Abstract
Background. Our interest in immunological effects produced by vitamin D3 (1,25(OH)2D3) and its therapeutic potential prompted us to examine the role of 1,25(OH)2D3 on cytokine production by Candida albicans.
Methods. Peripheral blood mononuclear cells (PBMC) with stimulated C. albicans and 1,25(OH)2D3, cytokine concentrations were measured in supernatant. Quantitative polymerase chain reaction (qPCR) was performed for T cell transcription factors, SOCS1 and 3. TLR2/4, Dectin-1, and mannose receptor expression was studied using flow cytometry and qPCR. An ex-vivo stimulation study was carried out in healthy volunteers to investigate the seasonality of immune response to C. albicans.
Results. Upon in vitro C. albicans stimulation, 1,25(OH)2D3 induced a dose-dependent, down-regulation of IL-6, TNFα, IL-17, and IFNγ. It also increased IL-10 production. The shift in cytokine profile was not due to 1,25(OH)2D3 augmenting expression of either Thelper differentiation factors or SOCS1 and SOCS3 mRNA. 1,25(OH)2D3 inhibited TLR2, TLR4, Dectin-1, and MR mRNA and protein expression. In our seasonality study, both IL-17 and IFNγ levels were suppressed in summer when 25(OH)D3 levels were elevated.
Conclusion. Vitamin D3 skews cytokine responses toward an antiinflammatory profile, mediated by suppression of TLR2, TLR4, Dectin-1, and MR transcription, leading to reduced surface expression. The biological relevance of these effects has been confirmed by the seasonality of cytokine responses.
Beyond its classical role on bone metabolism, vitamin D3 has now been widely accepted as a potent modulator of immune function affecting various cell types. This view is based on several fundamental observations [1]. First, vitamin D receptors (VDR), through which biologically active 1,25(OH)2D3 acts, are present in most immune cells, including T lymphocytes, neutrophils, macrophages, and dendritic cells [1–4]. Second, T lymphocytes, macrophages, and dendritic cells are equipped with enzymes to metabolize 25(OH)D3 into 1,25(OH)2D3 [5–7]. Third, 1,25(OH)2D3 can influence both innate and acquired immune cell types. Vitamin D3 has been shown to increase the proliferation and maturation of monocytes to macrophages in vitro [8, 9]. It also suppresses T cell proliferation and differentiation [10]. 1,25(OH)2D3 is known to induce a more tolerogenic phenotype in dendritic cells [11]. In addition, it can skew T cell response toward a T helper (Th) 2 profile [7, 12].
The above immunomodulatory properties of vitamin D3 have fueled much interest on vitamin D3 potential as a therapeutic agent in infectious diseases. Most notably, the clinical course of tuberculosis (MTB) [13], as well as viral influenza and HIV infection, have been associated with vitamin D3 status [14, 15]. However, the capability of vitamin D3 to modulate the host immune response to fungal infections is still unknown. Invasive candidiasis remains a disease with significant mortality and morbidity in hospitalized patients [16]. Both innate and acquired immunity are essential to optimal defense against fungal infections. Despite the current range of available antifungal therapy, mortality rates from invasive candidiasis remains high. This is partly due to the host's inability to mount a successful immune response to the invading fungi.
We know that vitamin D3 is a powerful immunomodulator. However we do not know its potential impact on Candida albicans infection. We hypothesize that 1,25(OH)2D3 can modulate the innate immune response of human leukocytes challenged with C. albicans. We therefore surmise that the knowledge gained in this study will increase our understanding of the potential of vitamin D3 as an adjunct immunotherapeutic agent against invasive fungal infections. In addition, due to the seasonal human variability of vitamin D3 stores, we hoped to further validate our in vitro findings in a non-interventional, ex vivo setting by measuring cytokine response to C. albicans stimulation in a small cohort of healthy volunteers over a 1-year period.
MATERIALS AND METHODS
Micro-organisms.
Heat-killed C. albicans blastoconidia, a strain of American Type Culture Collection MYA-3573 (UC820), were used at a concentration of 105 micro-organisms/mL as previously described [17, 18].
Reagents.
