Chloroquine began as a first-line antimalarial in 1946, the same year that the rock star Cher was born. Similar to Cher, chloroquine peaked in popularity in the early 1960s. Cher has famously made several major comebacks. Is it now chloroquine's turn?
For decades, chloroquine was a remarkably effective, safe, and inexpensive antimalarial. Optimism about effectiveness of chloroquine led public health professionals to predict the eradication of malaria by 2000 [1]. By 1979, the equivalent of >500 million tablets of chloroquine were used each year [2]. Unfortunately, Plasmodium falciparum gradually became resistant to chloroquine. After first appearing in Southeast Asia and South America in the late 1950s, resistance spread throughout Africa by the 1980s [3]. Meanwhile, alternative antimalarials were more expensive, and many countries continued to use chloroquine despite evidence that it was not effective. Recognition of this led to accusations of malpractice against the World Health Organization and the World Bank and a vigorous drive to replace chloroquine with more-effective artemisinin combination therapies [4].
Can chloroquine make a comeback? Evidence from Malawi suggests that chloroquine resistance faded a decade after it was withdrawn from use, restoring the clinical efficacy of the drug [5]. In addition, evidence presented by Ursing et al [6] suggests that, even in the presence of chloroquine resistance, a change in the dosing regimen restores efficacy.
Ursing et al. [6] reported in this issue of The Journal of Infectious Diseases and in previous articles [7-8] that the in vivo chloroquine failure rate can be decreased by giving the drug twice per day instead of once per day. Doubling the frequently used dose of chloroquine in this way achieved a high cure rate (95%) despite preexisting chloroquine resistance and did not result in an increase in adverse events. Of interest, these authors also showed that use of this modified chloroquine dosing regimen in Guinea-Bissau has stabilized the spread of chloroquine resistance, as measured by the prevalence of pfcrt 76T [7, 9–10].
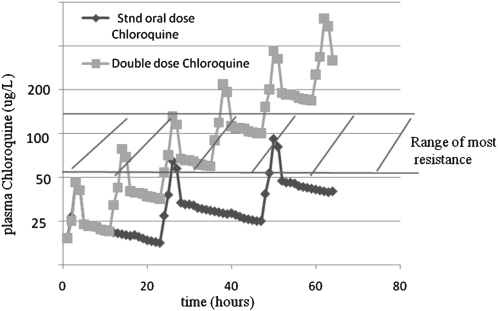
This increase in efficacy can be explained by the pharmacokinetics of chloroquine. Chloroquine can penetrate most tissues (eg, brain, eyes, heart, kidneys, leukocytes, liver, lungs, and spleen) and, therefore, has a large volume of distribution [11]. After oral administration, chloroquine is 85% absorbed in the plasma, with a time to peak plasma levels of 1–2 h, and then it is cleared in 2 phases. There is an initial brisk decrease in plasma concentration that is in accordance with first-order rate kinetics and occurs as the drug rapidly distributes throughout the body. This is followed by a second, slower phase. during which chloroquine moves from the body tissues to the plasma (Figure 1). This second phase results in the trough blood levels of chloroquine and may determine the efficacy of the dose given. By increasing the frequency of administration and dose of chloroquine, trough levels are progressively elevated.
Figure 1.
Predictive comparison of blood levels obtained after oral administration of chloroquine in 3 divided doses at 0, 24, and 48 h, for a total dose of 25 mg/kg (diamonds), and the blood levels obtained after oral administration of 6 divided doses of chloroquine at 0, 12, 24, 36, 48, and 72 h, for a total dose of 50 mg/kg (squares). (This figure shows hypothetical pharmacokinetics of chloroquine and is for illustrative purposes only; it and does not contain actual pharmokinetic data but is based loosely on data from [11, 25].)
Although chloroquine resistance is widespread, resistance is, in general, not very potent [12]. In vitro, parasites are considered to be chloroquine resistant the 50% inhibitory concentration (IC50) of the drug is > 160 nmol/L (51 μg/L) [13]. Recent reports from areas where malaria is endemic documented that the majority of chloroquine-resistant isolates of P. falciparum have IC50 values <400 nmol/L (128 μg/L) [14–21]. In contrast, typical trough levels are 80–125 nmol/L (25–40 μg/L). Thus, the majority of chloroquine-resistant isolates have IC50 values that are only 3–5-fold higher than typical trough plasma concentrations. In accordance with these findings, increasing the dose and frequency of administration of chloroquine can increase plasma concentrations to levels higher than the IC50 values of chloroquine-resistant parasites.
A second reason why the new regimen may be more effective is that trough levels of chloroquine vary substantially among individual patients, especially among those who have been previously infected with malaria [22]. The reason for the variability in these levels is not known but could depend on genetic factors, the nutritional status, or the amount of hemozoin (released during previous malaria infections) already present in tissues [23]. Thus, the double-dose chloroquine regimen might be especially effective, because it leads to increased plasma chloroquine trough levels in patients in whom trough levels might be subtherapeutic during the standard regimen.
Of course, doubling the dose of chloroquine might lead to increased adverse effects. Although none were seen in the study by Ursing et al [6], a larger study would be needed to rule out the possibility of low-frequency serious adverse events.
