Abstract
Alveolar bone loss associated with periodontal diseases is the result of osteoclastogenesis induced by bacterial pathogens. The mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP- 1) is a critical negative regulator of immune response as a key phosphatase capable of dephosphorylating activated MAPKs. In this study, rat macrophages transduced with recombinant adenovirus (Ad).MKP-1 specifically dephosphorylated activated MAPKs induced by lipopolysaccharide (LPS) compared with control cells. Bone marrow macrophages from MKP-1 knockout (KO) mice exhibited higher interleukin (IL)-6, IL-10, tumor necrosis factor (TNF)-α, and select chemokine compared with wild type (WT) mice when stimulated by LPS. In addition, bone marrow cultures from MKP-1 KO mice exhibited significantly more osteoclastogenesis induced by LPS compared with WT mice. Importantly, MKP-1 gene transfer in bone marrow cells of MKP-1 KO mice significantly decreased IL-6, IL-10, TNF-α, chemokine levels and formed fewer osteoclasts induced by LPS compared with control group of cells. Furthermore, MKP-1 gene transfer in an experimental periodontal disease model attenuated bone resorption induced by LPS. Histological analysis confirmed that periodontal tissues transduced with Ad.MKP-1 exhibited less infiltrated inflammatory cells, less osteoclasts and less IL-6 compared with rats of control groups. These studies indicate that MKP-1 is a key therapeutic target to control of inflammation-induced bone loss.
Keywords: MKP-1, cytokine, osteoclastogenesis, periodontal disease
INTRODUCTION
Periodontitis is a chronic inflammatory disease that is characterized by loss of the periodontal ligament and alveolar bone, and is a major cause of tooth loss. The bone loss associated with periodontal diseases is the most prevalent disease of the bone in human, ranging from 10 to 15% of adult 1,2. It has long been appreciated that periodontal bone loss results from inflammation-driven process associated with enhanced osteoclastogenesis 3. Osteoclasts, derived from hematopoietic monocyte/macrophage cell lineage 4, undergo cellular differentiation and maturation in response to many cytokines and hormones 5–7. Bacterial infection initiates innate immune responses that generate proinflammatory mediators, such as IL-1 8,9, IL-6 10, TNF-α 9, and prostaglandin E2 9, which are capable of stimulating osteoclastogenesis directly or indirectly leading to bone resorption.
The production of inflammatory cytokines is the consequence of activation key intracellular pathways, including MAPKs pathways 11,12 following activation of toll-like receptor (TLR) complexes that recognize bacterial constituents. MAPKs consist of three major families, including extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAPK. MAPK pathways relay, amplify and integrate signals, which modulate a series of physiological response including cellular proliferation, differentiation, development, inflammatory responses and apoptosis 13.
Although the activation of MAPK pathways is critical to initiate an innate immune response to help mount a protective immune response to eliminate invading pathogens, sustained production of proinflammatory cytokines can result in bone resorption. A number of studies have demonstrated that LPS from gram-negative bacteria was capable of inducing bone resorption in vivo 14–18. Therefore, modulating MAPK immune response to an appropriate level is essential to attenuate bone resorption associated with bacterial infection.
In mammalian cells, MAPKs are activated by phospohorylation on critical tyrosine, serine or threonine residues. Inactivation of activated MAPKs occurs via dephosphorylation by MKPs, a group of dual-specificity protein phosphatases (DUSP)19. To date, at least 11 MKP family members have been identified in mammalian cells, with MKP-1 being the founding member. Studies have shown that MKP-1 KO mice are susceptible to endotoxic shock; exhibited a marked increase in production of proinflammamtory cytokine TNF-α, IL-6, and an anti-inflammatory cytokine IL-10 as compared with WT animals 20. MKP-1 KO mice also had a marked increase in both the incidence and severity of experimentally induced autoimmune arthritis 21,22, and exhibited more bone loss after LPS challenge as compared with WT animals 23. Using an orally-active p38 MAPK inhibitor, we have shown p38 signaling is critical to LPS-induced inflammatory response, osteoclastogenesis, and alveolar bone loss 24. These results highlight the significance of the p38/MKP-1 axis of regulation on innate immune response to bacterial infections and maintaining bone homeostasis, suggesting that restriction of activated MAPKs is a potential therapeutic strategy for diseases associated with exaggerated MAPK responses.
In this study, we evaluated the therapeutic effects of local expression of MKP-1 in regulation of immune response and bone homeostasis following LPS challenge. We demonstrated that local adenoviral delivered MKP-1 restrained the activation of MAPKs, reduced proinflammatory/anti-inflammatory cytokine responses, reduced osteoclastogenesis and in vivo bone loss associated with experimental periodontitis. Data obtained from these studies suggest MKP-1 is a potential therapeutic target for diseases associated with increased MAPK activation.
RESULTS
MKP-1 specifically dephosphorylates MAPKs after LPS stimulation
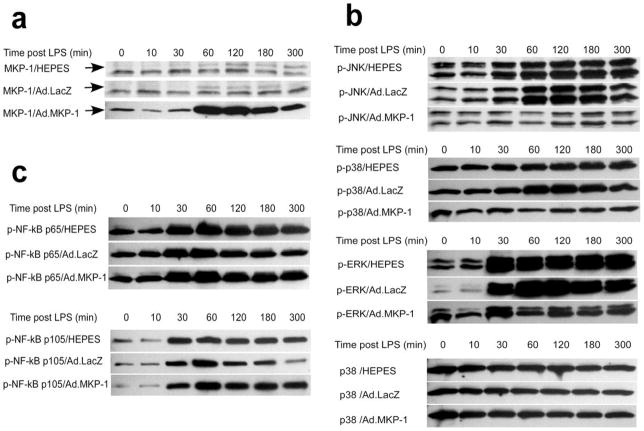
MKP-1 dephosphorylates critical tyrosine and serine/threonine residues of activated MAPKs, including p38 MAPK, ERK, and JNK 25. First, to test the efficacy of MKP-1 expression by Ad.MKP-1 transduction, rat macrophages (NR8383 cells) were transduced with Ad.MKP-1 or control vector, Ad.LacZ, at multiplicity of infection (moi) from 100 to 900 for 24 to 72 hours. Optimal MKP-1 protein expression was achieved at moi 300 for 48 hours (data not shown). The LacZ gene expression was confirmed by β-galactosidase staining in cells (data not shown). Following these preliminary experiments, rat macrophages were transduced either with Ad.MKP-1 or Ad.LacZ (moi 300), or treated with equal volume of HEPES buffered saline. Forty-eight hours after the adenovirus transduction, the cells were either unstimulated or stimulated with 1μg/ml of LPS for various time periods (10, 30, 60, 120, 180, and 300 minutes). The levels of MKP-1 protein expression in transduced cells were evaluated by western blot protein assay. As shown in Figure 1a, rat macrophages transduced with Ad.MKP-1 exhibited only mild level of MKP-1 protein expression (arrow shown, 39kda) before LPS stimulation. However, the level of MKP-1 protein expression significantly increased 60 to 300 minutes following LPS stimulation peaking from 60 min to 120 min. In contrast, cells transduced with Ad.LacZ or treated with HEPES buffered saline had undetectable level of MKP-1 protein expression at basal level. When incubated with a higher concentration MKP-1 antibody, cells transduced with Ad.LacZ or treated with HEPES buffered saline only displayed a slight increase of MKP-1 expression 60 to 300 minutes (arrow shown, peaking from 60 to 120 minutes) following LPS stimulation. The protein bands under the 39kda MKP-1 protein are non-specific protein bands due to incubation with a higher concentration of a polyclonal antibody.