Toll-like receptor (TLR)2 ligand lipopeptide (S)-(2,3-bis(palmitoyloxy)-(2RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser(S)-Lys4-OH, trihydrochloride (Pam3Cys), was purchased from EMC Microcollections (Tübingen, Germany). TLR4 ligand lipopolysaccharide (LPS; E. coli serotype 055:B5) was purchased from Sigma Chemical Co (St Louis, MO, USA). An extra purification step of LPS was performed as previously described [19]. Particulate β-glucan was a kind gift from Dr. David Williams (University of Tennessee) [20]. 1,25(OH)2D3 was purchased from Fluka Biochemika, Sigma-Aldrich (Missouri, USA) and dissolved in absolute ethanol.
Stimulation Assays.
Venous blood was drawn into EDTA tubes from healthy volunteers after informed consent. The study protocol was approved by institutional ethics committee. PBMC were isolated by density centrifugation on Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). Cells were washed twice in saline and counted. The cell counts were adjusted to 5x106 cells/mL. A 100 μL volume of PBMC, suspended in culture medium (RPMI 1640 DM; ICN Biomedicals, Costa Mesa, CA) and supplemented with 10 μg/mL gentamicin, 10 mM L-glutamine, 10 mM pyruvate, and 10% human pooled serum was added to flat-bottomed, 96-well plates (Greiner, The Netherlands).
PBMC were preincubated with 1,25(OH)2D3 (at the respective doses indicated) for 30 minutes, followed by addition of C. albicans at 105 micro-organisms/mL or RPMI (as unprimed control). This concentration of C. albicans has been predetermined in pilot experiments to yield optimal cytokine response. In the stimulation experiments using specific ligands, PBMC were incubated with the various stimuli individually (or in combination): TLR2 ligand (Pam3Cys 10 μg/mL), TLR4 ligand (LPS 1 ng/mL), Dectin-1 ligand (β-glucan 20 μg/mL), or combinations of β-glucan/Pam3Cys and β-glucan/LPS. Cell cultures were incubated in a 37°C, 95% humidity, 5% CO2 incubator. The culture supernatants were collected after 24 or 48 hours or 7 days of incubation, as appropriate, and stored at -20oC until cytokine assay.
Flow Cytometry.
Cells were phenotypically analyzed by 5-color flow cytometry (Coulter Cytomics FC 500, Beckman Coulter, Fullerton, USA) using Coulter Epics Expo 32 software. Cells were washed with PBS with 0.2% bovine serum albumin (BSA) before being labeled with fluorochrome-conjugated antibodies (mAb). After incubation for 20 minutes in the dark at room temperature, cells were washed twice to remove unbound antibodies and analyzed. For cell-surface staining, the following mAb were used: Dectin-1 receptor-PE (259931, R&D Systems, Minneapolis, MN), along with TLR2-FITC (TL2.1) and TLR4-PE (HTA125)—both from eBioscience, San Diego, CA—and mannose receptor (MR)-FITC (19.2, BD Bioscience, New York, NY).
Cytokine Measurements.
Interleukins (IL)-6, IL-1β, IL-10 and interferon (IFN)-γ concentrations were measured by commercial sandwich ELISA kits (Pelikine Compact, CLB, Amsterdam, The Netherlands) according to manufacturer instructions. Human tumor necrosis factor alpha (TNF-α) and IL-17 were measured by the appropriate commercial ELISA kits (R&D Systems, Minneapolis, MN). Detection limits were 8 pg/mL (IL-6 and IL-10), 20 pg/mL (IL-1β and IFN-γ), and 40 pg/mL (TNF-α and IL-17).
Quantitative PCR.
To determine mRNA expression, we extracted RNA from PBMC stimulated with C. albicans in both the presence and absence of 1,25(OH)2D3 for 24 hours. We extracted that RNA from 107 PBMC by using 1 mL TRIzol reagent (Sigma, St. Louis, MO). Subsequently, 200 μL chloroform and 500 μL 2-propanol (Merck, Darmstadt, Germany) were used to separate the RNA from DNA and proteins. Finally, after washing with 75% ethanol, we dissolved the dry RNA in 50 μL of diethylpyrocarbonate (DEPC) water. The amount and quality of mRNA were determined using a NanoDrop® ND-1000 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Complementary DNA was synthesized with a Superscript Reverse Transcriptase (Invitrogen).
