Introduction
The forces that are relentlessly pressuring wild animals are well-established and include the loss, fragmentation, pollution, and over-exploitation of habitat as well as emerging diseases, invasive species, and direct human activities, including hunting and urban sprawl. A major contemporary concern also is climate change, which alters how and where animals live. There now are objective data revealing that one in four mammal species and one in eight birds are at high risk of extinction, and one of every three amphibians and half of all tortoises are threatened [1].
Because modern extinctions appear to be occurring at remarkable rate [2], there is growing interest in ‘species’ and sustaining their viability and genetic integrity [3]. It is well known that a smaller amount of natural habitat almost is always detrimental for wildlife due to reduced food resources and too little space for dispersal of offspring or to find an unrelated mate. One consequence can be incestuous mating that homogenize the genome, causing the expression of deleterious alleles – also known as inbreeding depression. The impact of increasing homozygosity was first demonstrated in ex situ collections 30 years ago [4] when poorly managed, zooheld animals allowed to breed with relatives were found to experience high rates of neonatal and juvenile mortality. Subsequent ex situ and in situ studies have repeatedly demonstrated the insidious influences of increasing homozygosity, especially on reproductive fitness. For example, our laboratory has documented an increased incidence of cryptorchidism, pleiomorphic spermatozoa, and compromised fertilization in populations or species lacking genetic variation (e.g., African and Asian lion, Florida panther, black-footed ferret [5, 6]). The adverse impacts of decreasing gene diversity extend to other biological systems, including contributing to cardiac anomalies, compromised immune-suppression, and increasing vulnerability to environmental changes (climate and pathogens) [7, 8].
The gold standard strategy for preserving genetic variation and, thus, reproductive fitness in species has been retaining and protecting massive amounts of habitat. However, this approach becomes unrealistic in a modern world with unfettered, sprawling numbers of people demanding resources that make it impossible to preserve enough wild space to ensure self-sustaining, healthy populations of every species. Carnivores are especially susceptible to loss in space and inbreeding depression [8]. This awareness that saving habitat alone is insufficient has stimulated a groundswell of support for more species studies, including establishing ex situ security populations, especially those at high risk. These intensively managed animals serve as ‘insurance’ for wild counterparts, but also as an important source of biological (research) information impossible to collect under harsh, uncontrolled field situations. Ex situ operations are expensive, complex, and oriented toward ensuring the retention of all existing gene diversity for at least the next century to ensure species integrity [9]. Maintaining this robustness always is complicated by too few specimens that generally display stressful, self-destructive, and/or dangerous behaviors. Even so, these types of investigations are well worth the risk because there is almost nonexistent biological knowledge (even of the most general sort) for most of the world’s 55,000 vertebrate species [3]. In most cases, resulting data have direct (or indirect) application to improving the management and conservation breeding of rare species.
Value of Reproductive Studies and Fertility Preservation for Rare and Wild Species
Because reproduction is fundamental to species survival, understanding reproductive mechanisms is a high priority. It is well established that there are enormous differences in the specifics of how each species reproduces, even those in the same phylogenetic clade (i.e., family [10, 3]). Over the last 3 decades, our laboratory has studied more than 50 species, and we have concluded that there are as many mechanistic variations in reproduction as there are species [3]. This lack of data on how any given animal reproduces means that there is a need to characterize and describe common sexual patterns (including on the basis of breeding season, behavior, and endocrinology) for thousands of species. For example, a popular tactic in the field or in zoos is ‘behavioral endocrinology’ where investigators relate animal behaviors to hormonal patterns (gonadal/adrenal) using noninvasive fecal or urinary hormone metabolite monitoring, thereby avoiding animal disturbance [11]. When established, this fundamental scientific information fills a hole in the scholarly database on reproductive life history norms for individual species. It also serves as a source of voucher data that can be predictive of the normal (or abnormal) conditions of a species, population or even individual living in nature or in an ex situ security population. For example, having solid information on the normal breeding season, sexual behavior, and litter size for any given species can assist wildlife managers who may suspect abnormalities in contemporary populations under threat and then can undertake ‘adaptive’ management. Such information also is critical for risk assessment specialists whose task is to use sophisticated computer programs (e.g., VORTEX [12]), to calculate population status and then undertake research and mitigation priorities. Accuracy depends on knowing the reproductive norms for the target species. Finally, basic and species-specific reproductive data are essential for two types of reproductive management, the first being adapting human- and livestock-related assisted reproductive technologies to developing alternatives to natural mating for retaining all gene diversity [13]. The second involves ‘recovery,’ situations where a species has become severely threatened, reduced in population size, and it has become essential that every animal reproduce to protect all gene diversity. Both of these management tactics are largely focused on creating self-sustaining security populations in captivity, although recovery programs can eventually include reintroduction and release of animals back to the wild. There are a few models of success, especially using artificial insemination (AI), which allows transporting semen between breeding locations (without the need for moving stress-vulnerable, wild individuals) and overcoming the common problem of sexual incompatibility between computer-selected mates. Examples have been recently reviewed and include the giant panda [14], black-footed ferret [5] (see Fig. 7.1), and scimitar-horned oryx ([15] see Fig. 7.1), the latter two species being returned to the wild after intensive management that includes AI. Embryo-related technologies are not used currently for wildlife genetic management because of sorely lacking information on cross-species embryology [16]. There also is an issue of source of recipients for embryos produced from wildlife species, as inter-species embryo transfer is not viable [17]. Nonetheless, embryos have been produced from wild animals, often using in vitro oocyte maturation (IVM) and fertilization (IVF) and occasional offspring produced (see below [16]).
