Abstract
Acute inflammation is important for tissue repair; however, chronic inflammation contributes to neurodegeneration in Alzheimer’s disease (AD) and occurs when glial cells undergo prolonged activation. In the brain, stress or damage causes the release of nucleotides and activation of the Gq protein-coupled P2Y2 nucleotide receptor subtype (P2Y2R) leading to pro-inflammatory responses that can protect neurons from injury, including the stimulation and recruitment of glial cells. P2Y2R activation induces the phosphorylation of the epidermal growth factor receptor (EGFR), a response dependent upon the presence of a SH3 binding domain in the intracellular C terminus of the P2Y2R that promotes Src binding and transactivation of EGFR, a pathway that regulates the proliferation of cortical astrocytes. Other studies indicate that P2Y2R activation increases astrocyte migration. P2Y2R activation by UTP increases the expression in astrocytes of αVβ3/5 integrins that bind directly to the P2Y2R via an Arg-Gly-Asp (RGD) motif in the first extracellular loop of the P2Y2R, an interaction required for Go and G12 protein-dependent astrocyte migration. In rat primary cortical neurons (rPCNs) P2Y2R expression is increased by stimulation with interleukin-1β (IL-1β), a pro-inflammatory cytokine whose levels are elevated in AD, in part due to nucleotide-stimulated release from glial cells. Other results indicate that oligomeric β-amyloid peptide (Aβ1-42), a contributor to AD, increases nucleotide release from astrocytes, which would serve to activate upregulated P2Y2Rs in neurons. Data with rPCNs suggest that P2Y2R upregulation by IL-1β and subsequent activation by UTP are neuroprotective, since this increases the non-amyloidogenic cleavage of amyloid precursor protein. Furthermore, activation of IL-1β-upregulated P2Y2Rs in rPCNs increases the phosphorylation of cofilin, a cytoskeletal protein that stabilizes neurite outgrowths. Thus, activation of pro-inflammatory P2Y2Rs in glial cells can promote neuroprotective responses, suggesting that P2Y2Rs represent a novel pharmacological target in neurodegenerative and other pro-inflammatory diseases.
Keywords: Neurons, Neurodegeneration, Astrocytes, Growth factor receptors, Inflammation, P2Y2 receptors, Cofilin, Nucleotides, Proliferation, RGD motif, SH3 binding domain, Integrins
Introduction
Chronic neuroinflammation, associated with the pathogenesis and progression of Alzheimer’s disease (AD), occurs when glial cells (i.e., astrocytes and microglia) undergo prolonged activation in response to oxidative stress. Oxidative stress is postulated to be an early event in the development of AD that is due to increased production of reactive oxygen species from mitochondria and NADPH oxidase which can modify lipids, nucleic acids, and proteins [1–9]. Production of neurotoxic β-amyloid (Aβ) peptides, such as Aβ1-42, also is a widely accepted contributor to neurodegeneration in AD [10, 11], and oxidative stress can enhance Aβ production [12] leading to mitochondrial dysfunction and neuronal apoptosis [13]. Chronic inflammation occurs around β-amyloid plaques [14, 15], and has been associated with the activation by cytokines of receptors in glial cells that promote neuronal cell death [16–18]. Studies have shown that inflammation begins as a neuroprotective mechanism, but becomes neurodegenerative when sustained [19–22]. Chronic neuroinflammation occurs in brain pathologies including AD, trauma, and stroke and is characterized by increased glial cell migration and proliferation, and morphological changes, including extensive cellular hypertrophy, fiber extension and increased expression of glial fibrillary acidic protein (GFAP) [23, 24]. In the initial stages, neuroinflammation limits brain damage by promoting the clearance of neurotoxic soluble β-amyloid peptide [25, 26]. Activated glial cells migrate to the edge of an injured area and secrete cytokines, chemokines, and growth factors, and also upregulate antigens and cell adhesion molecules [27, 28]. Glial cell activation in the central nervous system under physiological conditions facilitates axonal growth during development [29]. In adult brain, glial cell activation is critical for structural plasticity and repair of damaged brain cells [24]. In the chronic stages, neuroinflammation may exacerbate neurotoxic effects induced by the formation of glial-derived amyloid plaques [24–30] that contribute to neurodegeneration and loss of brain function in AD. Anti-inflammatory drugs have been shown to alter Aβ deposition in an animal model of AD [31]. Among the agents that can contribute to glial cell activation in AD, nucleotides released from the cytoplasm of oxidatively stressed cells have garnered little attention despite the fact that multiple nucleotide receptor subtypes are expressed in glial cells and neurons. Studies have shown that ATP release due to stretch-induced injury increases GFAP expression and proliferation in astrocytes [32], and nucleotides cause responses indicative of astrogliosis in vivo [33] and in primary rat cortical astrocyte cultures [34]. Release of nucleotides has been proposed to occur by exocytosis of ATP/UTP-containing vesicles, facilitated diffusion by putative ABC transporters, cytoplasmic leakage, and electrodiffusional movements through ATP/nucleotide channels [35].
