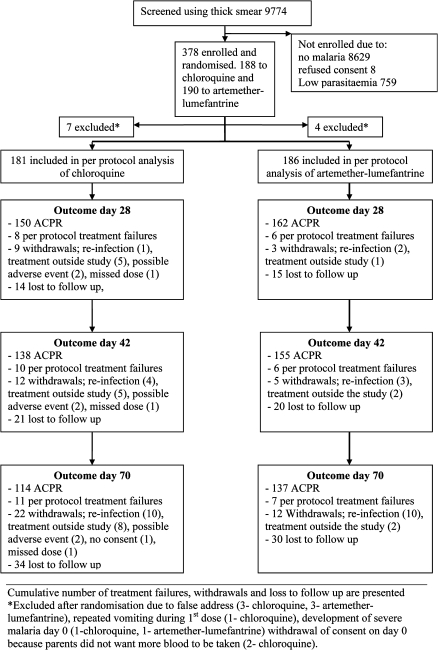
The study was stopped when 378 children were included, because artemether-lumefantrine was being introduced as part of the new national malaria treatment policy. Participant flow is shown in Figure 1. Baseline characteristics (Table 1) were similar between groups, even when stratified by age. Table 2 shows treatment outcomes. The PCR-adjusted day-42 ACPRs were 96.6% for artemether-lumefantrine and 93.8% for chloroquine (P = .28). The PCR-unadjusted day-42 ACPRs were 94.9% for artemether-lumefantrine and 91.3% for chloroquine (P = .35). The PCR-adjusted efficacy of chloroquine against the 60 P. falciparum strains with pfcrt 76T on day 0 was 86.7%, 82.3%, and 79.7% on days 28, 42, and 70. Children who experienced early treatment failure or late treatment failure were not significantly younger and did not have significantly higher parasitemia, compared with chidren who did not experience treatment failure. There were 10 reinfections in both study arms, with 3 and 4 occurring before day 42 in the artemether-lumefantrine and chloroquine arms, respectively.
Figure 1.
Trial profile.
Table 1.
Baseline Characteristics of Study Participants
| Characteristics | Chloroquine | Artemether-Lumefantrine |
| Age, median (IQR), mo | 87 (48–123) | 78 (52–123) |
| Male, No. (%) | 99 (55) | 108 (58) |
| Female, No. (%) | 82 (45) | 78 (42) |
| Weight, median (IQR), kg | 20 (14.5–28.5) | 19.4 (15.1–26.8) |
| Hemoglobin level, median (IQR),g/L | 112 (100–122) | 110 (99–123) |
| Temperature, median (IQR), °C | 38.0 (36.8–39) | 38.1 (37–39) |
| Parasite density, median (IQR), parasites/μL | 26400 (11360–57143) | 22346 (6800–62500) |
NOTE. IQR, interquartile range.
Table 2.
Polymerase Chain Reaction (PCR)–Adjusted and Unadjusted Treatment Outcomesa
| Reparasitemia Classification |
PCR-Unadjusted ACPR |
PCR-Adjusted ACPR |
|||||||
| Variable | ETF | LPF | LCF | Reinfection | ACPR, No. | ACPR, % | HR (95% CI) | ACPR, % | HR (95% CI) |
| Day 28 | 2 | 2 | 4 | 1 | 150 | 94.6 | 1.18 | 95.1 | 1.40 |
| Chloroquine | 3 | 0 | 3 | 2 | 162 | 95.5 | (0.46–3.07) | 96.6 | (0.49–4.03) |
| Artemether-Lumefantrine | |||||||||
| Day 42 | 2 | 2 | 6 | 4 | 138 | 91.3 | 1.65 | 93.8 | 1.76 |
| Chloroquine | 3 | 0 | 3 | 3 | 155 | 94.9 | (0.72–3.82) | 96.6 | (0.64–4.83) |
| Artemether-Lumefantrine | |||||||||
| Day 70 | 2 | 3 | 6 | 10 | 114 | 86.4 | 1.36 | 93.1 | 1.67 |
| Chloroquine | 3 | 1 | 3 | 10 | 137 | 89.8 | (0.72–2.57) | 96.0 | (0.65–4.32) |
| Artemether-Lumefantrine | |||||||||
NOTE. ACPR, adequate clinical and parasitological response; CI, confidence interval; ETF, early treatment failure; HR, hazard ratio; LCF, late clinical failure; LPF, late parasitological failure.