Figure 1.
MKP-1 gene transfer specifically dephosphorylated MAPKs in rat macrophages after LPS stimulation. Rat macrophages (NR8383 cells) were transduced with Ad.MKP-1 (moi 300), or Ad.LacZ (moi 300), or treated with equal volume of HEPES buffered saline for 48 hours. Cells were either unstimulated or stimulated with 1μg/ml of LPS (from A. actinomycetemcomitans) for various time periods (10, 30, 60, 120, 180, and 300 minutes). The MKP-1, p-JNK (p54, p46), p-p38 MAPK, p-ERK (p44, p42), p-38 MAPK, and p-NF-κB (p65, p105) protein expression were examined by western blot assays. The p-38 MAPK served as a loading control. (a) MKP-1 protein expression in rat macrophages. (b) Phosphorylated MAPKs protein expression in rat macrophages. (c) Phosphorylated NF-κB p65 and p105 protein expression in rat macrophages. The data is a representative from three separate experiments.
To investigate the effects of over-expression of MKP-1 on activated MAPKs, and NF-κB activity, phosphorylated (p)-MAPKs and p-NF-κB p65/p105 were analyzed by western blot protein assay. As shown in Figure 1b, there was significant induction of p-JNK (p54, p46), p-p38, and p-ERK (p44, p42) protein 60 to 300 minutes after LPS stimulation in cells either treated with HEPES buffered saline, or transduced with Ad.LacZ. Whereas in macrophages transduced with Ad.MKP-1, p-JNK (p54, p46), and p-p38 protein expression remained close to the basal levels following LPS stimulation. Although there was an increase level of p-ERK (p44, p42) expression in cells transduced with Ad.MKP-1 30 minutes after LPS stimulation, p-ERK (p44, p42) expression maintained at relatively lower level 60 to 300 minutes after LPS stimulation. This decrease of p-ERK (p44, p42) 60 to 300 minutes after LPS stimulation correlated with the increase levels of MKP-1 protein in cells transduced with Ad.MKP-1 60 minutes following LPS stimulation. In contrast to p-MAPKs, over-expression of MKP-1 by Ad.MKP-1 failed to dephosphorylate NF-κB p65 and p105 protein post LPS stimulation as demonstrated in Figure 1c. These data demonstrated that over-expression of MKP-1 specifically inhibited LPS-stimulated MAPKs activities and this effect last at least 5 hours following LPS stimulation.
MKP-1 gene transfer rescued the innate immune response in MKP-1 KO mice
Previous study has demonstrated that MKP-1 KO mice had compromised immune response, which exhibited enhanced production of IL-6, TNF-α, IL-10 after LPS challenge compared with MKP-1 WT mice 21. Studies in our lab also revealed that MKP-1 KO mice exhibited more alveolar bone loss after LPS challenge compared with MKP-1 WT mice 23. Therefore, a rescue experiment was conducted to determine the therapeutic effects of gene transfer of MKP-1 in modulating immune response and potentially restoring bone homeostasis after LPS challenge in MKP-1 deficient mice.
Since bone marrow macrophages (BMMφ) are the primary cells that both generate inflammatory cytokines in an autocrine manner differentiate into osteoclasts, we first analyzed the cytokine profile to verify if MKP-1 gene transfer in MKP-1 KO mice could correct their compromised immune response induced by LPS. Primary BMMφ cells from MKP-1 KO mice and WT mice were transduced by either Ad.MKP-1 or Ad.LacZ (moi 300) for 48 hours. The cells were stimulated with LPS (100 ng/ml) for 24 hours and cell culture supernatants were analyzed for IL-6, IL-10, TNF-α, and chemokine (C-X-C motif) ligand 1(CXCL1) levels by ELISA. As shown in Figure 2, bone marrow macrophages from MKP-1 KO mice displayed about 2-fold higher level of IL-6, 3-fold higher level of TNF-α and IL-10, and 4-fold higher level of CXCL1 following LPS stimulation compared with the cytokine/chemokine levels in WT mice. Importantly, BMMφ cells from MKP-1 KO mice treated with Ad.MKP-1 exhibited a 2-fold decrease of IL-6, 2.7-fold decrease of TNF-α, 1.6-fold decrease of IL-10 and 2.4-fold decrease of chemokine levels compared with the cytokine levels in cells treated with Ad.LacZ (n=4, *p<0.05, **p<0.01, ***p<0.001). In addition, the cytokine levels in bone marrow macrophages from MKP-1 KO mice treated with Ad.MKP-1 reduced to the similar cytokine levels as seen in cells from WT mice treated with Ad.LacZ.
Figure 2.