Fig. 7.1.

Wild species that are intensively managed ex situ by the Smithsonian’s National Zoological Park and partners: 1 black-footed ferret (Mustela nigripes), 2 cheetah (Acinonyx jubatus), 3 Eld’s deer (Cervus eldii thamin), 4 scimitar-horned oryx (Oryx dammah), 5 tufted deer (Elaphodus cephalophus), and 6 Przewalski horse (Equus ferus przewalskii). Ovarian tissue samples from these species have been cryopreserved and are currently stored in the Genome Resource Bank at the Conservation Biology Institute
Reproductive biologists studying wildlife benefit from advances in the human infertility and livestock production fields. However, the overall goals of these programs are substantially different – overcoming infertility (humans) versus more efficient/higher quality food production (livestock) versus retention of all gene diversity (wildlife). Nonetheless, these three groups share aligned interests in ‘ensuring reproductive health and preserving fertility.’ The emergence of the oncofertility field (which explores new approaches for preserving reproductive potential of cancer patients who may lose fertility due to chemical or radiation treatment) has intriguing applications for endangered species enthusiasts charged with conserving genetic variation. For example, there is strong interest in extending the reproductive longevity of a valuable wild animal indefinitely into the future, with the occasional re-infusion of its genes into the contemporary population. Such an approach contributes by avoiding (or mitigating) genetic drift and the tendency for inbreeding in small populations. In this same context, there has been significant effort to articulate the value of ‘genome resource banks,’ which are organized repositories of biomaterials to be stored and used for managing both heterozygosity and conducting basic and applied research [8]. For wildlife, there are other reasons to extend fertility potential, largely for animals that have not yet produced sufficient numbers of descendants to ensure the passing on of their genes. The specific targets include individuals that (1) are living but fail to natural reproduce, (2) unexpectedly die, (3) are nearing reproductive senescence, or (4) have been long-dead, but there is value in rescuing and re-infusing their genome into the modern population.
Value of Animal Models for Preserving and Extending Fertility in Wild Species
Some challenges related to understanding and protecting species biodiversity rival the concerns associated to the accessibility to biomaterials faced in field of human reproductive health. More than 20 years ago, we advocated the need for animal models to more efficiently develop assisted reproductive technologies for wildlife [18]. Due to the few numbers of individuals available within an endangered species, it is prudent (and safer) to first test approaches in a common species before applying to the rare counterpart. This philosophy actually emerged because of early failures to directly apply cattle AI techniques to the cheetah (i.e., the epiphany that a ‘cheetah is not a cow’ concept [10]). This led to the realization that little good information was available on the basic reproductive physiology of any of the existing 37 species of felids, which, in turn, resulted in our developing the domestic cat as a model system. This, in turn, has permitted making many fascinating discoveries on species-specific reproductive mechanisms, for example, a high rate of spontaneous ovulation in the clouded leopard (most felids are induced ovulators), resistance to exogenous gonadotropins in the ocelot, peculiar, protracted luteal function in the Iberian lynx, the ability of female cheetahs to mutually suppress their reproductive cycles, among other phenomena (see reviews [5, 13]). Such findings were the genesis for our encouraging the need for more species-specific research [3]. This point also is relevant if fertility preservation tools developed for humans are to have application to wildlife because it will likely be essential to conduct initial studies in an appropriate (usually taxonomically related) model. Besides the domestic cat as a target (for felids), other valuable models will include the domestic dog (for wild canids), red or white-tailed deer (for wild cervids), brushtail possum (for rare marsupials), or common frog (for near-extinct amphibians). However, there are many animals so specialized that there are no experimental species, for example, the two species of elephants, the five species of rhinoceroses, the giant panda, and killer whale (among hundreds of others). Such cases likely will require more bold and straightforward actions directly to the target species, which is supportable if adequate fundamental reproductive knowledge is available [17].