ACPR percentages were calculated using a survival analysis and, therefore, differ from the percentages calculated by dividing ACPR by ACPR and treatment failures.
Loss to follow-up did not differ significantly between treatment arms. All 3 withdrawals of consent were attributable to parents not wanting more blood samples obtained. The number of children treated outside the study protocol was not significantly different between the chloroquine and artemether-lumefantrine arms. Amongst children classified as receiving treatment outside the study protocol no P. falciparum were detected by microscopy or PCR during the week before and the week of treatment outside the study protocol.
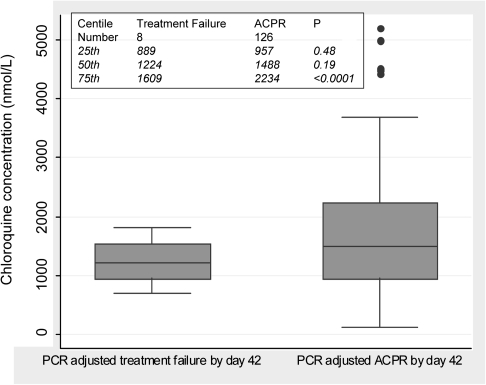
The median chloroquine concentration was 1408 nmol/L (interquartile range [IQR], 961–2190 nmol/L). Concentrations were higher in children who successfully cleared P. falciparum than in children in whom P. falciparum recrudesced (Figure 2). The median lumefantrine concentration was 813 nmol/L (IQR, 534–1200 nmol/L). Twenty-six children with ACPR on day 42 and 2 children with recrudescence on days 21 and 28 had lumefantrine concentrations <530 nmol/L. Chloroquine and artemether-lumefantrine concentrations were not significantly different between children subsequently reinfected and children with ACPR on day 42. The hemoglobin levels on days 0, 42, and 70 were 110, 117, and 120 g/L, respectively, in the artemether-lumefantrine arm and 112, 117 and 118 g/L, respectively, in the chloroquine arm.
Figure 2.
Box plot of whole blood chloroquine concentrations on day 7 in children with polymerase chain reaction (PCR)–adjusted treatment failure or adequate clinical and parasitological response (ACPR) by day 42.
Genotype proportions are shown in Table 3. Pfcrt 76T was associated with chloroquine treatment failure (P<.001). On day 0, pfcrt 76K and pfmdr1 86N were linked (odds ratio, 1.76, [95% confidence interval, 1.05–2.93] P = .03), and pfcrt 76T and pfmdr1 86Y were probably linked (odds ratio, 1.56, [95% confidence interval, 0.99–2.47] P = .06).
Table 3.
Proportions of pfcrt 76T and 76K and pfmdr1 86Y and 86N Alleles on Day 0 and by Day 42a
| Chloroquine |
Artemether-Lumefantrine |
|||||||||
| Allele Proportions on Day 0 and the Day of PCR-adjusted Treatment Failure (%) |
||||||||||
| Pfcrt 76T | Pfcrt 76K | Pfmdr1 86Y | Pfmdr1 86N | No. of Children in whom pfcrt and pfmdr1 Genotypes Were Identified | Pfcrt 76T | Pfcrt 76K | Pfmdr1 86Y | Pfmdr1 86N | No. of Children in whom pfcrt and pfmdr1 Genotypes Were Identified | |
| Day 0b | 45 (31) | 105 (72) | 58 (40) | 99 (68) | 146 | 38 (24) | 129 (82) | 50 (32) | 121 (78) | 157c |
| Treatment Failure | 8 (100) | 0 (0) | 5 (63) | 3 (37) | 8 | 0 (0) | 3 (100) | 0 (0) | 3 (100) | 3 |
| P value | <.001 | <. 001 | .18 | .09 | .44 | .56 | .32 | .47 | ||
| Allele Proportions on Day 0 and the Day of Reinfection | ||||||||||
| All Day 0 | 109 (29) | 283 (76) | 135 (37) | 268 (73) | 371d | 109 (29) | 283 (76) | 135 (37) | 268 (73) | 371d |
| Reinfections | 4 (40) | 6 (60) | 4 (40) | 6 (60) | 10 | 3 (30) | 7 (70) | 1 (11) | 8 (89) | 10e |
| P value | .34 | .20 | .53 | .29 | .60 | .44 | .11 | .25 | ||
NOTE. PCR, polymerase chain reaction.