Quantitative, real-time PCR was performed using the Bio-Rad iCycler and SYBR Green. The following primers were used (5’-3’):
AACCTCAGACAAAGCGTCAAATC (forward) and ACCAAGATCCAGAAGAGCCAA A (reverse) for TLR2, TTCCTTCAACCAAGAACATAGATC (forward) and TTGTTTCAATTTCACACCTGGATAA (reverse)
for TLR4, ACAATGCTGGCAACTGGGCT (forward) and GCCGAGAAAGGCCTATCCAAAA (reverse) for Dectin-1 receptor, TCAAGACAATCCACCAGTTACT (forward) and TTCTCTTTGCTGAAATAATACTGGTAGTC (reverse)
for MR, GCAGCCGACAATGCAGTCT (forward) and GAACGGAATGTGCGGAAGTG (reverse) for SOCS1, TGCGCCTCAAGACCTTCAG (forward) and GAGCTGTCGCGGATCAGAAA (reverse) for SOCS3 and
ATGAGTATGCCTGCCGTGTG (forward) and CCAAATGCGGCATCTTCAAAC (reverse) for β2 microglobulin (B2M) (Biolegio, The Netherlands) as housekeeping gene.
In another system, we quantified transcripts by real-time quantitative PCR on an ABIPRISM 7700 Sequence Detector using predesigned TaqMan GeneExpression Assays and reagents according to manufacturer instructions (Applied Biosystems, Foster City, CA). Probes with the following Applied Biosystems assay identification numbers were used: TBX21 (Tbet), Hs00203436_mL; GATA3, Hs00231122_mL; FOXP3, Hs00203958_mL; RORC1-2, Hs00172858_mL; RORC10-11, Hs01076112_mL, using human HPRT1 Endogenous Control (4333768T; Applied Biosystems) acted as housekeeping gene.
We validated all primers according to protocol. Mean relative mRNA expression was calculated using Pfaffl method. Values are expressed as ratio of fold increase to mRNA levels of vitamin D3-untreated cells.
Ex-vivo Study.
We recruited 15 healthy male volunteers from Radboud University Nijmegen Medical Centre, The Netherlands. Venous blood was drawn from all subjects on 4 occasions—in winter, spring, summer, and autumn of 2009. PBMC were isolated and stimulation assay was performed with C. albicans as discussed. This study has been approved by the local ethics committee. Informed consent was obtained from all volunteers.
Vitamin D3 Measurement.
Serum 25(OH)D3 levels were determined using high-performance liquid chromatography (HPLC) with UV detection, after prior extraction on small SepPak columns. Tritiated 25(OH)D3, collected from the HPLCsystem during passage of the UV peak, corrected for procedural losses.
Statistical Analysis.
Results from at least 5 experiments were pooled and analyzed using SPSS 16.0 statistical software. Data given as means + SEM and the Wilcoxon signed rank test compared differences between groups. Significance level was set at P < .05.
RESULTS
1,25(OH)2D3 Down-modulate Proinflammatory Cytokine Response to C. albicans
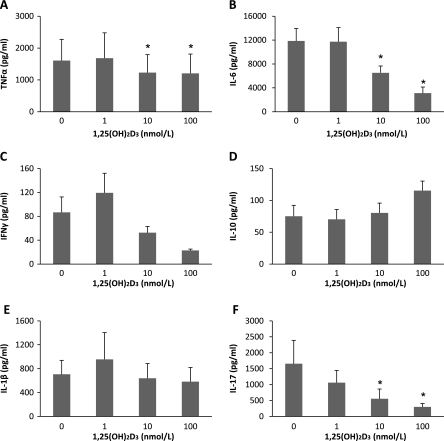
The influence of 1,25(OH)2D3 on immune response to C. albicans was assessed by stimulating PBMC in the presence of 1, 10, and 100 nmol/L of 1,25(OH)2D3. 1,25(OH)2D3 down-regulated TNFα secretion (25% drop) as well as IL-6 and IL-17 production (by more than 70%, in a dose-dependent manner (P < .05). IFNγ secretion was also attenuated by 1,25(OH)2D3. In contrast, IL-10 production is increased 1.5-fold (although this was statistically insignificant) (Figure 1). These observations suggested that 1,25(OH)2D3 has the capacity to modulate host inflammatory response to C. albicans toward an antiinflammatory response.
Figure 1.