It also is worth noting that some wildlife species could be interesting natural models for various human reproductive conditions. Such opportunities have recently been addressed and have ranged from the felids (for the ovarian tunica albuginea or for germinal vesicle characteristics [19, 3]) to elephants (for uterine pathologies in aging females, stress-related infertility in a social group, and impact of obesity on reproductive function [3]). Most of these managed animal populations are comprised of many individuals of exact known genetic provenance and variation, an advantage for providing new insights into the role of the individual effect. For example, one could examine an individual component in a reproductive response to a gonadotropin treatment, oocyte quality, or gamete sensitivity to cooling, freezing, or thawing.
Ways by Which Oocyte and Embryo Culture in Domestic Animals and Humans Can Help Preserve and Extend Fertility in Females of Wild Species
The first order priority for any fertility preservation approach is the capacity for successful in vitro culture of gametes or embryos. It is both technically and logistically possible to harvest follicular oocytes from selected wild female donors by (1) transvaginal or transabdominal laparoscopic recovery or (2) directly from the ovaries after ovariectomy or death [20]. In both instances, this approach requires in vitro maturation (IVM), which is known to produce less developmentally competent oocytes than counterparts matured in vivo [21]. However, the collection of in vivo matured eggs is highly challenging because of the need to (1) develop the appropriate protocols to stimulate folliculogenesis with exogenous hormones and (2) identify the optimum time for collecting oocytes from preovulatory follicles. Thus, in pragmatic terms, it is more reasonable to rely on recovering immature oocytes from antral follicles, a strategy that can be applied to prepubertal, pregnant or even dead specimens (‘gamete rescue’). For some domesticated mammals and humans, there have been common findings relative to oocyte IVM that likely will be relevant to wild animal applications. For example, it now is well established that the initial quality of the immature oocyte influences subsequent embryo developmental competence in vitro and after embryo transfer [22, 23]. Strict selection criteria are useful for ensuring future developmental success. For instance, some of the oocyte’s morphological traits (i.e., color and cytoplasm homogeneity and number of cumulus cell layers [24]) are important predictors for developmental competence and, more recently, follicle size [25], oocyte metabolism [26], and metabolomics [27]. These same tools are readily adaptable to effectively evaluate oocyte quality in wildlife species.
For genetic management programs involving endangered species, we would expect that IVM followed by IVF will be particularly useful for addressing issues related to aging. For example, cheetahs held in ex situ collections are well known for low reproduction success, which has resulted in many older, genetically important females in the population that still need to pass along their genes to the next generation [28]. Are there human-related fertility preservation tactics that could be useful to rescuing the maternal genome of older individuals? It is clear that oocytes isolated from aged mice and human donors are compromised in ability to complete meiotic maturation and support embryo development [29]. Furthermore, oocytes from older mice and women are developmentally sensitive to mitochondrial damage and exhibit a high incidence of aneuploidy [30]. There are perhaps alternatives to dealing with complete and ‘whole’ old oocytes, for example, focusing on the germinal vesicle (GV) as the target for rescuing valuable genetic materials. It now is known that the GV transferred into an enucleated counterpart oocyte can allow reconstituting a whole oocyte that (following electrofusion and culture) supports normal meiosis [31, 32]. This could also increase the source of ‘rescued’ maternal genomes from genetically valuable individuals that die in ex situ collection or even in nature. Additionally, we recently have demonstrated that there are diffusible factors produced by cumulus-enclosed oocytes that appear especially valuable in oocyte salvage. For example, we have observed in the cat model that the detrimental effects of too few or absent cumulus cells can be overcome to ensure that such non-ideal oocytes can fully mature, fertilize, and develop in vitro [33].
Interestingly, there are unique challenges to IVM/IVF for many wildlife species given the high prevalence of reproductive seasonality. Oocytes collected during the quiescent season(s) of the year are likely to be resistant to conventional developmental culture, with evidence already observed in the red deer [34] and domestic cat [35]. The result generally is low, or non-existent, embryo production during most of the year. However, there is recent evidence that seasonal impositions on oocyte quality can be circumvented by in vitro culture modifications. For example, in our cat model, we have found that supplementing IVMmedium with anti-oxidants and increased exogenous gonadotropin concentrations overcomes this seasonal compromise and enhances embryo production efficiency throughout the year [35].