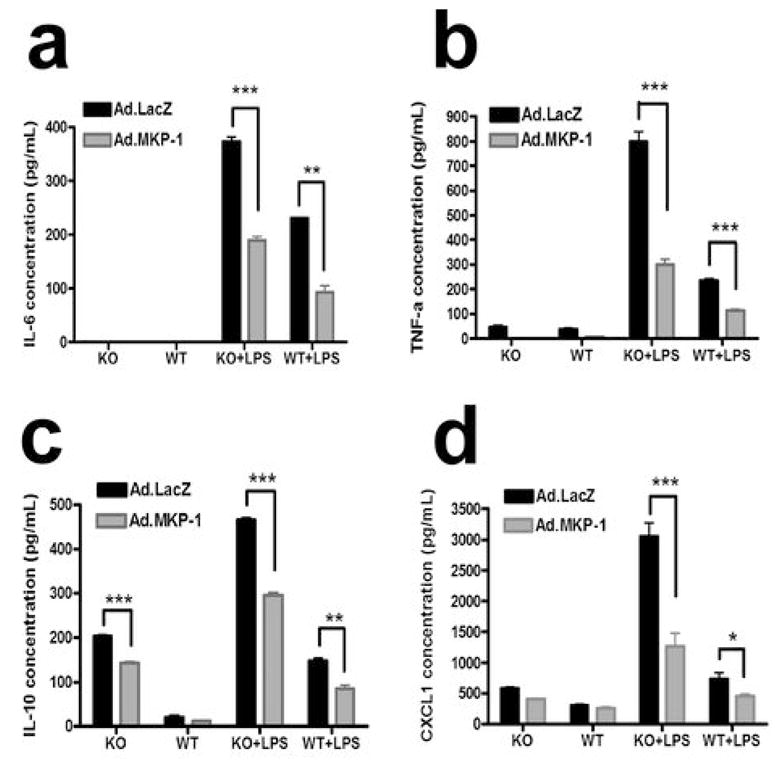
MKP-1 gene transfer corrected the defective immune responses seen in macrophages from MKP-1 KO mice after LPS stimulation. Bone marrow macrophages from MKP-1 KO mice or WT mice were transduced with Ad.MKP-1 or control Ad.LacZ (moi=300) for 48 hours, cells were stimulated with 100ng/ml of LPS (from A. actinomycetemcomitans) for 24 hours. Cytokine expression in supernatant was evaluated by ELISA assay and was normalized by the protein concentration in cell lysates. The data represent the average of three separate experiments. (a) IL-6 expression in mouse bone marrow macrophages (n=4, **p<0.01, ***p<0.001). (b) TNF- α expression in mouse bone marrow macrophages (n=4, ***p<0.001). (c) IL-10 expression in mouse bone marrow macrophages (n=4, **p<0.01, ***p<0.001). (d) CXCL1 expression in mouse bone marrow macrophages (n=4, *p<0.05, ***p<0.001).
To further explore the potential therapeutic effects of gene transfer of MKP-1 in bone homeostasis, the bone marrow cells from MKP-1 KO mice and MKP-1 WT mice were first transduced with either Ad.MKP-1 or control Ad.LacZ (moi 25) for 48 hours, then the cells were subsequently incubated with cell culture media containing M-CSF (50 ng/ml) +/− LPS (1 mg/ml) for additional 8 days. Osteoclastogenesis was measured in vitro as multinucleated (≥3 nuclei) osteoclasts that stained positive for tartrate resistant acid phosphatase (TRAP). Figure 3a showed that unstimulated cell cultures from both MKP-1 KO mice and WT mice failed to produce osteoclasts. In contrast, with LPS stimulation, there were significantly more multinucleated osteoclasts (arrows shown) in bone marrow cells from MKP-1 KO mice compared with cells from MKP-1 WT mice. Importantly, total bone marrow cultures treated with Ad.MKP-1 exhibited significant smaller and fewer numbers of TRAP+ osteoclasts in LPS containing cultures compared with control cells transduced with Ad.LacZ. The number of osteoclasts was quantified in each well. As shown in Figure 3b, there was a significant decrease number of mature osteoclasts in bone marrow cells from MKP-1 KO mice treated with Ad.MKP-1 compared with cells treated with Ad.LacZ (Figure. 3b, n=4, **p<0.01, ***p<0.001). We also noticed a decreased number of osteoclasts in bone marrow cells from MKP-1 KO mice treated with Ad.LacZ. However, this effect did not reach significant differences (p>0.05). Overall, these data demonstrate gene transfer of MKP-1 can modulate immune response and inhibit osteoclastogenesis following LPS challenge.
Figure 3.

MKP-1 gene transfer inhibited osteoclasts formation in bone marrow (BM) cells from MKP-1 KO mice after LPS stimulation. BM cells were extracted from either eight-week-old MKP-1 KO mice or WT mice. Cells were transduced with Ad.MKP-1 or control Ad.LacZ (moi 25) for 48 hours and then incubated with MEM-alpha medium containing 10% FBS, 2 mM glutamine, 50ng/ml recombinant mouse M-CSF (R&D systems) with or without LPS (1μg/ml, from A. actinomycetemcomitans) for 8 days. Osteoclasts were stained by tartrate resistant acid phosphatase (TRAP) staining. (a) Multinucleated osteoclasts (arrows shown) in bone marrow cells from MKP-1 KO mice and WT mice. (b) Number of osteoclasts (more than three nuclei) per well (96-well plate) in bone marrow cells from MKP-1 KO mice and WT mice (n=4, **p<0.01, ***p<0.001).
Local MKP-1 gene transfer alleviated inflammatory bone resorption
To test the therapeutic effect of MKP-1 gene transfer in normal animals under LPS stimulation, we initially needed to determine if gene expression adenoviral gene expression persisted during the experimental protocol. To optimize the dose of adenoviral delivery, eight-week-old of male Sprague-Dawley rats were injected with 4 μl of either 1×109 plague-forming unit (pfu) or 5×108 pfu of Ad.LacZ at palatal gingival tissues (21 rats/group). LacZ trans-gene expression from a single microinjection in gingival tissues was evaluated by β-galactosidase staining in frozen tissue sections 3, 21, and 28 days after adenoviral gene delivery (n=7/group). Figure 4 illustrates that there was prominent amount of LacZ transgene expression at day 3 following Ad.LacZ injection. The LacZ gene expression was still detectable at day 21 and 28 days following adenoviral delivery. These data are consistent with our previous reports26 and suggest that gene expression by local intraoral adenoviral delivery can persist over a long period. To evaluate the effects of local MKP-1 gene transfer in an experimental model of periodontitis, eight-week-old male Sprague-Dawley rats were either injected with 4 μl of either 1×109 pfu of Ad.MKP-1 (n=17), or control Ad.LacZ (n=17), or HEPES buffered saline (n=17) at interproximal palatal regions between the maxilla first and second molars. Forty-eight hours after the local adenoviral gene delivery, rats were injected with either 2 μl of PBS (n=7) or 2 μl/20 μg of LPS (n=10) three times a week for four weeks as previously established by our lab group24,26,27. The rats were sacrificed at the end of 4 weeks following LPS injection and the bone loss in the maxilla was evaluated by micro-computed tomography (μCT). As shown in Figure 5, rats transduced with Ad.MKP-1 exhibited significantly reduced bone resorption after LPS stimulation compared with rats treated with Ad.LacZ or HEPES control (n=10, *p<0.05). Although there was a trend showing that rats injected with Ad.LacZ reduced bone loss compared with rats injected with HEPES buffered saline, there was no statistical significant difference (p>0.05) between those two groups.