Our studies have shown that the Gq protein-coupled P2Y2 receptor subtype is an important mediator of neuroinflammatory responses mediated by astrocytes. Nucleotides are present at millimolar concentrations in the cytoplasm and when released activate a variety of P2 nucleotide receptors in the brain that have nanomolar to micromolar affinities for nucleotides [36]. Therefore, a small amount of nucleotide released from damaged or oxidatively-stressed cells can activate P2 receptors [37]. It has been demonstrated that ATP released from the leading edge of the cell surface amplifies chemotactic signals and directs neutrophil orientation by feedback through P2Y2 nucleotide receptors (P2Y2Rs) [38]. Our previous results indicate that the pro-inflammatory cytokine IL-1β upregulates P2Y2R expression in neurons [39], which can be activated by released nucleotides (unpublished data). Thus, the release of nucleotides in the brain is hypothesized to stimulate the generation of extracellular pro-inflammatory cytokines by astrocytes and microglial cells that promote the upregulation of neuronal P2Y2Rs. This review will discuss our findings relating to the mechanisms underlying the pro-inflammatory and neuroprotective effects mediated by P2Y2Rs in astrocytes and neurons and their potential relationship to the pathophysiology of AD.
The P2 Receptor Family
In the early 1970 s, it was reported that ATP was released into the extracellular space by stimulation of nonadrenergic, noncholinergic nerves to activate responses postulated to be mediated by P2 purinergic receptors for nucleotides [40, 41]. Over the next few decades, it was recognized that activation of P2 receptors can modulate a variety of responses in cells of the mammalian central nervous system (CNS), including neurotransmission, cell growth, and apoptosis [42–44]. It is now accepted that nucleotides are released from excitatory neurons, injured cells, cells undergoing mechanical or oxidative stress, aggregating platelets, degranulating macrophages, and astrocytes by exocytosis from ATP/UTP-containing vesicles, facilitated diffusion, or cytoplasmic leakage [35–38, 45–50]. Extra-cellular nucleotides activate cell surface P2 receptors belonging to two structurally distinct families: the G protein-coupled P2Y receptors (P2YRs) and P2X receptors (P2XRs) that are ligand-gated ion channels. Eight P2Y receptor subtypes have been cloned and characterized to date, including the Gq-coupled P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors, and the Gi-coupled P2Y12, P2Y13, and P2Y14 receptors [51]. Seven P2X receptors have been cloned and characterized as ligand-gated ion channels, including P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 receptors [52]. Activation of P2 receptors in neurons and glia under normal and pathological conditions regulates pro-inflammatory responses, ion transport, neurotransmission and cell apoptosis, proliferation, and migration [42–44, 52–54]. Therefore, P2 receptors in the CNS represent potential targets for pharmaceutical approaches to treat neurological disorders. Among these P2 receptor subtypes, our research has focused on the P2Y2R and its signaling pathways in the regulation of pro-inflammatory responses in astrocytes associated with reactive astrogliosis, and neuroprotective responses associated with neurite growth and stability and the non-amyloidogenic processing of amyloid precursor protein (APP).
