Abstract
Background. The use of a squalene-containing (AS03) pandemic vaccine for high-risk groups in England allowed vaccine effectiveness (VE) of such novel oil-in-water adjuvanted vaccine to be evaluated.
Methods. Cases of laboratory-confirmed pandemic (H1N1)2009 influenza in England between November 2009 and January 2010 were followed up for history of pandemic (H1N1)2009 or 2009/10 seasonal influenza vaccination and relevant comorbidities. Controls were patients similarly tested but negative for the virus. We estimated pandemic (H1N1)2009 VE from the relative reduction in the odds of confirmed pandemic (H1N1)2009 infection between vaccinated and unvaccinated individuals after adjustment for confounders.
Results. A total of 933 cases and 1220 controls were analyzed. VE from ≥14 days was 62% (95% CI 33% to 78%) with protection from 7 to 13 days post-vaccination (59%, 95% CI 12% to 81%). VE from ≥14 days differed by age (P=.03) being 77% (11% to 94%) in children <10 years, 100% (80% to 100%) in 10–24-year olds, 22% (-153% to 76%) in 25–49-year olds, and 41% (-71% to 80%) in 50-plus-year-olds.
Conclusion. Use of oil-in-water adjuvant contributed to a high VE with reduced antigen dosage in children and young adults. Our VE estimate supports the serological correlates of protection used for licensure in these age groups. However, the immunological basis of disappointing VE in older adults merits investigation.
A key element of the global response to an influenza pandemic is the rapid development, licensure, and deployment of monovalent pandemic strain vaccines [1]. Before the emergence of pandemic (H1N1) 2009 virus in April 2009, the most likely pandemic virus candidate was considered to be avian influenza H5N1, to which the population would likely be immunologically naïve [2]. To ensure a good antibody response to an H5N1 vaccine and to reduce the amount of antigen needed to prime the immune system, the strategy adopted by some manufacturers was to enhance immunogenicity by using novel adjuvants such as an oil-in-water emulsion [3]. However, the emergence of an H1N1 pandemic strain virus, to which preexisting, cross-reactive antibody could be demonstrated in many older individuals [4], suggested that conventional unadjuvanted vaccines may have been adequate for immunizing against this H1N1 pandemic-strain virus.
As part of its pandemic planning, the United Kingdom (UK) contracted with two manufacturers to supply monovalent pandemic-strain vaccine for the UK population. One vaccine was a split-virion vaccine grown in eggs and adjuvanted with ASO3, an oil-in-water adjuvant containing squalene [5]. The other was a whole-cell, unadjuvanted vaccine grown in Vero cells [6]. Both vaccines were licensed by the European Medicines Agency based on limited immunogenicity and reactogenicity data for a similar mock up vaccine containing an H5N1 strain. The use of a mock-up dossier for a novel influenza strain allowed a fast track approach to licensure by obviating the need to generate safety and immunogenicity data for the actual pandemic strain vaccine [7].
The UK pandemic vaccination program was initially targeted at individuals in clinical risk groups for whom seasonal influenza vaccine was already recommended, with the additional inclusion of otherwise healthy pregnant women [8]. Subsequently the vaccination program was extended to healthy children aged 6 months to 5 years [9]. The majority of the vaccine used in the UK was the ASO3 adjuvanted vaccine. But the use of the unadjuvanted whole-cell vaccine was restricted to those with a history of egg allergy and pregnant women preferring a thiomersal-free vaccine.
We report the effectiveness of the ASO3 adjuvant pandemic (H1N1) 2009 vaccine in preventing pandemic (H1N1) 2009 disease confirmed in patients who had underlying medical conditions and were therefore in high-risk priority groups targeted for vaccination in the UK. Protection against hospital admission as well as less serious disease not requiring hospitalization was assessed. We used an established case control method in which cases are those with laboratory-confirmed infection and controls are test-negative patients similarly investigated for suspected infection [10].