1,25(OH)2D3 attenuates the cytokine response to Candida albicans. PBMC were incubated in the absence or presence of various 1,25(OH)2D3 concentrations ranging from 1 to 100 nmol/L and stimulated with 105 HK C. albicans. Supernatants were collected and analyzed for A) TNFα, B) IL-6, C) IFNγ, D) IL-10 and E) IL-1β at 24 h, and F) IL-17 at day 7. Data show results from 6 independent experiments performed with cells obtained from different donors. *P < .05 as compared to respective cell culture without the addition of 1,25(OH)2D3.
Modulatory Effects of 1,25(OH)2D3 Not Mediated Through SOCS or Skewing of T Cell Differentiation
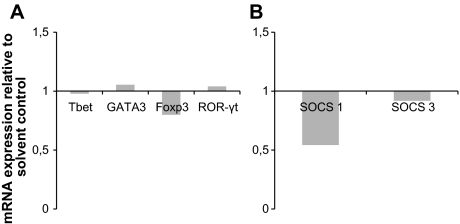
Two possible mechanisms through which 1,25(OH)2D3 could have attenuated proinflammatory response to C. albicans was through disruption of the suppressors of cytokine signaling (SOCS) pathway or a shift in T cell differentiation away from Th1 and Th17 toward Th2 or Treg profile. To assess the gene expression profiles of the various T cell transcription factors in response to 1,25(OH)2D3, we performed quantitative RT-PCR after stimulating PBMC with C. albicans. There were no significant changes in Tbet, GATA3, Foxp3, or ROR-γt mRNA expression (Figure 2A) 24 hours after cells were stimulated with C. albicans in the presence of 1,25(OH)2D3. We examined the effect of 1,25(OH)2D3 on SOCS1 and SOCS3 mRNA expression under the same conditions and found a moderate drop in SOCS1 at 4 hours (Figure 2B), but this cannot explain a decrease in the induction of proinflammatory cytokines.
Figure 2.
1,25(OH)2D3 does not act through modulation of Thelper differentiation or SOCS pathway. A) 1,25(OH)2D3 does not modulate of T-cell transcription factors. B) Modulation of SOCS1 and SOCS3 mRNA expression by 1,25(OH)2D3. Quantitative PCR for human Tbet, GATA3, Foxp3, ROR-γt, SOCS, and SOCS3 was conducted with PBMC and normalized to HPRT1 or β2M (for SOCS1 and 3) gene expression. Cells were incubated with 10 nmol/L 1,25(OH)2D3 or solvent for 24 h or 4 h (SOCS1 and 3). Data show results from 5 independent experiments and are expressed as fold change relative to solvent control.
Reduced TLR2 and TLR4 mRNA and Protein Expression by 1,25(OH)2D3
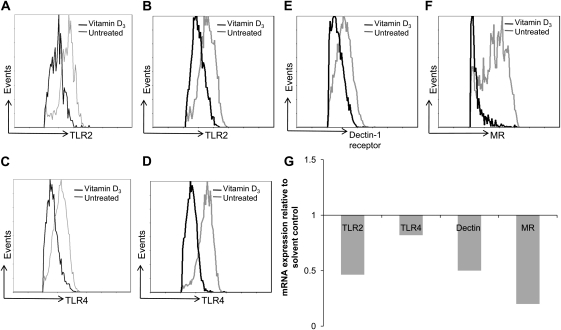
TLR2, TLR4, Dectin-1 and mannose receptors (MR) are well-established pattern recognition receptors (PRR) for detection of C. albicans. Therefore, we investigated whether 1,25(OH)2D3 could also modulate the expression of PRRs. In PBMC incubated with C. albicans, the expressions of both TLR2 and TLR4 were reduced by 1,25(OH)2D3 at 24 hours, 48 hours (Figure 3A-3D), and day 4 (not shown). In addition, TLR2 mRNA was decreased by 1,25(OH)2D3 after 24 hours incubation, while a less remarkable effect was apparent on the TLR4 expression (Figure 3G).
Figure 3.