Figure 4.
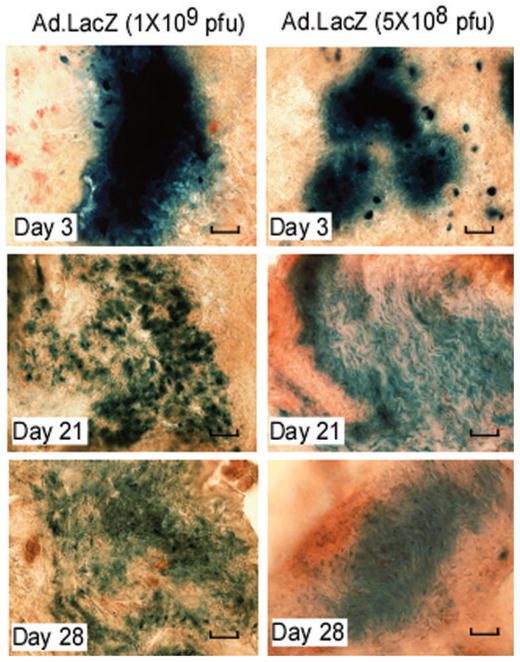
LacZ gene expression in rat gingival tissues after Ad.LacZ injection. Ad.LacZ (1×109 pfu, or 5×108 pfu in 4 μl) was injected in the gingival tissues of eight-week-old of male Sprague-Dawley rats (21 rats/group). Gingival tissues were harvested at 3, 21, and 28 days after Ad.LacZ injection (7 rats/group). LacZ gene expression was evaluated by β-galactosidase staining in frozen tissue sections. The scale bars represent 200 μm. Pictures represent average LacZ gene expression in each group.
Figure 5.
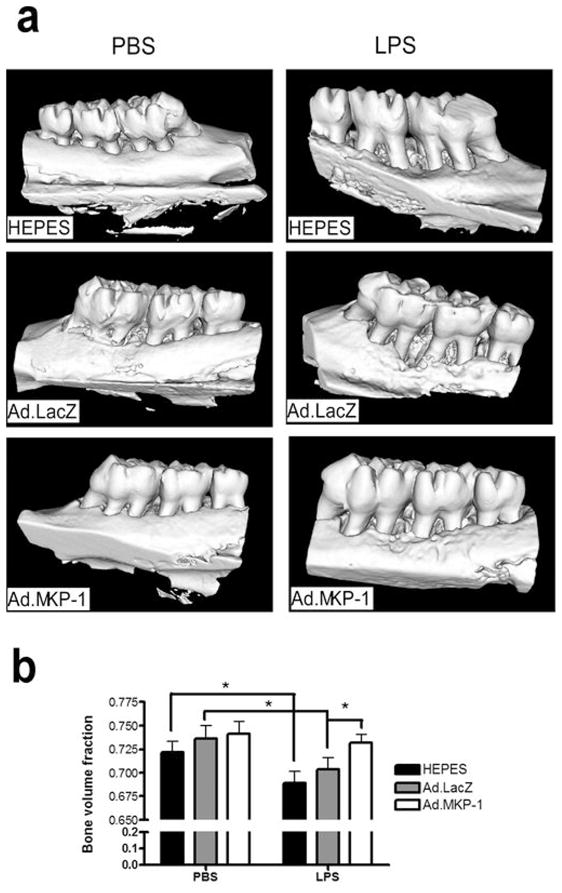
MKP-1 gene transfer alleviated bone resorption in rats after LPS challenge. Eight-week-old of male Sprague-Dawley rats (17 rats/group) were injected either Ad.MKP-1, or Ad. LacZ (1×109 pfu in 4 μl), or HEPES buffered saline (4 μl). Forty-eight hours after the adenovirus injection, the rats were injected with 2μl of either 20 μg of LPS (from A. actinomycetemcomitans) or PBS three times a week for four weeks. (a) Representative microcomputed tomography images of rat maxillae from indicated treatment groups. (b) Volumic analysis of bone loss levels (n=7 for PBS groups, n=10 for LPS groups, *p<0.05).
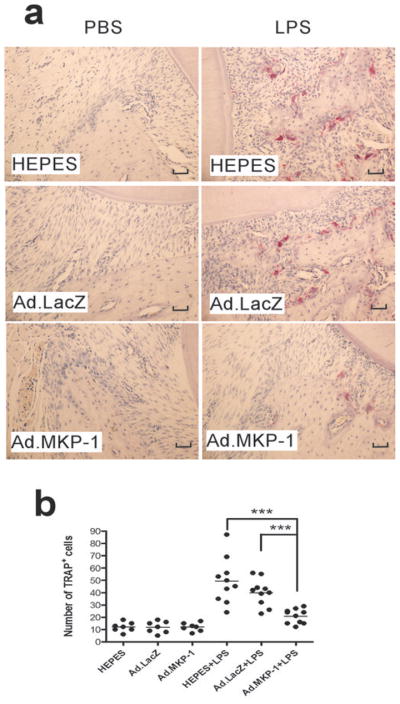
Histological analysis revealed that there were no significant inflammatory cells and few multinucleated osteoclasts on the alveolar bone surface in the periodontal tissues injected with PBS (Figure 6a). In contrast, there were significantly more inflammatory cells (neutrophils, lymphocytes, and macrophages), more fibroblasts, and more multinucleated osteoclasts (arrows shown) presented in the periodontal tissues injected with LPS (Figure 6b). Importantly, Ad.MKP-1 transduced rats showed significantly reduced the amount of inflammatory cells and multinucleated osteoclasts near the alveolar bone surface compared with rats injected with HEPES buffered saline or Ad.LacZ after LPS stimulation. The multinucleated osteoclasts were confirmed by TRAP staining (Figure 7a), where there were significantly fewer TRAP-positive osteoclasts on alveolar bone surfaces in periodontal tissues from rats injected with Ad.MKP-1 compared with the number of osteoclasts seen from rats injected with HEPES buffered saline or Ad.LacZ after LPS stimulation. Quantification of TRAP-positive osteoclasts confirmed that there was a significant reduction of the number of osteoclasts after LPS stimulation in Ad.MKP-1 group compared with control groups (Figure 7b, p<0.001). Immunohistological staining revealed that MKP-1 was present in the periodontal tissues of rats injected with Ad.MKP-1, whereas MKP-1 was undetectable in control groups of rats (Figure 8a). Furthermore, IL-6 expression was significantly decreased in periodontal tissues from rats injected with Ad.MKP-1 compared with IL-6 levels in rats injected with HEPES buffered saline or Ad.LacZ (Figure 8a). Rats treated with Ad.MKP-1 exhibited lower score of IL-6 expression (Figure 8b) compared with control groups of rats.