METHODS
Study Population
Cases and controls were individuals presenting to health care services in England with suspected influenza who had a respiratory swab tested for pandemic (H1N1) 2009 infection between 9 November 2009 and 4 January 2010. Only those with disease onset after 1 November 2009 were retained because this was the earliest date at which any vaccinated patient could have derived protection. During this period, diagnostic testing for suspected pandemic (H1N1) 2009 infection was restricted to patients hospitalized with an influenza-like illness or patients presenting in primary care with an influenza-like illness who were also in clinical risk groups for which seasonal vaccine was recommended [11]. A case was defined as a patient with laboratory-confirmed pandemic (H1N1) 2009 infection in the study period. A control was a patient investigated during this period but with a negative laboratory test. In the first week of the study, for those swabbed from 9 November 2009 to 15 November 2009, we sought information on all cases and controls. After this, to reduce workload, controls were frequency-matched one-to-one by age group, week sample was taken, and English region of residence. A questionnaire was sent to each patient's general practitioner (GP) for both cases and controls. This questionnaire requested information on date of symptom onset, whether or not the person was in a clinical risk group for influenza vaccination, and, if so, which one. It also asked for hospital admission history as well as dates and batch numbers of any pandemic (H1N1) 2009 or 2009/10 seasonal trivalent influenza vaccine doses given.
Laboratory Confirmation
We performed laboratory confirmation of pandemic (H1N1) 2009 virus by using respiratory swabs collected into virus transport medium. All samples were tested by an HPA Regional Microbiology Network (RMN) laboratory using real-time reverse transcriptase polymerase chain reaction (RT-PCR) assays for detection of influenza A and sub-typed for pandemic (H1N1) 2009 viruses [12, 13]. RMN laboratories followed the HPA National Standard Method RT-PCR testing protocol and reported test results weekly to the HPA Centre for Infections. The sensitivity of the RT-PCR method was 4–40 plaque forming units detectable per mL [J Ellis personal communication].
Vaccination Programme and Schedule
Starting in the last week of October 2009, vaccine was offered to heath care workers. Beginning in early November vaccine was offered to those in clinical risk groups and household contacts of immunosuppressed patients. It was offered to healthy children aged under 5 years from early January 2010. Children aged 6 months to 10 years of age were recommended to receive a single 0.25 mL dose of the AS03 adjuvanted vaccine (Pandemrix, Glaxo Smith Kline Vaccines, Rixensart, Belgium) manufactured from the A/California/7/2009 (H1N1) v-like strain antigen (New York Medical College x-179A). This vaccine contained 1.875 μg of hemagglutinin antigen, the oil-in-water emulsion based adjuvant AS03 (containing squalene (5.345mg), DL-α-tocopherol (5.93 mg), and polysorbate 80 (2.43mg), and thiomersal [9]. Individuals aged 10-plus years were recommended to receive a 0.5 mL dose of the same vaccine. A 2- dose schedule was recommended for individuals who were immunocompromised.
Sample-size Calculation
To obtain reasonable precision (± 20%) for a VE of about 70% approximately 1200 cases and controls were required, assuming 5% of controls were vaccinated.
Statistical Analysis
Multi-variable logistic regression was used to determine the association between vaccination and confirmed pandemic (H1N1) 2009 infection. A full model was developed that included all clinical risk factors for which the odds ratio of being a case was significantly different from one as well as pandemic (H1N1) 2009 and seasonal vaccine, age, time period (in weeks), pregnancy and gender. For estimation of the effectiveness of pandemic (H1N1) 2009 vaccine with maximum power, only significant variables that changed the odds ratio for the pandemic (H1N1) 2009 vaccine effect by 5% or more were retained along with period and age. The pandemic and seasonal vaccine variables were constructed as 4-level variables with unvaccinated as the baseline, vaccination within 6 days of onset as a period in which no protective effect is expected (results not shown), vaccination 7 to 13 days before onset, and vaccination 14 or more days before onset. The 6–13 days and 14 or more day levels were also combined to give estimates of effectiveness from 7 days. The adjusted odds ratio for a given level such as ≥14 days was used to calculate the vaccine effectiveness (VE) for that level using the formula (1- adjusted odds ratio)*100. The analyses were also performed stratified by age (<10, 10–24, 25–49, and 50+ years), hospitalized/not hospitalized and also according to whether the patient was immunosuppressed by disease or treatment. Finally VE was estimated by age (<25 and ≥ 25 years) and by immunosuppressive status within the hospitalized patients. Significance was taken at a 5% level. Analysis was performed using STATA software [StataCorp. 2007. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP].