These ideas and practices are emerging from the substantial advances being made in the human fertility field that, in turn, is being driven by vast resources. One of the major underpinnings of all human IVF was the original development of a reliable culture medium for IVF of hamster oocytes, which then was applied to human gametes in the laboratory [36]. Human IVF technology then has progressed extremely fast to a point where new techniques that have enormous potential have not yet been applied to wild animals (such as morphological selection of sperm before intra-cytoplasmic injection, IMSI [37]). Despite the significant use of oocyte and embryo-related technologies for enhancing reproduction in humans, livestock, and laboratory animals, IVF and embryo transfer have so far had a negligible impact on the genetic management of wildlife species [17]. In fact, there is an amazing lack of research attention on oocytes and embryos even for investigators who specialized in these non-traditional species. We recently surveyed more than 10 years of publications for ten major scientific journals, and, of the 1,330 reproductive papers generated on wildlife, only 19.3% were oriented to oocytes or embryos (compared to 31.3 and 21.3% for sperm and endocrine investigations, respectively; Songsasen, unpublished data). Finally, although there have been a few milestone births, including in the baboon, rhesus macaque, marmoset, gorilla, Indian desert cat, ocelot, tiger, African wild cat, Armenian red sheep, water buffalo, gaur, red deer, llama, and caracal (for a review, see [16]), these are mere hints of the potential of embryo technologies for protecting and preserving wild biodiversity.
Oncofertility Preservation Approaches that Have Special, Potential Value for Wildlife
Currently, there are four strategies being intensively investigated in the oncofertility field that are particularly attractive for helping achieve wildlife management goals.
Ovarian Tissue Cryopreservation
The ovarian cortex contains thousands of follicles at different developmental stages [38] that are recoverable from individuals at the time of ovariectomy. Of course, a major goal in oncofertility is to develop reliable methods for preserving this source of the maternal genome from women or girls that may lose the capacity to produce viable oocytes after therapeutic treatments. Whole tissue cryo-concepts are highly relevant to preserving fertility potential in wildlife as well (including from adult or prepubertal individuals that might die unexpectedly). We have incorporated this practice into our routine zoological management program at the Smithsonian Institution and with other institutional partners. In this way, the oncofertility consortium and networking process is a model for wildlife operations because excellent communication and interdisciplinary cooperation are critical. In our case, this typically involves close collaboration with curators and veterinarians who expeditiously provide information about a death or medical emergency and then cooperate in excising fresh ovarian tissue that is provided to the laboratory. Research staff then cut ovarian tissue into sufficiently small pieces to allow cryoprotectant permeation and cryopreservation. Others have demonstrated high survival of ovarian tissue (on the basis of cell integrity and grafting success) from most species studied to date [39, 40]. Our laboratory recently demonstrated the value of vitrification over slow cooling for preserving ovarian cortex and primordial follicles from prepubertal and adult cats [41]. Optimal techniques now are being used to routinely bank ovarian tissue samples from a host of rare species, including the black-footed ferret, cheetah, Eld’s deer, scimitar-horned oryx, tufted deer, and Przewalski horse among others (see Fig. 7.1). Early results have been quite encouraging, revealing that ~80% of these preantral follicles survive vitrification based on histological integrity, viability staining, and proliferation index (see Fig. 7.2).
Fig. 7.2.
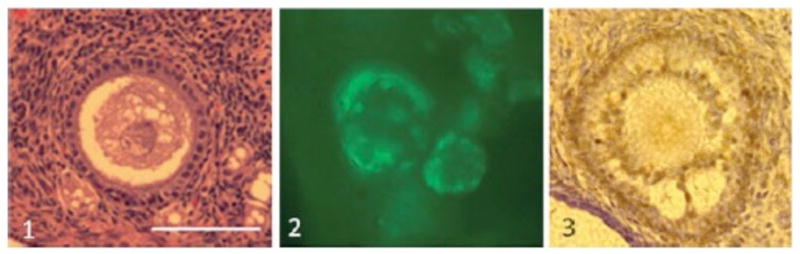
Assessment of 1 histological structure (eosine/hematoxylin staining), 2 cell viability (calcein-AM staining), and 3 cell proliferation (PCNA immune-staining) in follicles after vitrification of ovarian cortex in felids. For the three pictures, bar = 50 μm
Ovarian Tissue Grafting
The success of transplanting human ovarian tissue to produce viable oocytes (with the now subsequent birth of multiple babies [42]) offers excitement and strong incentive for similar studies in rare wildlife species. Ovarian tissue grafting also has been studied in the mouse [40], cat [43], dog [44], pig [45], sheep [46], rhesus monkey [47], wombat, and wallaby [39]. In all cases, it has been possible to obtain normal-appearing antral follicles from grafted tissues placed in immune-deficient mice. When inseminated in vitro, recovered oocytes from such ‘foreign’ follicles have the capacity to fertilize and form viable-appearing embryos. And occasionally living offspring have been produced after transfer – in the mouse, sheep, and macaque monkey – from oocytes derived from transplanted ovarian tissue [46, 47, 40]. The benefits of such ovarian tissue xenografting would be similar to those of testis tissue transplantations, specifically in species that take several years to attain sexual maturity like elephants [17]. Again, a major target of interest would be the rescue of the genome of rare, genetically valuable individuals (in combination with the cryopreservation and storage of ovarian tissues). There also is enormous potential for generating new insights into (1) the significance of naturally diverse oocyte morphotypes and mammalian follicular dynamics, (2) responsiveness to exogenous gonadotropins, and (3) the ability to achieve nuclear maturation and fertilization in varied culture conditions.