Proportions were calculated by dividing the number of samples with a specific allele by the number of samples in which PCR was successful. Mixed infections thus contributed to the proportion of both alleles
Alleles in children with PCR-corrected adequate clinical and parasitological responsea by day 42.
The pfmdr1 haplotype was identified in 156 children.
The pfcrt haplotype was not identified in 7 children, and the pfmdr1 haplotype was not identified in 9 children.
The pfmdr1 genotype was identified 9 children.
Parasite and fever clearances and assessed clinical conditions are shown in Table 4
Table 4.
Median Temperatures and Clinical Condition from Day 0 through Day 3
| Day 0 |
Day 1 |
Day 2 | Day 3 |
||||
| Variable | 1st Dose | Second Dose | Third Dose | Fourth Dose | Fifth Dose | Sixth Dose | After Treatment |
| No. of Plasmodium falciparum isolates/μL, median | |||||||
| CQ | 26,667 | 9080 | 280 | 0 | |||
| AL | 22,346 | 680 | 0 | 0 | |||
| P value | .40 | <. 001 | <. 001 | – | |||
| Temperature, median (95% CI), °C | |||||||
| CQ | 38.0 | 36.7 | 36.4 | 36.3 | 36.3 | 36.2 | 36.2 |
| AL | 38.1 | 36.7 | 36.5 | 36.3 | 36.2 | 36.2 | 36.1 |
| P value | .54 | .93 | .46 | >.99 | .09 | >.99 | .08 |
| Proportion of Children with Poor or Moderate Clinical Condition | |||||||
| CQ | 0.93 | 0.83 | 0.60 | 0.54 | 0.46 | 0.38 | 0.11 |
| AL | 0.93 | 0.83 | 0.65 | 0.53 | 0.40 | 0.29 | 0.15 |
| P value | .74 | .99 | .38 | .94 | .24 | .10 | .33 |
NOTE. AL, artemether-lumefantrine; CI, confidence interval; CQ, chloroquine.
Parasite clearance was faster with artemether-lumefantrine than with chloroquine (P < . 001)
Symptom Resolution
Symptoms resolved similarly in both treatment arms during days 0–3. In the artemether-lumefantrine arm, dizziness (25 of 184 vs 12 of 181; P = .03) and headache (92 of 184 vs 67 of 181; P = .01) were more common on day 1. Sleeping disorders were more common in the chloroquine arm on day 2 (36 of 177 vs 13 of 182; P = .003). Fever was cleared by 130 of 181 and 143 of 188 children by the second dose in the chloroquine and artemether-lumefantrine arms, respectively (P = .78).
Adverse Events after Artemether-Lumefantrine Treatment
One child developed convulsions on day 0 and subsequently died in the hospital. One child was admitted with convulsions on day 1, and 1 child was admitted because of delirium on day 3. Three additional children reported convulsions at home before the fourth dose but were well when assessed and remained in the study.
Adverse Events after Chloroquine Treatment
One child vomited repeatedly on day 0. Three children reported convulsions at home before the fourth dose but were well when assessed and remained in the study. One child who had responded well to treatment was admitted to the hospital on day 2 because of stomach pain, and 1 child was admitted on day 14 because of anemia but did not receive a diagnosis of malaria. One child died of cholera on day 21.
Other possible adverse events reported after the first dose included agitation and asthenia by 3 and 2 children, respectively, in the artemether-lumefantrine arm and by 0 and 2 children, respectively, in the chloroquine arm. Facial oedema was reported on day 3 in 1 child in the artemether-lumefantrine arm. Pruritus occurred in 36 children receiving chloroquine and 10 receiving artemether-lumefantrine (P < .001).