Ethical Permission
Informed consent for follow up was not sought from patients. The work was carried out under NHS Act 2006 (section 251), which provides statutory support for disclosure of such data by the NHS, and its processing by the Health Protection Agency for purposes of communicable disease control.
RESULTS
Descriptive
By 8 March 2010 a total of 4452 questionnaires (from 1905 cases and 2547 controls) had been returned by GPs in England of the 5942 sent out (74.9% response rate). Of the 4452 patients, 1976 were excluded because they had no underlying chronic conditions and were therefore not eligible for vaccination, although 3 (all controls) were reported to have received vaccine ≥ 14 days after disease onset. In addition, we excluded 299 individuals whose onset was before 1 November 2009 (week 45), considered the earliest date at which any vaccinated patient could have had protection from pandemic (H1N1) 2009 vaccine. Thirteen cases and 10 controls who had a swab taken ≥ 30 days after onset were also excluded. Finally, because only one individual received the unadjuvanted, whole-cell vaccine, this case was dropped so that all effectiveness estimates are for Pandemrix. The study population for the analysis of vaccine effectiveness was therefore restricted to 933 cases, with a confirmed infection with pandemic (H1N1) 2009 virus (Table 1) and 1220 controls—all with chronic conditions that made them eligible for vaccination.
Table 1.
Demographic, Clinical, and Vaccination Details of Pandemic (H1N1) 2009 Cases and Controls
| Variable | No. of cases (Total =933) | % | No. of controls (Total=1220) | % |
| Received pandemic vaccine | ||||
| 1st dose ≥14 days before estimated onset | 21 | 2.3 | 45 | 3.7 |
| 1st dose 7–13 days before estimated onset | 10 | 1.1 | 24 | 2.0 |
| 1st dose <7 days before estimated onset | 39 | 4.2 | 39 | 3.2 |
| 2nd dose ≥14 days before estimated onset | 0 | 0.0 | 5 | 0.4 |
| 2nd dose 7–13 days before estimated onset | 0 | 0.0 | 2 | 0.2 |
| Unvaccinated | 756 | 81.0 | 938 | 76.9 |
| Vaccination status unknown | 107 | 11.5 | 167 | 13.7 |
| Received seasonal vaccine | ||||
| Vaccinated ≥14 days before Estimated onset | 220 | 23.6 | 294 | 24.1 |
| Vaccinated 7–13 days before estimated onset | 24 | 2.6 | 35 | 2.9 |
| Vaccinated <7 days before estimated onset | 18 | 1.9 | 34 | 2.8 |
| Unvaccinated | 559 | 59.9 | 696 | 57.1 |
| Vaccination status unknown | 112 | 12.0 | 161 | 13.2 |
| Sex and Pregnancy | ||||
| Female – not pregnant | 396 | 42.4 | 533 | 43.7 |
| Female – pregnant | 130 | 13.9 | 53 | 4.3 |
| Female – pregnant unknown | 4 | 0.4 | 15 | 1.2 |
| Male | 403 | 43.2 | 619 | 50.7 |
| Age group (years) | ||||
| <5 | 123 | 13.2 | 203 | 16.6 |
| 5–9 | 110 | 11.8 | 126 | 10.3 |
| 10–14 | 85 | 9.1 | 66 | 5.4 |
| 15–24 | 120 | 12.9 | 135 | 11.1 |
| 25–34 | 146 | 15.7 | 130 | 10.7 |
| 35–49 | 152 | 16.3 | 198 | 16.2 |
| 50–64 | 146 | 15.7 | 216 | 17.7 |
| 65+ | 51 | 5.5 | 146 | 12.0 |
| Interval (days between onset and sample collection) | ||||
| 0–1 | 220 | 23.6 | 204 | 16.7 |
| 2–4 | 268 | 28.7 | 199 | 16.3 |
| 5–7 | 102 | 10.9 | 88 | 7.2 |
| 8–14 | 53 | 5.7 | 69 | 5.7 |
| 15–29 | 17 | 1.8 | 19 | 1.6 |
| Not stated, interval estimated from date of swab or hospitalization | 273 | 29.3 | 641 | 52.5 |
| Hospitalized | ||||
| No | 159 | 17.0 | 263 | 21.6 |
| Yes | 669 | 71.7 | 728 | 59.7 |
| Unknown | 105 | 11.3 | 229 | 18.8 |
| Risk factora | ||||
| Chronic respiratory | 151 | 16.2 | 272 | 22.3 |
| Chronic heart | 98 | 10.5 | 179 | 14.7 |
| Chronic renal | 64 | 6.9 | 112 | 9.2 |
| Chronic liver | 30 | 3.2 | 55 | 4.5 |
| Chronic neurological | 120 | 13.3 | 162 | 12.9 |
| Diabetes | 100 | 10.7 | 138 | 11.3 |
| Immunosuppressant disease | 118 | 12.7 | 259 | 21.2 |
| Immunosuppressant treatment | 139 | 14.9 | 268 | 22.0 |
| Any asthma | 403 | 43.2 | 429 | 35.1 |
| Severe asthma | 242 | 25.9 | 259 | 21.2 |
NOTE. If symptom onset date was not stated, onset date is presume to be date of hospitalization or 3 days before date of swab was taken.