Similar to the artemisinin derivatives, prospects for long-term chloroquine efficacy will be enhanced if it can be used in combination, such as chloroquine-azithromycin [24]. With the right dosage and combined agent, the prospects for a chloroquine comeback are good.
References
- 1.Pampana EA. Textbook of malaria eradication. London: Oxford University Press; 1963. [Google Scholar]
- 2.Kouznetsov RL. Antimalarial drug requirements for national malaria control programmes in developing countries, 1982-1989, second report. Geneva, Switzerland: World Health Organization; 1987. [Google Scholar]
- 3.Wellems Thomas E, Plowe Christopher V. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–6. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 4.Attaran A, Barnes KI, Curtis C, et al. WHO, the Global Fund, and medical malpractice in malaria treatment. Lancet. 2004;363:237–40. doi: 10.1016/S0140-6736(03)15330-5. [DOI] [PubMed] [Google Scholar]
- 5.Laufer MK, Thesing PC, Eddington ND, et al. Return of chloroquine antimalarial efficacy in Malawi. N Eng J Med. 2006;355:1959–66. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 6.Ursing J, Kofoed PF, Rodrigues A, et al. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: a randomized trial. J Infect Dis. 2010;203:109–116. doi: 10.1093/infdis/jiq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ursing J, Rombo L, Kofoed PE, Gil JP. Carriers, channels and chloroquine efficacy in Guinea-Bissau. Trends Parasitol. 2008;24:49–51. doi: 10.1016/j.pt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kofoed P-E, Ursing J, Poulsen A, et al. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans Royal Soc Trop Med Hyg. 2007;101:231–8. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ursing J, Kofoed P-E, Rodrigues A, Rombo L. No seasonal accumulation of resistant P. falciparum when high-dose chloroquine is used. PLoS ONE. 2009;4:e6866. doi: 10.1371/journal.pone.0006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ursing J, Schmidt B, Lebbad M, et al. Chloroquine resistant P. falciparum prevalence is low and unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infection, Genetics and Evolution. 2007;7:555–61. doi: 10.1016/j.meegid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine: focus on recent advancements. Clin Pharmacokinet. 1996;31:257–74. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Valderramos SG, Valderramos JC, Musset L, et al. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PLoS Pathog. 2010;6:e1000887. doi: 10.1371/journal.ppat.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringwald P. Susceptibility of Plasmodium falciparum to antimalarial drugs: report on global monitoring: 1996–2004. Geneva: : World Health Organization; 2005. [Google Scholar]
- 14.Bacon D, Jambou R, Fandeur T, et al. World Antimalarial Resistance Network (WARN) II: in vitro antimalarial drug susceptibility. Malaria J. 2007;6:120. doi: 10.1186/1475-2875-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibley CH, Ringwald P. A database of antimalarial drug resistance. Malar J. 2006;5:48. doi: 10.1186/1475-2875-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringwald P. Monitoring antimalarial drug efficacy. Clin Infect Dis. 2004;38:1192–3. doi: 10.1086/383152. author reply, 1193–4. [DOI] [PubMed] [Google Scholar]
- 17.Hatabu T, Kawazu S, Kojima S, et al. In vitro susceptibility and genetic variations for chloroquine and mefloquine in Plasmodium falciparum isolates from Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 4):73–9. [PubMed] [Google Scholar]
- 18.Borrmann S, Binder RK, Adegnika AA, et al. Reassessment of the resistance of Plasmodium falciparum to chloroquine in Gabon: implications for the validity of tests in vitro vs. in vivo. Trans Royal Soc Trop Med Hyg. 2002;96:660–3. doi: 10.1016/s0035-9203(02)90345-7. [DOI] [PubMed] [Google Scholar]
- 19.Moreno A, Cuzin-Ouattara N, Nebie I, Sanon S, Brasseur P, Druilhe P. Use of the DELI-microtest to determine the drug sensitivity of Plasmodium falciparum in Burkina Faso. Ann Trop Med Parasitol. 2001;95:309–12. doi: 10.1080/00034980020011750. [DOI] [PubMed] [Google Scholar]
- 20.Yang HL, Liu DQ, Yang YM, et al. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas. Southeast Asian J Trop Med Public Health. 1997;28:460–4. [PubMed] [Google Scholar]
- 21.Noedl H, Faiz MA, Yunus EB, et al. Drug-resistant malaria in Bangladesh: an in vitro assessment. Am J Trop Med Hyg. 2003;68:140–2. [PubMed] [Google Scholar]
- 22.Tett SE. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clinical pharmacokinetics. 1993;25:392–407. doi: 10.2165/00003088-199325050-00005. [DOI] [PubMed] [Google Scholar]
- 23.Obua C, Hellgren U, Gustafsson LL, et al. Population pharmacokinetics of chloroquine and sulfadoxine and treatment response in children with malaria: suggestions for an improved dose regimen. Br J Clin Pharmacol. 2008;65:493–501. doi: 10.1111/j.1365-2125.2007.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunne MW, Singh N, Shukla M, et al. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J Infect Dis. 2005;191:1582–8. doi: 10.1086/429343. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson LL, Walker O, Alvan G, et al. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983;15:471–9. doi: 10.1111/j.1365-2125.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]