Figure 6.
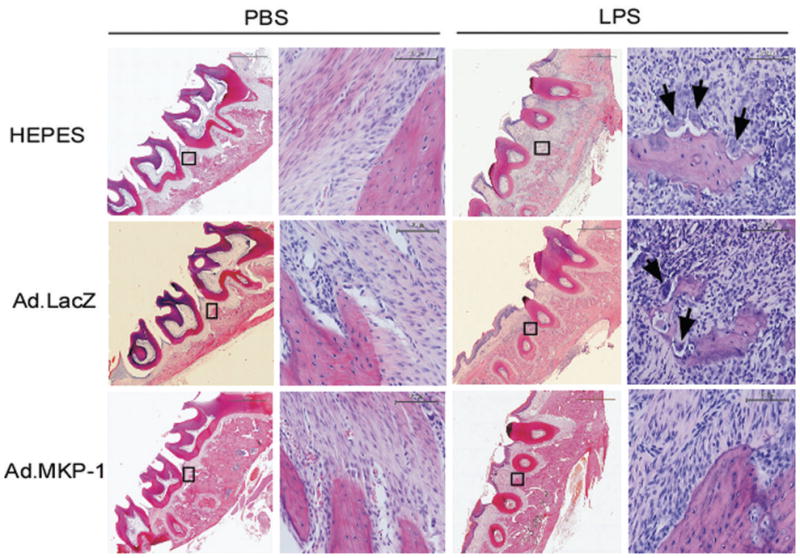
MKP-1 gene transfer reduced inflammatory infiltration and osteoclastogenesis in rats after LPS challenge. Rat tissue sections (7 μm) were stained by hematoxylin & Eosin (H.E.). Histological appearance of lower magnification (40 x) and higher magnification (400 x) of periodontal tissues from rat maxillae injected with PBS or LPS. Arrows show multinucleated osteoclasts near the surface of alveolar bone. Scale bars represent 1500 μm for lower magnification (40 x), and 90 μm for higher magnification (400 x).
Figure 7.
MKP-1 gene transfer attenuated osteoclastogenesis induced by LPS. Rat tissue sections (7 μm) were stained for tartate-resistant acid phosphatase (TRAP). (a) TRAP staining of representative tissue sections of rat maxillae from indicated treatment groups. TRAP-positive (TRAP+) osteoclasts were stained red. The scale bars represent 200 μm. (b) Number of TRAP+ cells in each tissue section. Horizontal bar indicates mean TRAP+ cell counts (n=7 for PBS groups, n=10 for LPS groups, *** p<0.001).
Figure 8.

MKP-1 gene transfer decreased IL-6 expression in rat tissues after LPS challenge. Rat tissue sections (7 μm) were immunohistochemically stained for MKP-1 and IL-6. (a) MKP-1 and IL-6 expression of representative tissue sections of rat maxillae from indicated treatment groups. The scale bars represent 200 μm. (b) Histological scores of IL-6 expression in tissue sections. (n=7 for PBS groups, n=10 for LPS groups).
DISCUSSION
Bone homeostasis is maintained through a process of coupled bone turnover where bone formation via osteoblasts is balanced with bone resorptive osteoclasts 28,29,30. Osteoclasts are the primary bone-resorbing cells, which play a pivotal role in creating bone marrow activity for hematopoiesis, and maintaining bone homeostasis 29,31. Systemic hormones and cytokines provide the molecular cues that control osteoclastogenesis and thus maintain homeostasis 30. Within the bone microenvironment, T and B lymphocytes, bone marrow stromal cells, macrophages, and osteoblasts all can produce cytokines, which impact osteoclastogenesis30. Since activation of MAPKs is critical for production of proinflammatory cytokines and osteoclastogenesis, dephosphorylaing activated MAPKs by MKP-1 is a critical target to control inflammatory diseases associated with exuberant cytokine production and bone resorption. In this proof-of-concept study, we demonstrated that local MKP-1 gene transfer by adenoviral gene delivery reduced inflammatory mediators and inflammatory bone loss in vivo. Ad.MKP-1 inhibited exaggerated MAPK responses where cells transduced with Ad.MKP-1 specifically dephosphorylated activated JNK, ERK, and p38 MAPK after LPS stimulation, but not activated NF-κB signaling pathway. Ad.MKP-1 attenuated proinflammatory cytokine IL-6, TNF-α, CXCL1, as well as an anti-inflammatory cytokine IL-10 response after LPS stimulation. MKP-1 gene delivery corrected the compromised immune responses seen in MKP-1 KO mice compared with MKP-1 WT mice. In addition, Ad.MKP-1 reduced osteoclastogenesis in bone marrow cells of MKP-1 KO mice compared controls. Furthermore, Ad.MKP-1 attenuated bone resorption in rats post LPS stimulation compared with bone loss in rats of control groups.