Note that individuals may have multiple risk factors.
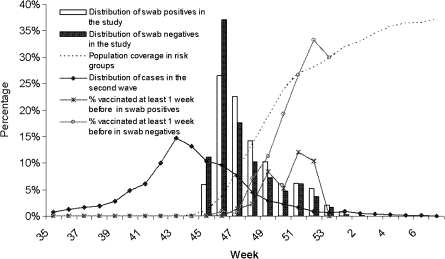
Most cases and controls were young adults and children (Table 1). The proportions admitted to hospital by age were similar in cases and controls: 80%, and 81% respectively among children aged 0–9 years of age, 71% and 65% among 10–24 year olds, 79% and 67% among 25–49 year olds and 90% and 76% among 50+ year olds. Pregnancy was reported more often in cases than in controls. Influenza onset date was known more often for cases than for controls. Where known, the median interval from onset to swab was 3 days, with 89% of swabs taken within 7 days for cases and 85% within 7 days for controls. For those individuals without a reported onset date, we used the hospitalization date or date the swab was taken minus 3 days (the median interval from onset to swab). Inclusion of all controls at the start of the study, followed by age/period/region matching, led to relatively more controls at the start and also more controls in the older age groups and children younger than 5 years. Therefore, it was necessary to adjust for age and period in our analysis. The vaccine was introduced just after the peak of the second pandemic wave (Figure 1). This meant many cases and controls in the study had onset close to the time of vaccination. Figure 1 also shows that the proportion of PCR-negative individuals (controls) who were vaccinated followed the coverage in risk groups in the population as assessed by a national survey [14].
Figure 1.
Distribution by week and year of illness onset and proportion vaccinated for the PCR swab positives and negatives in the vaccine effectiveness analyses. Also shown is the cumulative pandemic (H1N1) 2009 vaccine coverage in risk groups in England by week of administration, and estimated distribution of cases* in the second wave by week of onset from week 35, 2009 to week 7, 2010. * Estimated from influenza-like illness reports from general practice data (Q surveillance) combined with pandemic (H1N1) 2009 positivity rates from routine swabbing of influenza-like illness cases.
A total of 31 cases and 76 controls had received at least 1 dose of pandemic (H1N1) 2009 vaccine ≥7 days before the onset date. Of these, no cases and 7 controls had received 2 doses ≥ 7 days before; 6 of these 7 were reported to be immunosuppressed. This gives a crude 2-dose vaccine effectiveness (VE) from ≥7 days of 100% (95% CI 32% to 100%). Because of the small numbers, we dropped from our multivariable analysis those individuals receiving 2 doses.
Logistic Regression Modeling Results
In the full multivariable model there was an increased odds of being a case if pregnant (odds ratio 2.85, 95% CI 1.81 to 4.49) and a nonsignificant increase if given seasonal influenza vaccine (odds ratio 1.29, 95% CI .99 to 1.68). The odds were decreased for those with chronic respiratory disease (odds ratio 0.66, 95% CI 0.50 to 0.90), those immunosuppressed by disease (odds ratio 0.62, 95% CI 0.46 to 0.83), and those immunosuppressed by treatment (odds ratio 0.66, 95% CI 0.50–0.88). In this full-model pandemic, (H1N1) 2009 vaccine showed a protective effect with an effectiveness estimate of 60% (95% CI 27% to 78%) from ≥14 days after vaccination (Table 2).