The P2Y2 Nucleotide Receptor
Activation of the Gq-coupled P2Y2R stimulates phospholipase C (PLC) and leads to the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) [54, 55], second messengers for calcium release from intracellular storage sites and protein kinase C (PKC) activation, respectively. Interestingly, we have found that the P2Y2R by virtue of a Arg-Gly-Asp (RGD) motif in its first extracellular loop (Fig. 1) can bind to αVβ3/5 integrins and enable UTP to stimulate Go and G12 proteins leading to the activation the small GTPases Rac and Rho, respectively (Fig. 2) [56, 57]. Mutation of the RGD sequence to Arg-Gly-Glu (RGE), prevents both integrin binding and UTP-induced activation of Go, G12, Rac and Rho by the mutant P2Y2R expressed in human 1321N1 astrocytoma cells that lack endogenous P2Y receptors. In 1321N1 astrocytoma cells, activation of the wild-type P2Y2R, but not the RGE-mutant P2Y2R, leads to cytoskeletal rearrangements and increases in cell migration, suggesting that association with αVβ3/5 is required for these P2Y2R-mediated responses. The P2Y2R also contains 2 PXXP motifs in the intracellular C-terminal domain that represent consensus Src-homology-3 (SH3) binding sequences (Fig. 1). Activation of the wild type P2Y2R expressed in 1321N1 astrocytoma cells induces the phosphorylation of Src and EGFR, responses that are attenuated for a mutant P2Y2R in which the SH3 binding domains for Src in the intracellular C-terminus of the P2Y2R have been deleted [58]. Since the activated P2Y2R co-localizes with EGFR in the plasma membrane [58], these findings suggest that the previously reported ability of the P2Y2R to regulate EGFR phosphorylation [59, 60] is due to Src-dependent recruitment of the P2Y2R to a signaling complex containing EGFR, thereby inducing EGFR phosphorylation in response to P2Y2R ligands. These studies used kinase inhibitors to demonstrate that P2Y2R-mediated activation of the mitogen-activated protein kinases ERK1/2, is dependent on the kinase activities of Src [58] and EGFR [59]. Whereas the activities of ERK1/2 are important for P2Y2R-mediated cell/astrocyte proliferation [61], the activity of another MAP kinase, p38, is important for P2Y2R-mediated upregulation of adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), which is involved in tight binding of monocytes to endothelial cells [62] and lymphocytes to epithelial cells [63]. In astrocytic cells, the p38 signaling pathway is also required for the P2Y2R to inhibit trauma-induced cell death [64]. Other studies indicate that EGFR signaling regulates neuronal survival by promoting cortical but not midbrain astrocyte apoptosis [65], which suggests an endpoint for P2Y2R activation in the CNS. Additionally, it has been shown that the P2Y2R interacts directly with filamin A (FLNa) [66], a crosslinking cytoskeletal maintenance protein [66].
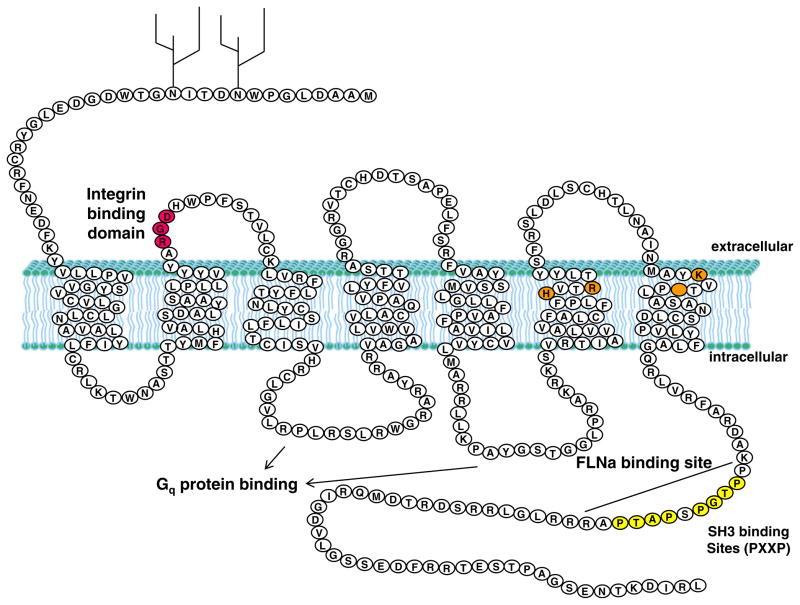
Fig. 1.