A previous study in Raw 264.7 cells has shown that LPS induced a moderate level of MKP-1 expression 60 to 120 minutes following LPS stimulation, which dephosphorylated p-JNK and p-p38 effectively 32. In this study, endogenous induction of MKP-1 by LPS in rat macrophages was very weak as shown in Figure 1a. The level of MKP-1 peaked around 60 to 120 minutes following LPS stimulation when incubated with a higher concentration of MKP-1 antibody. Apparently, weak induction of MKP-1 was not potent to dephosphorylate p-JNK, p-p38 and p-ERK as shown in Figure 1b. In contrast, macrophages transduced with Ad.MKP-1 had significant higher level of MKP-1 expression 60 minutes following LPS stimulation. The over-expression of MKP-1 significantly inhibited the p-JNK and p-p38 expression, and to a less extent p-ERK expression 60 minutes following LPS stimulation. This effect lasted at least 5 hour following LPS stimulation. These data demonstrate the potential therapeutic effect of over-expression of MKP-1 in regulation of activated MAPKs both at magnitude and duration levels. The discrepancy between these studies might account for the different cell lines, different strains of LPS, and the different dose of LPS that used in those studies.
Many inflammatory cytokines play a synergic role in osteoclastogenesis including IL-1 8,9, IL-6 10, TNF-α 9, and prostaglandin E2 9 which have been all been reported to stimulate osteoclastic differentiation and bone resorption. Chemokines are chemotactic signals for monocytes that can facilitate the fusion of monocytes into multi-nucleated osteoclast 33. Therefore, elevated levels of proinflammatory cytokine and chemokines are osteolytic factors are associated with inflammatory bone loss. In this study, bone marrow cells from MKP-1 KO mice displayed significant higher levels of IL-6, TNF-α, and chemokine compared with those levels in MKP-1 WT mice (Figure 2). This observation was correlated with more osteoclasts seen in bone marrow cells of MKP-1 KO mice compared with osteoclasts from WT mice in response to LPS stimuli (Figure 3). In addition, this increase osteoclastogenesis in bone marrow cells from MKP-1 KO mice after LPS stimulation is in line with our previous finding that MKP-1 KO mice displayed more bone loss after LPS challenge compared with WT mice 23.
In this study, bone marrow cells from MKP-1 KO mice transduced with Ad.MKP-1 significantly decreased of IL-6, TNF-α, IL-10 and CXCL1 levels compared with cells transduced with Ad.LacZ. Our observation demonstrated that MKP-1 plays a key role in negatively regulation of IL-6, TNF-α, IL-10, and CXCL1 expression. This observation was in line with a previous study by Zhao et al. 20 showing that MKP-1 KO mice produced significantly higher levels of IL-6, TNF-α and IL-10 than WT mice after LPS challenge. By down-regulation of osteolytic pro-inflammatory cytokine and chemokine expression, delivery of Ad.MKP-1 significantly reduced the number of osteoclasts induced by LPS stimulation. Our study emphasized the important role of MKP-1 in maintaining bone homeostasis, suggesting the potential therapeutic effects of MKP-1 gene transfer in diseases associated with inflammatory bone loss. A recent study showed that MKP-1 KO mice had equivalent bone loss, but fewer osteoclasts compared with WT mice after ovariectomy 34. Instead they showed that osteoclasts from MKP-1 KO mice had higher resorptive activity than osteoclasts from WT mice. Their observation is contradictory to our result that MKP-1 KO mice have more osteoclasts compared with WT mice. The discrepancy among those studies might account for the ovariectomy, which is not a strong stimulator of immune response. A previous study conducted by Li et al. 35 revealed that p38 MAPK signals are required for osteoclast differentiation, but not for osteoclastic function. They demonstrated that p38 MAPK inhibitor SB203580 only inhibited osteoclast differentiation, but not affect the survival of osteoclasts or dentine-resorption activity induced by RANKL.
Our lab previous showed that inhibiting p38 MAPK suppressed oral bone loss after LPS challenge in rats 24. Other investigators 36 also confirmed the therapeutic role of p38 MAPK inhibitor in inflammatory arthritis. They showed that systemic administration of two different p38 MAPK inhibitors significantly alleviated the TNF-α-mediated inflammatory bone destruction in arthritis. Since MKP-1 not only regulates p38 MAPK, but also JNK and ERK activity, over-expression of MKP-1 has more advantages in restraining an overwhelming immune response and osteoclastogenesis in response to stimuli compared with p38 MAPK inhibitors. By local gene transfer of MKP-1, this study demonstrated that MKP-1 is a key factor in maintaining immune and bone homeostasis, MKP-1 gene transfer is a potential therapy in preventing inflammatory bone loss.
It is noteworthy that MKP-1 gene transfer in unstimulated cells only displayed a low level of MKP-1 protein expression. The MKP-1 protein expression was significantly increased 60 min after LPS stimulation. MKP-1 gene transfer did not raise the basal level of proinflammatory cytokine (IL-6, TNF-α), or anti-inflammatory cytokine IL-10, or chemokine levels. MKP-1 gene transfer only restrained an exaggerated immune response after LPS stimulation. Moreover, MKP-1 gene transfer not only restrained proinflamamtory cytokines (IL-6, TNF-α), and chemokine, but also inhibited an anti-inflammatory cytokine IL-10 after stress stimulation. These data specify the important role of MKP-1 in maintaining immune homeostasis. MKP-1 gene is only activated upon stress stimuli, which plays a critical role in restraining an exaggerated immune response and maintaining immune homeostasis after stress stimuli.
Recombinant adenovirus may offer an advantage for local intraoral usage since the target gene can be expressed over a relatively long period of time compared with chemical drugs, or protein based drugs, which can be degraded quickly. In a previous study in oral delivery of an adenoviral vector carrying a luciferase-tagged gene 26, we demonstrated that luciferase activity persisted over 21 days after the adenoviral vector delivery. In this study, we demonstrated that the LacZ gene expression last 28 days following Ad.LacZ single injection. In this study, we noticed a trend of less number of osteoclasts and less bone loss in cells and animals treated with Ad.LacZ after stimulated by LPS, although it is not statistically significant different. This observation might be attributed to the property of adenovirus in inhibiting cell growth, as we noticed less cell number/and less protein concentration with increasing amount of adenovirus in cells.
In summary, exuberant MAPK response is associated with increasing production of inflammatory cytokines, which leads to inflammatory bone loss. MKP-1 gene transfer by recombinant adenovirus is a potential strategy for inflammatory bone loss and for those diseases associated with exuberant MAPK responses.