Table 2.
Adjusted Vaccine Effectiveness (VE) Estimates for a Single Dose of AS03 Adjuvanted Pandemic (H1N1) 2009 Vaccine by Age Group, Risk Group and Whether Admitted to Hospital
| Analysis (No. of Observations) | VE (95% CI) |
|
| Onset ≥ 7 days since vaccination | Onset ≥ 14 days since vaccination | |
| Full model (1758)a | 60% (33% to 75%) | 60% (27% to 78%) |
| Simple model (1872)b | 61% (38% to 75%) | 62% (33% to 78%) |
| Risk group | ||
| Immunosuppressant disease/therapyb (504) | 29% (-53% to 67%) | 33% (-76% to 75%) |
| Others (1368) | 68% (41% to 82%) | 65% (29% to 83%) |
| Age groupb | ||
| 0–9 (502) | 37% (-50% to 74%) | 77% (11% to 94%) |
| 10–24 (351) | 100% (85% to 100%)c | 100% (80% to 100%)c |
| 25–49 (527) | 61% (-9% to 86%) | 22% (-153% to 76%) |
| 50+ (492) | 52% (-18% to 80%) | 41% (-71% to 80%) |
| Hospitalizedb | ||
| No (383) | 52% (-47% to 84%) | 68% (-60% to 94%) |
| Yes (1246) | 45% (3% to 69%) | 42% (-14% to 70%) |
NOTE. aAdjusting for age, period, sex, pregnancy, seasonal vaccine, immunosuppressant disease, immunosuppressant treatment, and chronic respiratory disease.
Adjusting for age and period only.
No cases exposed, so unadjusted exact 95% CI calculated.
In the simpler model where variables were dropped if non-significant, or did not confound the pandemic (H1N1) 2009 vaccine effect, only period and age were retained. In this model the pandemic (H1N1) 2009 VE from ≥ 14 days post-vaccination was 62% (95% CI 33% to 78%), Table 2. Within the 7–13 day post-vaccination period a significant protective effect was also found with VE = 59% (95% CI 12% to 81%). When this period was combined with the ≥14 day period the VE from ≥7 days post-vaccination was 61% (95% CI 38% to 75%).
Our sub-analyses showed evidence that VE differed by age (P = .03) for the age-vaccine interaction. To increase precision, age groups were combined to <25 years and 25+ years. The VE from ≥ 7 days post vaccination was 73% (44% to 87%) in those aged <25 years compared to 50% (6% to 74%) in those aged 25+ years. VE from ≥ 14 days post-vaccination was 89% (66% to 96%) in those aged <25 compared to 27% (−54% to 65%) in those aged 25+ years. There were no significant interactions between vaccine and hospitalization (P = .29) or vaccine and immunosuppressant disease/therapy (P= .11).
In analyses restricted to hospitalized patients (Table 3) VE from 14 days was higher in those without immunosuppression (P = .02) and in those aged <25 (P=.02). Among those without immunosuppression, VE was 89% (47% to 98%) for <25 and −13% (−303% to 68%) for ≥ 25 year olds, similar to the overall estimates by age.
Table 3.
Vaccine Effectiveness (VE) Estimates for a Single Dose of AS03 Adjuvanted Pandemic (H1N1) 2009 Vaccine by Age Group and Risk Group in Hospitalized Patients
| Analysis (No. of Observations) | VE (95% CI) |
|
| Onset ≥ 7 days since vaccination | Onset ≥ 14 days since vaccination | |
| Full model (1181)a | 52% (12% to 74%) | 49% (-4% to 75%) |
| Simple model (1246)b | 45% (3% to 69%) | 42% (-14% to 70%) |
| Risk groupb | ||
| Immunosuppressant disease/therapy (278) | 20% (-107% to 69%) | -54% (-463% to 52%) |
| Others (968) | 49% (-6% to 75%) | 56% (-1% to 81%) |
| Age groupb | ||
| 0–24 (567) | 59% (1% to 83%) | 80% (32% to 94%) |
| 25+ (679) | 40% (-30% to 73%) | 1% (-156% to 62%) |
NOTE.aAdjusting for age, period, sex, pregnancy, seasonal vaccine, immunosuppressant disease, immunosuppressant treatment, and chronic respiratory disease.