1,25(OH)2D3 inhibits expression of TLR2, TLR4, Dectin-1 receptor and MR. Representative histogram overlay plots show reduced A and B) TLR2 (at 24 h and 48 h), C and D) TLR4 (at 24 h and 48 h), E) Dectin-1 (at 48 h) and F) MR (day 4) with and without 1,25(OH)2D3 treatment as assessed by flow cytometry. G) 1,25(OH)2D3 down-modulates TLR2, TLR4, Dectin-1 and MR mRNA expression. PBMC were incubated in the absence or presence of 100 nmol/L 1,25(OH)2D3 and stimulated with 105 HK Candida albicans. Flow cytometry data shown was performed at 24 h and 48 h for TLR2, TLR4; 48 h for Dectin-1; and day 4 for MR. Quantitative PCR for human TLR2, TLR4, Dectin-1, and MR (normalized to β2M gene expression) was performed at 24 h. Data represent 5 independent experiments performed with cells obtained from different donors.
1,25(OH)2D3 Diminished Dectin-1 and Mannose Receptors Expression at the Level of Transcription
The surface expression of Dectin-1 was inhibited by 1,25(OH)2D3 upon stimulation with C. albicans, and this was most evident at 48 hours (Figure 3E); data at 24 hours and day 4 is not shown. Down-regulation of mannose receptors (MR) surface expression by 1,25(OH)2D3 is clearly seen by day 4 (Figure 3F) even though MR protein expression on PBMC is usually only apparent after 72 hours of incubation. Likewise, the mRNA levels of both receptors were also reduced in the vitamin D3-treated cells (Figure 3G).
Functional Consequence of 1,25(OH)2D3-Induced Suppression of TLRs and C-Type Lectin Receptor Expression
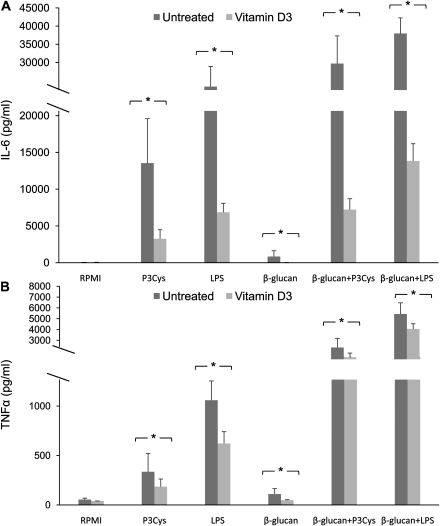
To validate our findings and to assess the functional consequences of vitamin D3 on TLRs and C-type lectin receptor (CLR) expression, we carried out stimulation using purified TLR2, TLR4, and Dectin-1 ligands in PBMC in the presence of 1,25(OH)2D3. A significant drop in IL-6 and TNFα secretion was observed in cells treated with 1,25(OH)2D3. In addition, we used β-glucan combined with Pam3Cys or LPS to test the effect of 1,25(OH)2D3 on synergy between TLR2 or TLR4 and Dectin-1. Notably, we found reduced synergistic effects mediated by Dectin-1 when 1,25(OH)2D3 was added to the culture (Figure 4).
Figure 4.
1,25(OH)2D3 inhibited proinflammatory cytokines induced by purified TLR/Dectin-1 ligands. PBMC were incubated in the presence or absence of 100 nmol/L 1,25(OH)2D3 and stimulated with 10 μg/mL Pam3Cys, 2 ng/mL LPS, 20 μg/mL β-glucan, and β-glucan in combination with Pam3Cys or LPS. Supernatants were collected and analyzed for A) IL-6 and B) TNFα at 24h. Data show results from five independent experiments performed with cells obtained from different donors. *P < .05 as compared to respective cell culture without the addition of 1,25(OH)2D3.