P2Y2R structure and domains: the P2Y2R is a seven pass transmembrane G protein-coupled extracellular nucleotide receptor. It is activated equipotently by ATP and UTP and has been shown to be upregulated in response to stress or injury in various cell types. Highlighted features include the consensus RGD integrin-binding domain (in pink), positively-charged amino acid residues known to be involved in ATP/UTP binding (in orange), two consensus PXXP SH3 domain binding sites (in yellow), the FLNa binding site, the intracellular loops that regulate Gq protein binding, and two glycosylation sites
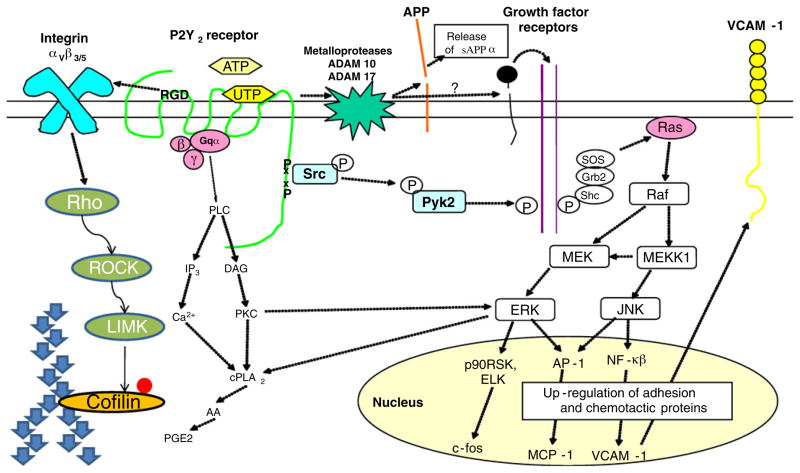
Fig. 2.
P2Y2 receptor-mediated signal transduction: activation of the P2Y2 receptor (P2Y2R) is coupled to several intracellular signal transduction pathways including: a Gq❮-dependent activation of phospholipase C (PLC) that generates inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG), second messengers for intracellular calcium mobilization and protein kinase C activation, respectively; b Src-mediated transactivation of growth factor receptor phosphorylation that stimulates mitogen-activated protein kinase cascades to regulate gene transcription; c association with and activation of αvβ3/5 integrins that stimulates Rho kinase leading to cofilin phosphorylation; and d activation of metalloproteases (i.e., ADAM10/17) to stimulate the non-amyloidogenic processing of amyloid precursor protein (APP). Other abbreviations: AA arachidonic acid, PGE2 prostaglandin E2, VCAM-1 vascular cell adhesion molecule-1
The ability of the P2Y2R to regulate signal transduction via activation of integrins and growth factor receptors, in addition to PLC, suggests that P2Y2R activation could have significant physiological and pathophysiological consequences in a variety of cell types that express the P2Y2R. P2Y2Rs are expressed in epithelial cells, smooth muscle cells, endothelial cells, monocytes, macrophages, neutrophils, and cardiomyocytes and in brain, heart, kidney, liver, spleen, placenta, and skeletal muscle tissue [35, 36, 55, 67–70]. In cells derived from the peripheral and central nervous systems, P2Y2Rs also are expressed in immortalized astrocytes, NG108-15 neuroblastoma × glioma hybrid cells, Schwann cells, dorsal horn and cortical astrocytes, astrocytoma cells, rat cortical neurons, microglia and oligodendrocytes [37, 54, 71–74]. The P2Y2R subtype is upregulated in activated thymocytes, in response to pro-inflammatory cytokines including IL-1β, interferon-γ, and tumor necrosis factor-α, and in animal models of injury or disease of the salivary gland epithelium or the vasculature [63, 67, 75–77] and nucleotides have been reported to activate promonocytic cells [78]. For