Adjusting for age and period only.
DISCUSSION
Our study has shown that the pandemic (H1N1) 2009 strain split virion AS03 adjuvanted vaccine protects against pandemic (H1N1) 2009 infection in the high-risk groups targeted for immunization in the UK with significant protection (61%, 95% CI 38% to 75%) demonstrated from as early as 7 days after vaccination. For children younger than 10 years, as permitted under the licensed indication, the UK recommended a single 0.25 mL dose based on concerns about the high proportion with fever after a second 0.25 mL dose in a small manufacturer- sponsored study [5]. The effectiveness estimate for this age group from 14 days after vaccination was 77% (95 % CI 11% to 94%). This compares with a Cochrane review efficacy estimate of 59% (95% CI 41% to 71%) for unajuvanted seasonal influenza vaccines against confirmed influenza in healthy children [15]. The high effectiveness we found in young children was consistent with the limited immunogenicity data available for this vaccine. In a small manufacturer-sponsored study, 99% and 81% of children aged 6–35 months had a 4-fold rise in titer by hemagglutination inhibition (HI) or microneutralization assay respectively after a single dose [5]. Although serological correlates of protection for children have not been established, for the purposes of licensure of pandemic strain vaccine, correlates established in adults for seasonal influenza vaccines were adopted. Our data suggest that these putative serological correlates predict protection in young children.
In contrast, protection was poor in individuals aged 25+ years, with VE only 27% (−54% to 65%) in those aged 25+ years from ≥14 days after vaccination. The VE estimate in 25+ year olds compares unfavorably with that of unadjuvanted seasonal influenza vaccine in this age group against a matched strain. A recent Cochrane systematic review found an efficacy of 80% (95% CI 56% to 91%) for unadjuvanted, trivalent seasonal vaccine against virologically confirmed influenza in adults aged 16–64 years, with an efficacy of 50% (95% CI 27% to 65%) against an unmatched strain [16]. Unlike these earlier studies, ours was in individuals with high- risk chronic conditions, many of whom were admitted to a hospital. The high vaccine effectiveness we found in hospitalized patients under 25 years of age who had chronic conditions suggests that these factors per se are not the cause of the low effectiveness in older age groups. Although we did not have enough power to stratify VE estimates by individual clinical conditions, exclusion of immunocompromised patients did not alter the age-effect with the VE estimate among hospitalized patients, 89% (47% to 98%) for those aged <25 years and -13 % (−303% to 68%) for those aged 25+ years.
To date, the immunogenicity data available in adults for the AS03 adjuvant H5N1 and pandemic (H1N1) 2009 vaccines has been generated in healthy individuals and has shown at least 95% of those aged 18–60 years seroconverting by HI after a single 0.5 mL dose of the Pandemic (H1N1) 2009 vaccine with somewhat lower rates (∼80%) in those aged 60+ years [5]. Patients in our effectiveness study had comorbidities that may have had a negative impact on their immune response to vaccination but removal of those who were immunosuppressed did not materially affect the VE estimates. Because of their underlying clinical conditions, many patients in our study are likely to have had repeated doses of seasonal influenza vaccine in the past, because this has been the UK policy for over 20 years. Recent studies in Canada reported that receipt of 2008/9 influenza vaccine increased the risk of pandemic (H1N1) 2009 disease [17]. This suggests that prior seasonal influenza vaccination may have a negative effect on generation of protective antibody responses to the pandemic (H1N1) 2009 hemagglutinin. In our study, some evidence was found of an increased risk (odds ratio 1.29, 95% CI 0.99 – 1.69) for those who had received the 2009/10 seasonal influenza vaccine. We did not collect information on receipt of previous years' seasonal vaccine, but it is likely that since our study was in high-risk groups, receipt of vaccine in 2009/10 would be a predictor of vaccination in earlier years.