Increased In vivo 1,25(OH)2D3 Led to Attenuated Inflammatory Response to C. albicans Ex-vivo in Human Volunteers
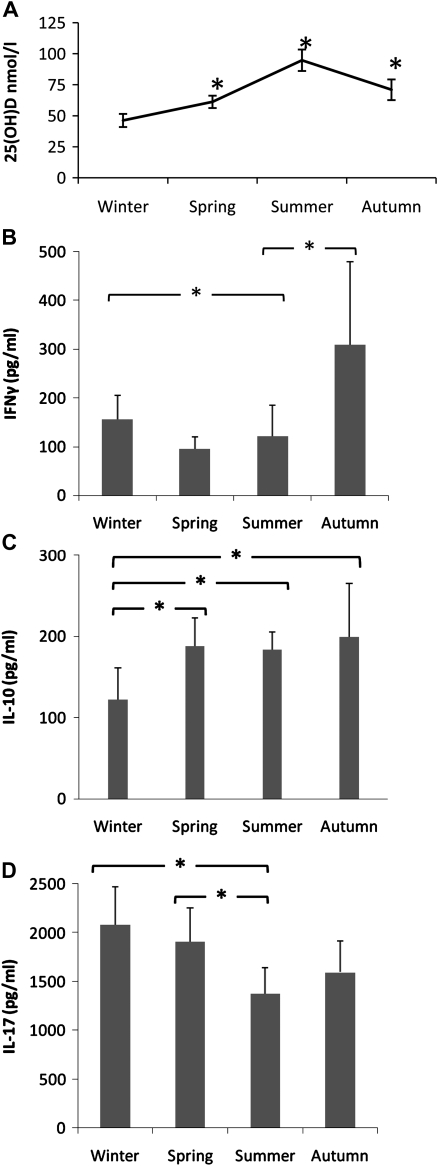
To validate our in vitro findings, we performed an ex vivo study on 15 healthy male volunteers (median age 36, range 28–60 years old). We examined how physiological seasonal variation in sun exposure (through winter, spring, summer, and autumn) could influence host innate immunity against C. albicans infections. PBMC from subjects isolated during each season of the year were stimulated with C. albicans. It was of great interest to us that we observed a significant drop in IFNγ and IL-17 secretion in spring and summer months, while the IL-10 levels were higher in summer as compared to winter (Figure 5B-5D). These observations correlated with the serum 25(OH)D3 concentrations, which doubled in summer compared to winter (Figure 5A). Trends in IL-6 and TNFα production, however, displayed wider variation and were not statistically significant (data not shown).
Figure 5.
Seasonal variation of host immune response to ex-vivo Candida albicans stimulation. A) Mean serum 25(OH)D3 concentrations of 15 healthy volunteers at different seasons of the year *P < .05 as compared to winter. PBMC were stimulated with 105 HK C. albicans and supernatants collected and analyzed for B) IFNγ, C) IL-10, and D) IL-17 at day 7. Data show results from 15 subjects at the different seasonal time points.
DISCUSSION
Despite its well-known immunomodulatory effects, nothing to date has been discovered about the effects of vitamin D on the innate immune response to Candida. We demonstrate in this study that 1,25(OH)2D3 can significantly modulate host proinflammatory and Th17 response to C. albicans, both in vitro and ex vivo. This biologically active component of vitamin D3 exerted its effect through modulation of the expression of PRR, recognizing fungi—both TLR2 and TLR4—and C-type lectin receptors, Dectin-1 and MR. Until now, the effect of 1,25(OH)2D3 on CLR has not been recognized.
The constitutive expression of the VDR on human immune cells, including monocytes, macrophages, and lymphocytes, has been well-established [2–4]. This is a prerequisite for vitamin D3 to exert any immunomodulatory effect. We have shown that 1,25(OH)2D3 has a distinct propensity to skew host response to C. albicans away from a ”proinflammatory” profile through attenuation of TNF-α, IL-6, IFN-γ as well as production of IL-17. Other researchers have reported inhibition of Th1 cytokine production with 1,25(OH)2D3, although not in the setting of Candida infection and polarization toward a Th2-type response [21–23]. In our system using human PBMC stimulated by C. albicans, we also observed an increase in IL-10 production in vitamin D presence. We initially hypothesized that this IL-10 might originate from skewing toward Th2 differentiation as seen elsewhere [24, 25] or from the potentiation of regulatory T cell function [26, 27]. However , we were unable to demonstrate any significant shift in the Thelper or Treg transcription factors—(Tbet, GATA3, Foxp3 or ROR-γt) mRNA expression —in our Candida-stimulation model under the influence of 1,25(OH)2D3. Our observation that 1,25(OH)2D3 had the characteristic capability to suppress IL-6 production, and to some extent, IFN-γ also led us to investigate whether the underlying mechanism was due to novel 1,25(OH)2D3 activity on the respective SOCS3 and SOCS1 regulatory pathways [28]. However, we found no evidence of direct or indirect 1,25(OH)2D3-related interference on SOCS regulatory function. Stimulation of SOCS1 and SOCS3 mRNA would have been an anticipated response following the suppression of proinflammatory cytokine production by 1,25(OH)2D3. Slight drops in SOCS1 mRNA expression is a consequence of decreased TLR 2/4 and Dectin-1 signaling rather than the direct effect of 1,25(OH)2D3.