MATERIALS AND METHODS
Cells and Lipopolysaccharide (LPS)
Rat macrophage NR8383 cell line (ATCC CRL-2192) was obtained from American Type Cell Collection (ATCC, Manassas, VA, USA). NR8383 cells were cultured with Ham’s F12K medium (ATCC), supplemented with 15% heat inactivated fetal bovine serum (FBS), 100 IU/ml penicillin, and100μg/ml streptomycin in a 37°C incubator with 5% CO2. The human embryonic kidney (HEK) 293A cell line was purchased from Invitrogen (Carlsbad, CA, USA). 293A cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with high glucose, supplemented with 10% cosmic calf serum, 100 IU/ml penicillin, and 100μg/ml streptomycin in a 37°C incubator with 5% CO2. LPS from A. actinomycetemcomitans (strain Y4, serotype B) was extracted by the hot phenol-water method as described37 and diluted in PBS.
Adenovirus
Ad5-CMV-MKP-1 phosphatase, which expressing the full-length of MKP-1 gene under the cytomegalovirus (CMV) promoter, and control Ad5-CMV-LacZ were obtained from Seven Hills Bioreagents (Cincinnati, Ohio, USA). Adenovirus were propagated in HEK 293A cells, purified by cesium chloride density gradient ultracentrifugation method, and desalted by PD-10 column (GE Healthcare, Pittsburgh, PA, USA) in HEPES buffered saline.
Western blot analysis
Protein was extracted from NR8383 cells 48 hours after adenovirus transduction with/or without LPS stimulation by cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Total protein (30 μg) was loaded on 10% Tris-HCl Ready Gel (Bio-Rad Laboratories, Hercules, CA, USA) and electrotransferred to nitrocellulose membranes, blocked, and then incubated overnight at 4 °C with primary antibodies. The primary antibody for MKP-1 (M-18) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The primary antibody of p-p38 MAPK; p-SAPK/JNK; p-p44/42 MAPK; p38 MAPK; and p-NF-κB p65 were purchased from Cell Signaling Technology. The presence of the primary antibodies was detected on radiographic film by using HRP-conjugated secondary antibodies (Cell Signaling Technology) and SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). Digitalized images of the radiographic films were obtained in a gel documentation system (Bio-Rad Laboratories).
ELISA
Mouse IL-6, IL-10, TNF-α, and chemokine (C-X-C motif) ligand 1 (CXCL1) were measured in culture medium by ELISA kits purchased from R & D systems (Minneapolis, MN, USA). The protein concentration in cell lysates was determined by DC protein Assay Kit (Bio-Rad Laboratories). The concentration of cytokines was normalized by protein concentrations in cell lysates.
Animals
MKP-1 knock out (KO) mice and wild type (WT) mice were provided by Bristol-Myers Squibb Pharmaceutical Research Institute and bred at Medical University of South Carolina. These mice are maintained on a mixed C57/129 background. Eight-week-old male Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA, USA) with food and tap water ad libitum. All animal-related work was performed in accordance with NIH guidelines. The Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina approved all experimental protocols. For adenoviral gene expression experiment, 8-week-old male Sprague-Dawley rats were injected with 4 μl of either 1×109 plague-forming unit (pfu) dose (21 rats/group) or 5×108 pfu dose (21 rats/group) of Ad.LacZ at palatal gingival tissues. The rats were sacrificed at 3, 21, or 28 days following adenovirus delivery. For bone resorption experiment, 8-week-old male Sprague-Dawley rats were injected with 4 μl of either 1×109 pfu of Ad.MKP-1 (n=17) or control Ad.LacZ (n=17), or HEPES buffered saline (n=17) at interproximal palatal regions between the maxilla first and second molars. Forty-eight hours after delivery of adenovirus, the rats were injected with 2 μl of PBS (n=7) or 2 μl/20 μg of LPS (n=10) three times a week for four weeks. The rats were sacrificed at the end of 4 weeks following LPS injection.
Bone marrow extraction & macrophage separation
Bone marrow cells were harvested from 8-week-old MKP-1 KO/WT mice. The femurs and tibias were flushed with Minimal Essential Alpha Medium (MEM-alpha; Invitrogen) The bone marrow cells were grown in MEM-alpha medium containing 10% FBS, 2 mM glutamine, 50 ng/ml recombinant murine granulocyte/macrophage colony stimulating factor (GM/CSF) (R&D systems), 100 IU/ml penicillin and 100 μg/ml of streptomycin. Cells were incubated at 37°C in 5% CO2 overnight. Non-adherent cells were collected. CD11b positive cells were magnetically selected using CD11b MicroBeads by an autoMACS™ Separator (Miltenyi Biotec, Auburn, CA, USA). Subsequently, the CD11b positive cells were grown in DMEM medium containing 10% FBS, 2 mM glutamine, 50 ng/ml recombinant murine M-CSF (R&D systems, Minneapolis, MN), 100 U/ml penicillin and 100 μg/ml of streptomycin for additional 7 days.
β-galactosidase staining in tissue sections
Thirty μm of tissue sections were fixed with 0.5% glutaraldehyde in PBS for 15 minutes at room temperature. After washing with PBS twice, the tissue sections were stained in staining solution at 37 °C overnight. The staining solution contains 1 mg/ml X-gal (Sigma Aldrich, St. Louis, MO, USA), 2 mM MgCl2, 5mM potassium hexacyanoferrate (Sigma Aldrich) and 5 mM potassium hexacyanoferrate trihydrate (Sigma Aldrich). After washing with PBS, the sections were counterstained with nuclear fast red solution (Sigma Aldrich) at room temperature for 3 minutes. Finally the slides were washed in water and mounted with aqueous mounting medium.
Microcomputed tomography (μ-CT)
Non-demineralized rat maxillae (n=10 per group) were scanned by a cone beam μ-CT system (GE Healthcare BioSciences, Chalfont St. Giles, UK) as previously described 24. Each scan was reconstructed at a mesh size of 18×18×18 μm, and three-dimensional digitized images were generated for each specimen. Using GEHC MicroView software (version viz.+2.0build 0029), the images were rotated into a standard orientation and threshold to distinguish between mineralized and non-mineralized tissue. For each specimen, a grayscale voxel value histogram was generated to determine an optimal threshold value. Volumic alveolar bone losses expressed as bone volume fraction (BVF) were measured as previously described 24.
Hematoxylin & eosin staining and immunohistological staining in tissue sections
Formalin-fixed specimens were decalcified in a 10% EDTA solution for 2 weeks at 4°C. The EDTA solution was changed three times per week. The maxillas were paraffin-embedded, and 7 μm sagittal sections were prepared. Some sections were stained with hematoxylin and eosin (HE) for descriptive histology.