DISCUSSION
The >95% efficacies of artemether-lumefantrine and double-dose chloroquine on days 42 and 28, respectively, fulfill WHO efficacy recommendations for antimalarials that will be adopted as policy and for antimalarials in use [25]. The high prevalence of tolerance associated single-nucleotide polymorphisms did not adversely affect the efficacy of artemether-lumefantrine that was similar to the efficacy reported from nearby countries [26, 27]. Two treatment failures in the artemether-lumefantrine arm were probably caused by low drug concentrations [6] despite intake of milk with artemether-lumefantrine. Unobserved intake of artemether-lumefantrine has resulted in lower lumefantrine concentrations [28], and the 50% inhibitory concentration of lumefantrine has been shown to be 5.5 times higher with the P. falciparum genotypes (pfcrt 76K and pfmdr1 86N) that are most common in Guinea-Bissau [29], emphasizing the importance of effectiveness studies.
The 95% efficacy of chloroquine by day 28 is contrary to the situation in all other African countries that have continued to use chloroquine. The high efficacy is attributable to the 87% efficacy of double-dose chloroquine against P. falciparum strains with pfcrt 76T (potentially resistant to standard-dose chloroquine) and to the low proportion of P. falciparum strains with pfcrt 76T; the latter may be an effect of the routine use of high doses of chloroquine in clinical practice [9, 10]. Chloroquine was given as monotherapy but, if the drug was combined with a rapidly acting artemisinin, efficacy would probably improve. The efficacy of chloroquine was greater than the minimum amodiaquin monotherapy efficacy (80%) required for artesunate and amodiaquine combination therapy to be recommended [25].
Standard doses of chloroquine are well tolerated, but peak concentrations are toxic [30] and can cause circulatory collapse [31]. Smoother concentration profiles that avoid toxic peak concentrations are achieved when repeated smaller doses are given [30, 32]. Because chloroquine has a very large volume of distribution [30], a large total amount of chloroquine may be received this way before toxic concentrations are reached. Our results confirm that double-dose chloroquine is well tolerated [11, 12, 33]. Only pruritus, a well-known adverse effect of chloroquine, was attributed to chloroquine. Artemether-lumefantrine was also generally well tolerated, in line with published data [34].
The 95% efficacy of double-dose chloroquine cannot be generalized to areas with high prevalence of pfcrt 76T. However, the 87% efficacy against P. falciparum strains with pfcrt 76T can be generalized to most of Africa, because chloroquine resistance appears to have a common genetic basis in Africa [35, 36]. Because chloroquine concentrations were lower in children experiencing treatment failure, an even higher dose is likely to further increase chloroquine efficacy. In support of this hypothesis, chloroquine resistance is mediated by a saturable efflux mechanism [37, 38], and double-dose chloroquine had a higher efficacy than did standard-dose chloroquine [12]. Furthermore, parasite clearance was shown to be faster when multiple daily doses were given, suggesting that the dosage regime also affects efficacy [39]. In Guinea-Bissau, ∼3 times the standard dose of chloroquine is frequently administered as multiple doses without obvious adverse events [13]. It is therefore likely that doses >50 mg/kg, as used in the present study, can be well tolerated and result in even higher levels of efficacy than the 87% seen in the present study.
The >90% efficacy of both treatments on day 42, when all children with recurrent parasitemia were considered to have experienced treatment failure, is considerably higher than the expected efficacy of chloroquine. The same number of reinfections occurred at approximately the same time, suggesting that both treatments had a similar posttreatment protective effect. Pfcrt 76T was associated with chloroquine treatment failure and was linked to pfmdr1 86Y. In the artemether-lumefantrine arm, pfmdr1 86N and pfcrt 76K were the only genotypes identified in recrudescent infections by day 42, supporting previous findings, even though the changes in proportion were not statistically significant [16, 17]. Recrudescing P. falciparum infection after treatment with chloroquine thus involved alleles associated with artemether-lumefantrine susceptibility, and recrudescing P. falciparum infection after treatment with artemether-lumefantrine involved alleles associated with chloroquine susceptibility. As suggested elsewhere [14], chloroquine and artemether-lumefantrine may protect each other from development of resistance to a certain extent.