Another mechanism that could account for our observation is vitamin D3-mediated suppression of PRR expression, followed by modulation of response by host immune cells to Candida. 1,25(OH)2D3 indeed diminished surface expression of TLR2 and TLR4, which on follow-up investigation was attributable to its effect on inhibition of upstream TLR2 and TLR4 mRNA transcription. A similar observation had also been reported by Sadeghi et al [29] in the setting of bacterial cell-wall components. In addition, 1,25(OH)2D3 had further influence on functioning of other PRRs not previously recognized. Besides TLR2 and TLR4, the C-type lectin receptors Dectin-1 and MR are known to play pivotal roles in triggering the immune signaling cascade upon activation by C. albicans [30].We found that 1,25(OH)2D3 activity also exerted inhibition of upstream Dectin-1 and MR protein synthesis. This latter finding was especially significant relative to that of the TLRs, because the extent of 1,25(OH)2D3-induced inhibition of Dectin-1 and MR functioning was suggestively more pronounced than on TLR2 and TLR4. We have also recently reported that Candida recognition by CLRs such as Dectin-1 and MR is a more constant feature of antifungal innate immunity than recognition by TLRs [31].
Furthermore, we ascertained the functional consequences of synergistic effects resulted from 1,25(OH)2D3-related suppression of TLR2, TLR4, and Dectin-1 in the setting of C. albicans infection. This is important because it has been well-described that ”cross-talk” between the TLR2/4 and Dectin-1 is responsible for the synergy of proinflammatory response mounted by the host [32–34]. Our use of pure TLR2, TLR4, and Dectin-1 ligands, as well as TLR2/Dectin-1 and TLR4/Dectin-1 ligand combination, illustrated the profound influence that 1,25(OH)2D3 exerted beyond its effect on the individual PRR. The results demonstrated how use of 1,25(OH)2D3 has also led to ablation of the TLR-Dectin-1 synergy, which in the setting of C. albicans infection especially, would be very significant. Resultant suppression of MR function most likely further contributes to attenuation of host IL-17 response to Candida [35]. Hence, the net effect of 1,25(OH)2D3 is a critical shift away from the proinflammatory response, which would have been anticipated upon C. albicans challenge.
We know that production of the vitamin D3 precursor in the skin depends on UV radiation exposure. A seasonal variation in vitamin D3 status in temperate climates is widely accepted [36]. To validate our in vitro findings, we carried out an ex vivo study in 15 healthy subjects to investigate the variation in cytokine response to C. albicans through the year’s 4 seasons. Compared to winter, during spring and summer months IFN-γ and IL-17 response to Candida is significantly reduced. This correlates inversely with serum 25(OH)D3 levels. Conversely, IL-10 production is higher in summer. Interleukin-6 and TNFα trends were found to be less consistent. This might be attributed to other factors inducing wider biological variation in the ex vivo study. Such factors might be UV radiation exposure and the levels of vitamin D3 necessary to effect a modulation in these cytokine responses. It has been suggested that a higher vitamin D level (>100 nmol/L) may be required to sustain immune function [37]. Therefore, we ought to be mindful that while certain cytokine trends in the seasonality study correlate with our in vitro work, the physiological levels of vitamin D3 required to comprehensively attenuate the immune responses to Candida infection remain to be determined. Nevertheless, our study is the first clear demonstration of seasonal variation in innate immune responses to an important human pathogen.
Research has been ongoing to study the potential role of vitamin D as a therapeutic agent [38]. It has been proposed that in invasive fungal infections such as candidiasis and aspergillosis, disease pathology may be attributable to an exaggerated or dysregulated host inflammatory response that results in undue tissue damage [39, 40]. While a proinflammatory-type immune response will be desirable at disease onset, an unnecessarily prolonged hyperinflammatory phenotype may be averse to outcome. Likewise, Th1, and especially Th17, responses are known to exacerbate and often cause autoimmune phenomena [41, 42]. Hence, given the immunomodulatory profile of 1,25(OH)2D3, it is tempting to speculate that in specific clinical settings, 1,25(OH)2D3 may have a role in modulating and regulating any unnecessary and prolonged inflammatory response generated by the host. Also, the significant effect of seasonal variation on Thelper responses makes it tempting to speculate whether vaccination programs during winter months may be more efficient than those during the summer period. Although speculative at the moment, research on this subject is certainly needed. An encouraging answer would have far-reaching implications for health policy.