Follicle In Vitro Culture
The abundance of primordial follicles within the ovary is a significant resource for fertility preservation [48]. A capacity to culture these follicles in vitro to the point of recovering viable oocytes that can achieve nuclear maturation and then fertilize offers enormous opportunities for maternal genome conservation (in association with cryo-banking of gonadal tissues). This approach has been used in the laboratory mouse to produce offspring from cultured primordial follicles derived from both fresh and thawed ovarian tissue [49]. Advances also are gradually being made in both rodent and non-rodent species using isolated preantral follicles. Particularly inspiring have been studies in humans [50] where secondary follicles were able to grow, maintain architecture, and produce steroids in vitro for 15–30 days. The challenges for developing the follicle culture strategy are mostly technical and information-based, but laborious, including (1) matching culture medium and environment to physiological needs of each species, aligning as closely as possible to conditions in vivo; (2) maintaining cell-to-cell communication and signaling; and (3) understanding the influence of epigenetics and the genetic and fertility status of in vitro-derived mature oocytes. It may well be that larger-sized animals (like in the human) will require a long (2 or 3 months) and multi-stage process, whereby primordial follicle growth is initiated in situ by culturing ovarian cortex fragments, and then pre-antral follicles are isolated and grown to advanced stages before steroidogenic function is elicited in somatic cells. The final stage in this complex would be oocyte recovery followed by IVM/IVF and then embryo transfer. To our knowledge, early stage follicular culture has been attempted in only a few non-laboratory species (i.e., sheep, goat, and cattle [51, 52, 53]. Recently, our laboratory has had encouraging preliminary results by adapting ‘mouse methods’ to early stage, preantral domestic dog and cat follicles (in collaboration with Dr. Teresa Woodruff’s laboratory). Over the course of a 10-day culture, follicle size routinely increased by 1.5- to 2-fold (see Fig. 7.3).
Fig. 7.3.
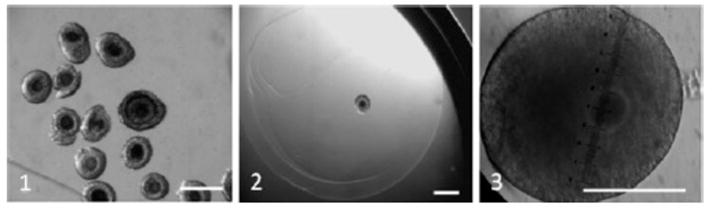
Domestic dog preantral follicles and enclosed oocytes 1 before culture, 2 after encapsulation in alginate, and 3 after 8 days of in vitro culture. Bars = 200 μm
Oocyte Preservation
There has been extensive progress in both fundamental knowledge and practical application of cryopreserving mammalian oocytes [54]. Although the cooling, freezing, and thawing of an ovum is much more challenging than the spermatozoon or embryo, oocytes have been consistently cold-stored and used to produce offspring in several species, with most success in mouse and human [54]. Furthermore, while conventional slow-cooling has been extensively used, both mature and immature oocytes have been cryopreserved recently using ultra-rapid protocols, such as vitrification on electron microscope grids and cryo-loops [55]. Importantly, immature oocytes appear to be more resistant to cryo-damage than mature counterparts because cells at the germinal vesicle stage do not contain a temperature-sensitive meiotic spindle [56]. This characteristic to withstand the stress of extremely low temperature is a significant reason to center more attention on the storage of immature oocytes. But, as with other approaches, there have been few comparative cryo-studies in wildlife species, largely due to the lack of access to good quality oocytes [57]. Regardless, progress for wildlife continues to be linked with parallel studies of taxonomically related domestic animal models and humans [58]. Certainly, continued advancements with the common cow, sheep, goat, cat, dog, and white-tailed deer would have relevance to more rapid progress with wild bovids, small ruminants, felids, canids, and cervids, respectively. It also would be prudent to explore novel approaches for oocyte/maternal genome storage. For example, desiccation has been successful for spermatozoa [59] and could be adapted for the oocyte’s germinal vesicle, thereby allowing the stockpiling of female genomes at room temperature.