Considering the limited number of available and affordable antimalarials, the high efficacy of a double standard dose of chloroquine should not be ignored. The development of artemisinin resistance [3, 4], rapid selection of genotypes associated with artemether-lumefantrine tolerance and/or resistance [16, 17, 29], and overuse of artemether-lumefantrine for unproven clinical malaria [19] are of concern. High-dose chloroquine (with artemisinin) that selects for opposite genotypes to artemether-lumefantrine could be a treatment option for concurrent use with artemether-lumefantrine and might decrease the total amount of artemether-lumefantrine used and delay resistance development [14, 40].
In summary, we report that artemether-lumefantrine and a double dose of chloroquine are efficacious and well tolerated. Furthermore, both drugs provided similar protection from reinfection and selected for opposite tolerance- and resistance-associated haplotypes in Guinea-Bissau.
Acknowledgments
We thank the clinical study team in Guinea-Bissau, for their invaluable work, Ulrika Fjelling, for drug concentration analysis, and Peter Aaby and Andreas Mårtensson, for valuable comments. Johan Ursing, Poul-Erik Kofoed, Amabelia Rodrigues, and Lars Rombo contributed to the design and coordination of the study, supervised the enrollment and follow-up of patients, assisted with data entry and interpretation, and prepared the manuscript. Anders Björkman contributed to the design of the study and participated in the preparation of the manuscript. Johan Ursing conducted molecular analyses. Rikke Thoft Nielsen contributed to coordination of the study, supervised the enrollment and follow-up of patients, and participated in the preparation of the manuscript. Daniel Blessborn contributed to the design of the study, designed and participated in drug concentration analyses, and participated in the preparation of the manuscript.
This work was supported by the Clinical Research Center at Sörmland County Council and the Department of Infectious Diseases at Eskilstuna, Sweden. Recip provided chloroquine tablets, and the WHO provided Coartem tablets.
References
- 1.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–18. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World malaria report. World Health Organization; 2009. [Google Scholar]
- 3.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim P, Alker AP, Khim N, et al. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar J. 2009;8:11. doi: 10.1186/1475-2875-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–77. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achan J, Tibenderana JK, Kyabayinze D, et al. Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ. 2009;339:b2763. doi: 10.1136/bmj.b2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am J Trop Med Hyg. 2007;76:844–8. [PubMed] [Google Scholar]
- 9.Ursing J, Kofoed PE, Rodrigues A, Rombo L. No seasonal accumulation of resistant P. falciparum when high-dose chloroquine is used. PLoS One. 2009;4:e6866.. doi: 10.1371/journal.pone.0006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ursing J, Schmidt BA, Lebbad M, et al. Chloroquine resistant P. falciparum prevalence is low and unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infect Genet Evol. 2007;7:555–61. doi: 10.1016/j.meegid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Kofoed PE, Lopez F, Johansson P, et al. Treatment of children with Plasmodium falciparum malaria with chloroquine in Guinea-Bissau. Am J Trop Med Hyg. 2002;67:28–31. doi: 10.4269/ajtmh.2002.67.28. [DOI] [PubMed] [Google Scholar]
- 12.Kofoed PE, Ursing J, Poulsen A, et al. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans R Soc Trop Med Hyg. 2007;101:231–8. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Ursing J, Kofoed PE, Rodrigues A, Bergqvist Y, Rombo L. Chloroquine is grossly overdosed and overused but well tolerated in Guinea-bissau. Antimicrob Agents Chemother. 2009;53:180–5. doi: 10.1128/AAC.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warhurst DC, Duraisingh MT. Rational use of drugs against Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2001;95:345–6. doi: 10.1016/s0035-9203(01)90177-4. [DOI] [PubMed] [Google Scholar]