example, placement of a silicone collar around a rabbit carotid artery upregulates P2Y2R expression in smooth muscle and endothelium and upon activation of the P2Y2R in vivo promotes intimal thickening and monocyte infiltration due to increased smooth muscle cell proliferation and VEGF receptor-2-dependent upregulation of VCAM-1, respectively [62, 67]. P2Y2R-mediated VCAM-1 expression also promotes lymphocyte adherence to salivary epithelial cell monolayers, a potential consequence of P2Y2R upregulation detected in a mouse model of Sjögren’s syndrome, an autoimmune exocrinopathy that leads to salivary gland dysfunction [63, 77]. The P2Y2R agonists ATP and UTP have been shown to stimulate the adherence of monocytes and neutrophils to endothelial cell monolayers [62, 79]. P2Y2R activation also regulates the synthesis of superoxide, prostaglandins, nitric oxide, and cytokines in response to the elicitors IFN-γ and LPS [34, 37, 38, 55, 80, 81].
Very few studies have investigated the consequences of P2Y2R expression in the brain. We utilized in situ hybridization and reverse transcriptase–polymerase chain reaction to identify P2Y2R messenger RNA (mRNA) expression in normal rodent (i.e., rat, mouse, and gerbil) brain slices, where expression levels were highest in the hippocampus (i.e., dentate gyrus) and cerebellum [34]. P2Y2R mRNA expression was also detected in rat primary astrocytes and microglial cells, although rat primary neurons express very low levels of P2Y2R mRNA [37, 39]. Under non-inflammatory conditions, P2Y2R expression in neurons and oligodendrocytes is low, therefore, these cells are unresponsive to UTP [82], unless the presence of the pro-inflammatory cytokine IL-1β increases functional expression of the P2Y2R in neurons [39].
P2Y2 Receptors Regulate Neuroinflammatory Responses
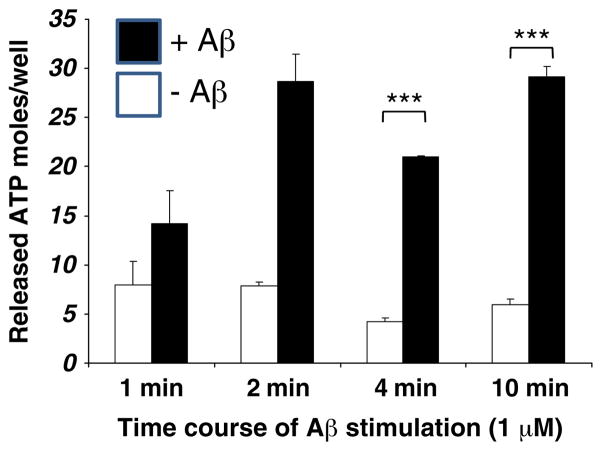
It is well accepted that nucleotides can be released into the extracellular milieu from aggregating platelets, degranulating macrophages, excitatory neurons, and injured cells [35, 49, 50]. Under pathophysiological conditions in the brain and other tissues, extracellular nucleotides can be released in response to oxidative stress, ischemia, hypoxia or mechanical stretch [45–50], consistent with the ability of released ATP and UTP to induce migration [67, 68, 83, 84] and chemotaxis of microglial cells [85] and primary rat cortical astrocytes [86]. We have also determined that the amyloidogenic peptide, oligomeric Aβ42, whose levels are elevated in Alzheimer’s brain, induces the release of ATP from mouse primary cortical astrocytes (Fig. 3). Primary rat cortical astrocytes were isolated from postnatal 2- to 3-day old rat pups. Briefly, cerebral cortices were cut into