Conclusion and Prospects
Fertility preservation strategies used to ensure human reproductive health, including in the field of oncofertility, have significant secondary advantages for conserving biodiversity. This is especially important because there is a growing portfolio of species management and recovery stories benefiting from assisted reproductive technologies and because the highest priority in conservation breeding is to retain gene diversity. Fertility preservation approaches that are in place (or in development) for humans in fact already are protecting the maternal genome of individuals. Thus, there is compatibility and common purpose to these widely diverse targets (humans and wildlife). We can envision laboratories devoted exclusively to the organized collection, culture, storage, and use of ovarian biomaterials from rare species. Furthermore, we foresee the staff of these facilities exploiting the methods developed by colleagues who are working to ensure fertility in human patients. Perhaps there could be direct collaborations with mutual benefits. We also argue that human reproductive specialists could well take advantage of new fundamental knowledge on biological insights from studies of far-from-traditional animal species.
The major limiting factors for advancing fertility preservation in diverse animals will continue to be the significant variance among even closely related species in specific reproductive mechanisms. This will extend to uniqueness in ability to survive cryopreservation and culture of tissues, follicles, and oocytes as well as dealing with the many complexities related to IVF, selecting/managing recipients, and conducting embryo transfer. However, this should not prevent us from exploring innovative approaches such as desiccation and storage of female gametes at room temperature (which also could benefit numerous non-mammalian species, such as birds and fishes).
Important, near-term priorities are clear, starting with more studies on readily available and probably domesticated species that can serve as appropriate models for wild counterparts. There also is a strong need to gain access to rare specimens that die or present opportunities for ovarian recovery during medical procedures in zoological collections or in the field. Finally, it seems wise to promote more interaction among stakeholders in all areas – whether human, livestock, laboratory animal, or wildlife-oriented. For example, there could be significant benefits from the establishment of a fertility preservation network, with benefits ranging from active communication for sharing critical (or simply interesting) information to opportunities for direct collaboration.
Acknowledgments
This research was supported by the oncofertility consortium NIH 8UL1DE019587, 5RL1HD058296.
References
- 1.IUCN. [Accessed September 17, 2009];International Union for Conservation of Nature. 2009 www.iucn.org/. Accessed September 6, 2009. IUCN Red List of Threatened Species. www.iucnredlist.org.
- 2.Wilson EO. The diversity of life. Cambridge: Harvard University Press; 1992. pp. 349–51. [Google Scholar]
- 3.Wildt DE, Comizzoli P, Pukazhenthi BS, Songsasen N. Lessons from biodiversity – the value of non-traditional species to advance reproductive science, conservation and human health. Mol Reprod Dev. 2010;77:397–409. doi: 10.1002/mrd.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralls K, Brugger K, Ballou J. Inbreeding and juvenile mortality in small populations of ungulates. Science. 1979;206:1101–03. doi: 10.1126/science.493997. [DOI] [PubMed] [Google Scholar]
- 5.Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology. 2009;71:130–48. doi: 10.1016/j.theriogenology.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Pukazhenthi BS, Wildt DE, Howard JG. The phenomenon and significance of teratospermia in felids. J Reprod Fertil. 2001;57:423–33. [PubMed] [Google Scholar]
- 7.Roelke ME, Martenson JS, O’Brien SJ. The consequences of demographic reduction and genetic depletion in the endangered Florida panther. In: Comizzoli P, et al., editors. Curr Biol. Vol. 3. 1993. pp. 340–50.pp. 98 [DOI] [PubMed] [Google Scholar]
- 8.Wildt DE, Rall WF, Critser JK, Monfort SL, Seal US. Genome resource banks: “Living collections” for biodiversity conservation. BioScience. 1997;47:689–98. [Google Scholar]
- 9.Ballou JD. Genetic and demographic modeling for animal colony and population management. ILAR J. 1997;38:69–75. doi: 10.1093/ilar.38.2.69. [DOI] [PubMed] [Google Scholar]