- 15.Mwai L, Kiara SM, Abdirahman A, et al. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob Agents Chemother. 2009;53:5069–73. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisowath C, Petersen I, Veiga MI, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750–7. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisowath C, Stromberg J, Martensson A, et al. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem) J Infect Dis. 2005;191:1014–7. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 18.Duraisingh MT, Roper C, Walliker D, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol. 2000;36:955–61. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues A, Schellenberg JA, Kofoed PE, Aaby P, Greenwood B. Changing pattern of malaria in Bissau, Guinea Bissau. Trop Med Int Health. 2008;13:410–7. doi: 10.1111/j.1365-3156.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 20.Blessborn D, Romsing S, Annerberg A, et al. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J Pharm Biomed Anal. 2007;45:282–7. doi: 10.1016/j.jpba.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Lindegårdh N, Forslund M, Green MD, Kaneko A, Bergqvist Y. Automated solid-phase extraction for determination of amodiaquine, chloroquine and metabolites in capillary blood on sampling paper by liquid chromatography. Chromatographia. 2002:5–12. [Google Scholar]
- 22.WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva, world Health Organization. 2007 [Google Scholar]
- 23.Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–63. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 24.Veiga MI, Ferreira PE, Bjorkman A, Gil JP. Multiplex PCR-RFLP methods for pfcrt, pfmdr1 and pfdhfr mutations in Plasmodium falciparum. Mol Cell Probes. 2006;20:100–4. doi: 10.1016/j.mcp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Guidelines for the treatment of malaria. 2006 [Google Scholar]
- 26.Zongo I, Dorsey G, Rouamba N, et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet. 2007;369:491–8. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 27.Sagara I, Dicko A, Djimde A, et al. A randomized trial of artesunate-sulfamethoxypyrazine-pyrimethamine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2006;75:630–6. [PubMed] [Google Scholar]
- 28.Piola P, Fogg C, Bajunirwe F, et al. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet. 2005;365:1467–73. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 29.Mwai L, Kiara SM, Abdirahman A, et al. In vitro activity of piperaquine, lumefantrine and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in Pfcrt and Pfmdr1. Antimicrob Agents Chemother. 2009;53:5069–73. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looareesuwan S, White NJ, Chanthavanich P, et al. Cardiovascular toxicity and distribution kinetics of intravenous chloroquine. Br J Clin Pharmacol. 1986;22:31–6. doi: 10.1111/j.1365-2125.1986.tb02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith ER, Klein-Schwartz W. Are 1-2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers. J Emerg Med. 2005;28:437–43. doi: 10.1016/j.jemermed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 32.White NJ, Miller KD, Churchill FC, et al. Chloroquine treatment of severe malaria in children: pharmacokinetics, toxicity, and new dosage recommendations. N Engl J Med. 1988;319:1493–500. doi: 10.1056/NEJM198812083192301. [DOI] [PubMed] [Google Scholar]
- 33.Kofoed PE, Co F, Johansson P, et al. Treatment of uncomplicated malaria in children in Guinea-Bissau with chloroquine, quinine, and sulfadoxine-pyrimethamine. Trans R Soc Trop Med Hyg. 2002;96:304–9. doi: 10.1016/s0035-9203(02)90107-0. [DOI] [PubMed] [Google Scholar]
- 34.van Vugt M, Brockman A, Gemperli B, et al. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–9. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wootton JC, Feng X, Ferdig MT, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–3. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 36.Ariey F, Fandeur T, Durand R, et al. Invasion of Africa by a single pfcrt allele of South East Asian type. Malar J. 2006;5:34. doi: 10.1186/1475-2875-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680–2. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez CP, Rohrbach P, McLean JE, Fidock DA, Stein WD, Lanzer M. Differences in trans-stimulated chloroquine efflux kinetics are linked to pfcrt in Plasmodium falciparum. Mol Microbiol. 2007;64:407–20. doi: 10.1111/j.1365-2958.2007.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pussard E, Lepers JP, Clavier F, et al. Efficacy of a loading dose of oral chloroquine in a 36-hour treatment schedule for uncomplicated plasmodium falciparum malaria. Antimicrob Agents Chemother. 1991;35:406–9. doi: 10.1128/aac.35.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci U S A. 2008;105:14216–21. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]