In conclusion, we have shown that 1,25(OH)2D3 plays a novel role for the modulation of innate host response to C. albicans, by modifying the proinflammatory cytokine responses both in vitro and ex vivo. By exerting its influence on almost all the major PRRs involved in Candida recognition and immune signaling, namely TLR2, TLR4, Dectin-, and MR, its antiinflammatory effect is profound in Candida infection. Seasonal variation of cytokine responses seems notably important. This opens up a new area of clinical research. We expect that the epidemiological and clinical consequences of our findings could be significant. Future studies are warranted.
Funding
This work is supported by The Nutricia Research Foundation, The Netherlands.
L.C. was supported by the Health Manpower Development Plan (HMDP) Fellowship, Ministry of Health, Singapore and the International Fellowship, Agency for Science, Technology and Research (A*STAR)/National Medical Research Council (NMRC), Singapore.
M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research.
Acknowledgments
The authors are grateful to Prof. Fred Sweep and Mr. André Brandt for performing the assay on vitamin D levels. The authors are also thankful to Dr. Xuehui He for her technical assistance.
References
- 1.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 4.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 5.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 7.van EE, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457–61. [PubMed] [Google Scholar]
- 9.Ohta M, Okabe T, Ozawa K, Urabe A, Takaku F. 1 alpha,25-Dihydroxyvitamin D3 (calcitriol) stimulates proliferation of human circulating monocytes in vitro. FEBS Lett. 1985;185:9–13. doi: 10.1016/0014-5793(85)80730-4. [DOI] [PubMed] [Google Scholar]
- 10.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–55. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penna G, Amuchastegui S, Giarratana N, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 13.Fielding J, Maloney JJ. Calciferol, streptomycin, and para-aminosalicylic acid in pulmonary tuberculosis. Lancet. 1951;2:614–7. doi: 10.1016/s0140-6736(51)92889-9. [DOI] [PubMed] [Google Scholar]
- 14.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–71. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DL, McNamee RB, Jones EL, et al. A method for the solubilization of a (1–--3)-beta-D-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res. 1991;219:203–13. doi: 10.1016/0008-6215(91)89052-h. [DOI] [PubMed] [Google Scholar]
- 21.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 22.Overbergh L, Decallonne B, Waer M, et al. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49:1301–07. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 23.Imazeki I, Matsuzaki J, Tsuji K, Nishimura T. Immunomodulating effect of vitamin D3 derivatives on type-1 cellular immunity. Biomed Res. 2006;27:1–9. doi: 10.2220/biomedres.27.1. [DOI] [PubMed] [Google Scholar]
- 24.Ardizzone S, Cassinotti A, Trabattoni D, et al. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol. 2009;22:63–71. doi: 10.1177/039463200902200108. [DOI] [PubMed] [Google Scholar]
- 25.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–34. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolders J, Thewissen M, Peelen E, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 30.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Gow NA, Joosten LA, Verschueren I, van der Meer JW, Kullberg BJ. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol. 2010;49(7):897–903. doi: 10.3109/13693781003621575. [DOI] [PubMed] [Google Scholar]
- 32.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–66. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 34.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van dV, Marijnissen RJ, Kullberg BJ, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host. Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Vieth R. What is the optimal vitamin D status for health? Prog Biophys Mol Biol. 2006;92:26–32. doi: 10.1016/j.pbiomolbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89–90:575–9. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 39.Miceli MH, Maertens J, Buve K, et al. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: Proof of principle, description, and clinical and research implications. Cancer; 2007;110:112–20. doi: 10.1002/cncr.22738. [DOI] [PubMed] [Google Scholar]
- 40.Zelante T, De LA, D'Angelo C, Moretti S, Romani L. IL-17/Th17 in anti-fungal immunity: what's new? Eur J Immunol. 2009;39:645–8. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 41.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann NY Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]