very small pieces and incubated with trypsin-EDTA at 37°C for 7 min. The suspension was filtered through 85 μm nylon mesh and centrifuged at ~250 g for 5 min. The cell pellet was resuspended in DMEM with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 7.5 μg/ml fungi-zone, and transferred to T75 culture flasks. Cells were maintained in an incubator with 5% CO2 at 37°C and the medium was changed every two days. When cells reach ~80–90% confluence, flasks were shaken at 225 rpm for 6 h at room temperature to remove microglial cells. Then, 106 cells were seeded into 12-well plates and cultured for 2 days when ATP release assays were performed. Our results showed that oligomeric Aβ42 induces the release of endogenous ATP from rat primary cortical astrocytes (Fig. 3). The basal release of ATP, determined after incubation of cells in HEPES buffer supplemented with 200 μM AOPCP, an inhibitor of 5′-nucleotidase, was 7.9, 7.8, 4.2 and 5.9 nmoles/well for 1, 2, 4 and 10 min, respectively. After stimulation of the cells with oligomeric Aβ, the endogenous ATP release was14.2, 28.7, 21 and 29.2 nmoles/well, for 1, 2, 4 and 10 min, respectively, and results compared with controls were significantly different at 4 and 10 min (p<0.01). Thus, pro-inflammatory conditions in AD that include oxidative stress and the increased production of Aβ42 [4–14], are likely to induce the release of P2Y2R agonists. Once released, these agonists will activate P2Y2Rs expressed in astrocytes and microglial cells to induce integrin-dependent activation of Rho and Rac to promote glial cell migration, and trans-activation of growth factor receptors to increase glial cell proliferation, responses associated with neuroinflammation, [34, 37, 43, 56–58] (Fig. 4), although nucleotides have been suggested to exert anti-inflammatory effects in LPS-treated microglial cells [74].
Fig. 3.
Effect of oligomeric Aβ42 on ATP release from primary cultured rat cortical astrocytes: the cells were incubated for 15 min at 37°C with HEPES buffer supplemented with 200 μM AOPCP, an inhibitor of 5′-nucleotidases, to retard ATP breakdown. Cells were washed 3 times using the same buffer and then incubated for different time periods at 37°C with or without oligomeric Aβ. Supernatants were collected and released ATP was measured with the ATP Bioluminescence Assay kit HSII (Roche). The ATP levels were calculated based on an ATP standard curve. The results are expressed as nmoles of ATP released per well of 12-well plate and are presented as means±S.E.M.; n=3. White bars are basal levels at each time point (without oligomeric Aβ) and black bars are stimulated ATP release (with oligomeric Aβ). **p<0.01
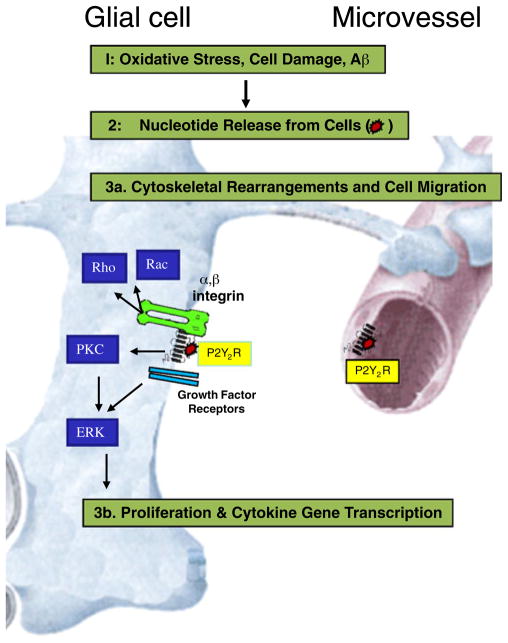
Fig. 4.