For immunohistological staining, tissue sections were deparaffinized in xylene and rehydrated in graded ethanol. After antigen retrieval in citric buffer at 100 °C for 10 minutes, the slides were incubated with 3% H2O2 for 10 minutes to quench endogenous peroxidase activity. After washing and incubation with block serum for 30 minutes at room temperature, the sections were incubated with anti-MKP-1 (M-18 1:200, Santa Cruz Biotechnology), or with anti-rat IL-6 (1:800, R & D systems) at 4°C overnight. IL-6 expression was detected using a VECTASTAIN Universal ABC kit (Vector Laboratories Inc, Burlingame, CA, USA) following manufacturer’s instruction. Finally, the sections were counterstained with hematoxylin; dehydrated in graded ethanol; and covered with coverslips in mounting media. The control sections were incubated with preimmune serum to assess background staining. Histological slides were evaluated and graded by an experienced pathologist.
Tartate-resistant acid phosphatase (TRAP) staining
TRAP staining was performed in cultured bone marrow cells and tissue sections using a leukocyte acid phosphatase kit (Sigma Aldrich). The tissue sections were counterstained with hematoxylin after TRAP staining. Active osteoclasts were defined as multinucleated TRAP-positive cells in contact with bone surface.
Statistical Analysis
Data were analyzed by Student’s t test with Welch’s correction for unequal variances. All statistical tests were performed using a GraphPad Prism software (GraphPad Software Inc, La Jolla CA, USA). P values of 0.05 or less were considered significant.
Acknowledgments
This study is supported by NIH 1R01DE018290 and 2P20 RR017696. This study was conducted in a facility constructed with support from the NIH C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
The authors would like to acknowledge the continued support of the University of Michigan Orthopedic Research Labs and the Muscloskeletal Core for the micro-computed tomography.
Footnotes
Conflict of interest
The authors declare no conflict interest.
References
- 1.Brown LJ, Loe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000. 1993;2:57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 2.Pilot T, Miyazaki H. Periodontal conditions in Europe. J Clin Periodontol. 1991;18:353–357. doi: 10.1111/j.1600-051x.1991.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 3.Hall TJ, Chambers TJ. Molecular aspects of osteoclast function. Inflamm Res. 1996;45:1–9. doi: 10.1007/BF02263497. [DOI] [PubMed] [Google Scholar]
- 4.Reddy SV. Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr. 2004;14:255–270. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.20. [DOI] [PubMed] [Google Scholar]
- 5.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 7.Mundy GR. Inflammatory mediators and the destruction of bone. J Periodontal Res. 1991;26:213–217. doi: 10.1111/j.1600-0765.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanazawa S, Amano S, Nakada K, Ohmori Y, Miyoshi T, Hirose K, et al. Biological characterization of interleukin-1-like cytokine produced by cultured bone cells from newborn mouse calvaria. Calcif Tissue Int. 1987;41:31–37. doi: 10.1007/BF02555128. [DOI] [PubMed] [Google Scholar]
- 9.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002;17:1211–1218. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- 10.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 11.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 12.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. Epub 2006 Nov 1217. [DOI] [PubMed] [Google Scholar]
- 13.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 14.Orcel P, Feuga M, Bielakoff J, De Vernejoul MC. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol. 1993;264:E391–397. doi: 10.1152/ajpendo.1993.264.3.E391. [DOI] [PubMed] [Google Scholar]
- 15.Nishida E, Hara Y, Kaneko T, Ikeda Y, Ukai T, Kato I. Bone resorption and local interleukin-1alpha and interleukin-1beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2001;36:1–8. doi: 10.1034/j.1600-0765.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Mehta CK, Hsu TY, Alsulaimani FF. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect Immun. 2002;70:3143–3148. doi: 10.1128/IAI.70.6.3143-3148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang L, Jung JY, Wang EW, Houlihan P, Ramos L, Pashia M, et al. Pseudomonas aeruginosa lipopolysaccharide induces osteoclastogenesis through a toll-like receptor 4 mediated pathway in vitro and in vivo. Laryngoscope. 2007;117:841–847. doi: 10.1097/MLG.0b013e318033783a. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki Y, Ukai T, Yamaguchi M, Yokoyama M, Haro ER, Kaneko T, et al. Locally administered T cells from mice immunized with lipopolysaccharide (LPS) accelerate LPS-induced bone resorption. Bone. 2009 doi: 10.1016/j.bone.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 19.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 23.Sartori R, Li F, Kirkwood KL. MAP Kinase Phosphatase-1 Protects against Inflammatory Bone Loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA, et al. A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol. 2007;78:1992–1998. doi: 10.1902/jop.2007.070101. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil CS, Liu M, Zhao W, Coatney DD, Li F, VanTubergen EA, et al. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther. 2008;16:1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Kreider JM, et al. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther. 2007;320:56–63. doi: 10.1124/jpet.106.112466. Epub 2006 Oct 2013. [DOI] [PubMed] [Google Scholar]
- 28.Marie PJ. Human endosteal osteoblastic cells: relationship with bone formation. Calcif Tissue Int. 1995;56 (Suppl 1):S13–16. [Google Scholar]
- 29.Miyamoto T, Suda T. Differentiation and function of osteoclasts. Keio J Med. 2003;52:1–7. doi: 10.2302/kjm.52.1. [DOI] [PubMed] [Google Scholar]
- 30.Yavropoulou MP, Yovos JG. Osteoclastogenesis--current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 31.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 33.Kim MS, Magno CL, Day CJ, Morrison NA. Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J Cell Biochem. 2006;97:512–518. doi: 10.1002/jcb.20649. [DOI] [PubMed] [Google Scholar]
- 34.Carlson J, Cui W, Zhang Q, Xu X, Mercan F, Bennett AM, et al. Role of MKP-1 in osteoclasts and bone homeostasis. Am J Pathol. 2009;175:1564–1573. doi: 10.2353/ajpath.2009.090035. Epub 2009 Sep 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143:3105–3113. doi: 10.1210/endo.143.8.8954. [DOI] [PubMed] [Google Scholar]
- 36.Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS, et al. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum. 2006;54:463–472. doi: 10.1002/art.21626. [DOI] [PubMed] [Google Scholar]
- 37.Wilson ME, Hamilton RG. Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect Immun. 1992;60:1806–1812. doi: 10.1128/iai.60.5.1806-1812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]