A negative effect of prior vaccination with an unadjuvanted influenza vaccine on the serological response to subsequent booster vaccination with a heterologous strain ASO3 adjuvanted vaccine has been reported for an H5N1 vaccine [18]. In this study, the HI response to 2 booster doses with ASO3 adjuvanted A/Indonesia/5/2005) vaccine in individuals primed with unadjuvanted H5N1 A/Vietnam/1194/2004 vaccine was significantly lower than in un-primed individuals. A negative effect of prior vaccination with unadjuvanted trivalent seasonal influenza vaccine on the response to an alum-adjuvanted H5N1 vaccine has also been reported in children under 10 years of age [19]. If the lower effectiveness of the ASO3 adjuvanted H1N1 vaccine in adults aged 25+ years is associated with a poorer serological response due to prior receipt of unadjuvanted heterologous A influenza strains in seasonal vaccines, then a possible explanation is the original antigenic sin (OAS) hypothesis. In this situation, new influenza strains evade surveillance when memory B cells reactive to previous strains dominate the serological response [20,21]. Such an effect would be more likely in older individuals with greater cumulative exposure to different influenza strains. This phenomenon may be the immunological basis of the reduced response to an adjuvanted heterologous pandemic strain vaccine seen after priming with an unadjuvanted vaccine. It does not seem to occur when unadjuvanted, trivalent subunit seasonal vaccines are given sequentially—at least not to the B influenza component of such vaccines [22].
For future pandemic vaccine development it is important to understand the immunological basis for poor protection with the ASO3 adjuvanted vaccine in older individuals. For this, immunogenicity data, including studies of the clonality and affinity of antibodies produced by the early antibody-secreting plasma cells and later memory B cells, would allow hypotheses about the immunological basis of poor protection from the adjuvanted H1N1 pandemic vaccine to be tested.
Ours was an observational study, not a randomised controlled trial. Therefore it is subject to bias. However, the test-negative design we employed has been used to estimate influenza vaccine effectiveness in a number of studies with robust and plausible results [10, 16, 23, 24, 25]. Our study's strength is that both cases and controls have presented with an influenza-like illness, and their true status is unknown at the time of swabbing. Lack of PCR assay sensitivity could lead to underestimation of effectiveness, because cases are classified as controls. This seems unlikely, however, because the HPA RT-PCR is highly sensitive, most samples were taken within 7 days, and we saw a high VE estimate of 89% from ≥ 14 days was obtained in those aged <25 years. Collection of information on comorbidities and pregnancy enabled adjustment for these factors. However, with the exception of pregnancy, other clinical conditions were no more common among cases than controls. A retrospective collection of vaccination history has the potential to introduce bias because it is done after the result is known. However, our study's vaccination status was based on dates of vaccination and batch numbers recorded in GP notes rather than patient recall, making biased reporting less likely.
In conclusion, our study has shown that one dose of AS03 adjuvanted pandemic (H1N1) 2009 vaccine is highly protective in children and young adults. Its results therefore support the putative serological correlates of protection used for licensure in this age group. Effectiveness in adults aged 25+ years was, however, disappointing. This finding merits further investigation to understand its immunological basis. It also highlights the need to conduct immunological studies in high-risk groups, including those who have had repeated exposure to unadjuvanted seasonal vaccines, as part of the dossier used to support licensure of pandemic strain vaccines in the future.
Funding
This study was funded by the Health Protection Agency.
Acknowledgments
We thank the many General Practitioners in England who completed the questionnaires and the HPA Regional Microbiology Network laboratories (St Bartholomew's and the London Trust, Birmingham, Cambridge, Leicester, University College London, Southampton, Leeds Manchester, Newcastle, Royal Free Hospital, Kings and Bristol) for providing information on the patients they tested. We are grateful to Joanna Ellis and Alison Bermingham for their scientific contribution in developing and validating the RT-PCR used in this study. We also thank Rashmi Malkani of the HPA Immunisation, Hepatitis and Blood Safety Department of the Centre for Infections for her dedicated administrative support.