- 10.Wildt DE, Ellis S, Howard JG. Linkage of reproductive sciences: from ‘quick fix’ to ‘integrated’ conservation. J Reprod Fertil Suppl. 2001;57:295–307. [PubMed] [Google Scholar]
- 11.Monfort SL. Non-invasive endocrine measures of reproduction and stress in wild populations. In: Wildt DE, Holt W, Pickard A, editors. Reproduction and integrated conservation science. Cambridge: Cambridge University Press; 2002. pp. 147–65. [Google Scholar]
- 12.Lacy RC. VORTEX: A computer simulation model for Population Viability Analysis. Wildl Res. 1993;20:45–65. [Google Scholar]
- 13.Wildt DE, Swanson WF, Brown J, Sliwa A, Vargas A. Felids ex situ for managed programs, research and species recovery. In: Macdonald D, Loveridge AJ, editors. The biology and conservation of wild felids. Oxford: Oxford University Press; 2010. p. 450. [Google Scholar]
- 14.Wildt DE, Zhang A, Zhang H, Janssen D, Ellis S. Giant pandas: biology, veterinary medicine and management. Cambridge: Cambridge University Press; 2006. p. 586. [Google Scholar]
- 15.Morrow CJ, Penfold LM, Wolfe BA. Artificial insemination in deer and non-domestic bovids. Theriogenology. 2009;71:149–65. doi: 10.1016/j.theriogenology.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Pukazhenthi BS, Wildt DE. Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reprod Fertil Dev. 2004;16:33–46. doi: 10.10371/RD03076. [DOI] [PubMed] [Google Scholar]
- 17.Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- 18.Wildt DE, Schiewe MC, Schmidt PM, Goodrowe KL, Howard JG, Phillips LG, O’Brien SJ, Bush M. Developing animal model systems for embryo technologies in rare and endangered wildlife. Theriogenology. 1986;25:33–51. [Google Scholar]
- 19.Bristol-Gould S, Woodruff TK. Folliculogenesis in the domestic cat (Felis catus) Theriogenology. 2006;66:5–13. doi: 10.1016/j.theriogenology.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Comizzoli P, Mermillod P, Mauget R. Reproductive biotechnologies for endangered mammalian species. Reprod Nutr Dev. 2000;40:493–504. doi: 10.1051/rnd:2000113. [DOI] [PubMed] [Google Scholar]
- 21.Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68:71–6. doi: 10.1016/j.theriogenology.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 22.Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann NY Acad Sci. 2004;1034:132–44. doi: 10.1196/annals.1335.016. [DOI] [PubMed] [Google Scholar]
- 23.Mtango NR, Potireddy S, Latham KE. Oocyte quality and maternal control of development. Int Rev Cell Mol Biol. 2008;268:223–90. doi: 10.1016/S1937-6448(08)00807-1. [DOI] [PubMed] [Google Scholar]
- 24.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil. 1997;110:355–60. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- 25.Songsasen N, Wildt DE. Size of the donor follicle, but not stage of reproductive cycle or seasonality, influences meiotic competency of selected domestic dog oocytes. Mol Reprod Dev. 2005;72:113–9. doi: 10.1002/mrd.20330. [DOI] [PubMed] [Google Scholar]
- 26.Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56:163–71. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online. 2009;18:219–25. doi: 10.1016/s1472-6483(10)60259-3. [DOI] [PubMed] [Google Scholar]
- 28.Crosier AE, Comizzoli P, Baker T, Pukazhenthi BS, Howard JG, Marker LL. Wildt DEOocyte fertilization and uterine morphology in aged female cheetahs (Acinonyx jubatus) are similar to younger counterparts. Biol Reprod. 2008;77(Suppl):79. [Google Scholar]
- 29.Tatone C. Oocyte senescence: a firm link to age-related female subfertility. Gynecol Endocrinol. 2008;24:59–63. doi: 10.1080/09513590701733504. [DOI] [PubMed] [Google Scholar]
- 30.Thouas GA, Trounson AO, Jones GM. Effect of female age on oocyte developmental competence following mitochondrial injury. Biol Reprod. 2005;73:366–73. doi: 10.1095/biolreprod.105.040956. [DOI] [PubMed] [Google Scholar]
- 31.Comizzoli P, Godard-Glasnapp N, Keefer CL, Wildt DE, Pukazhenthi BS. Impact of reconstruction by germinal vesicle transfer on nuclear maturation of domestic cat oocytes. Biol Reprod. 2007;77(Suppl):486. [Google Scholar]
- 32.Moffa F, Comoglio F, Krey LC, Grifo JA, Revelli A, Massobrio M, Zhang J. Germinal vesicle transfer between fresh and cryopreserved immature mouse oocytes. Hum Reprod. 2002;17:178–83. doi: 10.1093/humrep/17.1.178. [DOI] [PubMed] [Google Scholar]
- 33.Godard NM, Pukazhenthi BS, Wildt DE, Comizzoli P. Paracrine factors from cumulusenclosed oocytes ensure the successful maturation and fertilization in vitro of denuded oocytes in the cat model. Fertil Steril. 2009;91:2051–60. doi: 10.1016/j.fertnstert.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg DK, Asher GW. New developments reproductive technologies in deer. Theriogenology. 2003;59:189–205. doi: 10.1016/s0093-691x(02)01272-4. [DOI] [PubMed] [Google Scholar]
- 35.Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–16. [PubMed] [Google Scholar]
- 36.Bavister BD. How animal embryo research led to the first documented human IVF. Reprod Biomed Online. 2002;4:24–9. doi: 10.1016/s1472-6483(12)60008-x. [DOI] [PubMed] [Google Scholar]
- 37.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–90. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 38.Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009 doi: 10.1093/molehr/gap073. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paris MC, Snow M, Cox SL, Shaw JM. Xenotransplantation: a tool for reproductive biology and animal conservation? Theriogenology. 2004;61:277–91. doi: 10.1016/s0093-691x(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 40.Shaw JM, Trounson AO. Ovarian tissue transplantation and cryopreservation. Application to maintenance and recovery of transgenic and inbred mouse lines. Methods Mol Biol. 2002;180:229–51. doi: 10.1385/1-59259-178-7:229. [DOI] [PubMed] [Google Scholar]
- 41.Comizzoli P, Martinez-Madrid CB, Pukazhenthi BS, Wildt DE. Vitrification of ovarian cortex from prepubertal and adult cats induces less damage to the primordial follicles than slow freezing. Biol Reprod. 2009;81(Suppl):187. [Google Scholar]
- 42.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Andersen CY. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy – a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Bosch P, Hernandez-Fonseca HJ, Miller DM, Wininger JD, Massey JB, Lamb SV, Brackett BG. Development of antral follicles in cryopreserved cat ovarian tissue transplanted to immunodeficient mice. Theriogenology. 2004;61:581–94. doi: 10.1016/s0093-691x(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe SS, Shaw JM, Gunn IM. Xenografting of canine ovarian tissue to ovariectomized severe combined immunodeficient (SCID) mice. J Reprod Fertil Suppl. 2001;57:323–29. [PubMed] [Google Scholar]
- 45.Kaneko H, Kikuchi K, Noguchi J, Hosoe M, Akita T. Maturation and fertilization of porcine oocytes from primordial follicles by a combination of xenografting and in vitro culture. Biol Reprod. 2003;69:1488–93. doi: 10.1095/biolreprod.103.017038. [DOI] [PubMed] [Google Scholar]
- 46.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at –196!C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 47.Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP. Live birth after ovarian tissue transplant. Nature. 2004;428:137–8. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 48.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–15. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- 49.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue engineered follicles produce live fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muruvi W, Picton HM, Rodway RG, Joyce IM. In vitro growth and differentiation of primary follicles isolated from cryopreserved sheep ovarian tissue. Anim Reprod Sci. 2009;112:36–50. doi: 10.1016/j.anireprosci.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Silva JR, Tharasanit T, Taverne MA, van der Weijden GC, Santos RR, Figueiredo JR, van den Hurk R. The activin-follistatin system and in vitro early follicle development in goats. J Endocrinol. 2006;189:113–25. doi: 10.1677/joe.1.06487. [DOI] [PubMed] [Google Scholar]
- 53.Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75:924–32. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- 54.Songsasen N, Comizzoli P. A historic overview of embryos and oocyte preservation in the world of Mammalian in vitro fertilization and biotechnology. In: Borini A, Coticchio G, editors. Preservation of human oocytes. London: Informa Healthcare; 2009. pp. 1–11. [Google Scholar]
- 55.Stachecki JJ, Cohen J. An overview of oocyte cryopreservation. Reprod Biomed Online. 2004;9:152–63. doi: 10.1016/s1472-6483(10)62124-4. [DOI] [PubMed] [Google Scholar]
- 56.Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–54. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leibo SP, Songsasen N. Cryopreservation of gametes and embryos of non-domestic species. Theriogenology. 2002;57:303–26. doi: 10.1016/s0093-691x(01)00673-2. [DOI] [PubMed] [Google Scholar]
- 58.Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod. 2009;24:2114–23. doi: 10.1093/humrep/dep182. [DOI] [PubMed] [Google Scholar]
- 59.Meyers SA. Dry storage of sperm: applications in primates and domestic animals. Reprod Fertil Dev. 2006;18:1–5. doi: 10.1071/rd05116. [DOI] [PubMed] [Google Scholar]