References
- 1.World Health Organisation. Global pandemic influenza action plan to increase vaccine supply. Immunization, Vaccines and Biologicals. Epidemic and Pandemic Alert and Response. http://www.who.int/vaccines-documents/DocsPDF06/863.pdf Accessed 26 April 2010. [Google Scholar]
- 2.WHO Scientific Consultation. Options for the use of human H5N1 influenza vaccines and the WHO H5N1 vaccine stockpile. http://www.who.int/csr/resources/publications/WHO_HSE_EPR_GIP_2008_1/en/index.html. Accessed 26 April 2010. [Google Scholar]
- 3.El Sahly HM, Keitel WA. Pandemic H5N1 influenza vaccine development: an update. Expert Rev Vaccines. 2008;7:241–7. doi: 10.1586/14760584.7.2.241. [DOI] [PubMed] [Google Scholar]
- 4.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–08. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 5. Summary of Product Characteristics for Pandemrix™. Pandemic influenza vaccine (H1N1)v (split virion, inactivated, adjuvanted) last updated on the electronic Medicines Compendium 04/03/2010. http://www.medicines.org.uk/emc/medicine/22352/SPC/Pandemrix+suspension+and+emulsion+for+emulsion+for+injection/ Accessed 26 April 2010. [Google Scholar]
- 6.Summary of Product Characteristics for Celvapan. http://www.ema.europa.eu/humandocs/PDFs/EPAR/celvapan/emea-combined-h982en.pdf Accessed 26th April 2010. [Google Scholar]
- 7.Committee for Proprietary Medicinal Products (CPMP) Guideline on dossier structure and content for pandemic influenza vaccine marketing authorisation application. 5 April 2004. http://archives.who.int/prioritymeds/report/append/62EMEAguidelines.pdf. Accessed 26th April 2010. [Google Scholar]
- 8.Department of Health (DH) Chief Medical Officer's Update, http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Dearcolleagueletters/DH_107169 15-10-2009. Accessed 26 April 2010. [Google Scholar]
- 9.Department of Health (DH) Chief Medical Officer's Update, http://www.nelm.nhs.uk/en/NeLM-Area/News/2009–-December/16/CMO-letter-announcing-amendment-of-Pandemrix-license-to-allow-a-one-dose-schedule-in-children-/ Accessed 26 April 2010. [Google Scholar]
- 10.Fleming DM, Andrews NJ, Ellis JS, et al. Estimating Influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. doi: 10.1136/jech.2009.093450. doi:10.1136/jech.2009.093450. [DOI] [PubMed] [Google Scholar]
- 11.The Green Book. Immunisation against infectious disease. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_107754.pdf. Accessed 26 April 2010. [Google Scholar]
- 12.Curran MD, Ellis JS, Wreghitt TG, Zambon MC. Establishment of a UK National Influenza H5 Laboratory Network. J Med Micro. 2007;56:1263–1267. doi: 10.1099/jmm.0.47336-0. [DOI] [PubMed] [Google Scholar]
- 13.Ellis J, Iturriza M, Allen R, et al. Evaluation of four real-time PCR assays for detection of influenza A(H1N1)v viruses. Eurosurveillance. 2009;14 doi: 10.2807/ese.14.22.19230-en. [DOI] [PubMed] [Google Scholar]
- 14.Gates P, Noakes K, Begum F, Pebody R, Salisbury D. Collection of routine national seasonal influenza vaccine coverage data from GP practices in England using a web-based collection system. Vaccine. 2009;27:6669–77. doi: 10.1016/j.vaccine.2009.08.094. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008:CD004879. doi: 10.1002/14651858.CD004879.pub3. Review. PubMed PMID: 18425905. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007:CD001269. doi: 10.1002/14651858.CD001269.pub3. Review. [DOI] [PubMed] [Google Scholar]
- 17.Skowronski DM, De Serres G, Crowcroft NS, et al. Canadian SAVOIR Team. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010;7:e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–57. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Nolan T, Richmond PC, Formica NT, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 20.De St Fazekas S, Webster RG. Disquisitions on original antigenic Sin 1. Evidence in man. J Exp Med. 1966;124:331–45. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skowronski DM, Masaro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005-2006 season of dual A and B vaccine mismatch in Canada. Vaccine. 2007;25:2842–51. doi: 10.1016/j.vaccine.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Skowronski DM, De Serres G, Dickinson J, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006-2007. J Infect Dis. 2009;199:168–79. doi: 10.1086/595862. [DOI] [PubMed] [Google Scholar]
- 25.Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]