P2Y2Rs in astrocytes and microvessels: chronic inflammation caused by oxidative stress in brain is mediated by P2Y2Rs via cytokine-like actions of nucleotides in astrocytes and microvessels through transactivation of integrins and growth factor receptors. Nucleotides are released from oxidatively-stressed brain cells activating P2Y2Rs in astrocytes and microvessels. Activation of endogenously expressed P2Y2Rs in glial cells leads to integrin-mediated cell migration (via the P2Y2R RGD domain), which has been shown to be necessary for cell migration. Nucleotideinduced and integrin-dependent activation of Rho and Rac promotes glial cell migration, and P2Y2R-induced transactivation of growth factor receptors increases cell proliferation and pro-inflammatory gene expression
P2 receptor activation in vascular smooth muscle and glial cells also has been shown to increase the release of pro-inflammatory cytokines, including IL-1β and IFNγ [76, 87, 88]. Since cytokine release is dependent on metalloprotease activation, we postulate that IL-1β release from astrocytes is dependent upon P2Y2R-mediated metal-loprotease activation (see Fig. 2). Consistent with this hypothesis, P2Y2R activation has been shown to activate the metalloproteases ADAM10 and ADAM17 in astrocytoma cells, primary neurons and salivary epithelial cells [39, 89].
P2Y2Rs Mediate Neuroprotective APP Processing
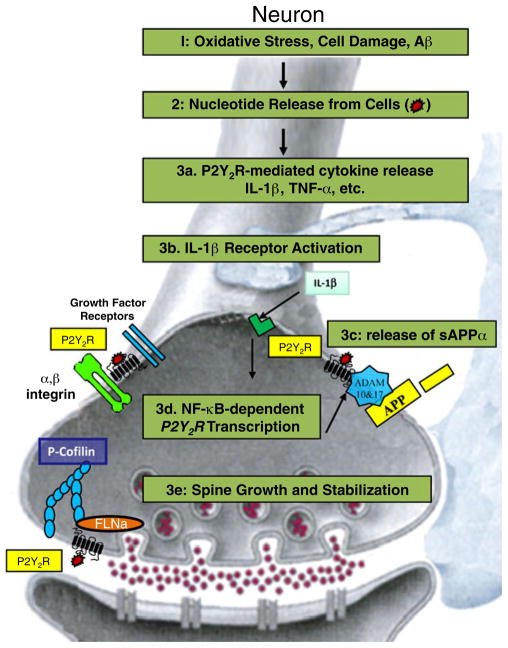
The inflammatory cytokine IL-1β whose levels are elevated in AD [90] has been shown to upregulate functional expression of the P2Y2R in rat primary cortical neurons [39]. IL-1β release from astrocytes and microglia has been shown to be induced by exogenous ATP acting through the P2X7 receptor, however, the contribution of other P2 purinergic receptors was not excluded [91]. In primary rat and mouse neuronal cultures, the P2Y2R is expressed at very low levels (39, unpublished data). However, Il-1β induces an increase in P2Y2R expression by activating the NF-κB signaling pathway, since Bay-11-7085, an irreversible inhibitor of IκB-α phosphorylation and thus NF-κB activation, decreases IL-1β-induced P2Y2R expression levels in rat primary cortical neurons [39]. These results are consistent with the finding that the P2Y2R promoter contains an NF-κB binding site that regulates P2Y2R transcription in intestinal epithelial cells [92]. Since the pro-inflammatory cytokine IL-1β upregulates P2Y2R expression in neurons, it was somewhat surprising to find that the P2Y2R serves a potential neuroprotective role by stimulating the non-amyloidogenic processing of APP [89] and the activation of cofilin [56], a cytoskeletal actin-binding protein that is known to promote dendritic spine growth and stabilization [26, 93–95] (Fig. 5).
Fig. 5.
P2Y2Rs in neurons: nucleotides released from oxidatively stressed brain cells activate P2Y2Rs on neurons. P2Y2R activation induces release of cytokines, which upregulate the expression of the P2Y2R. Additionally, extracellular nucleotides activate matrix metal-loproteases to increase production of the non-amyloidogenic APP fragment, sAPP-α. Activation of the P2Y2R also promotes binding of FLNa to the C-terminal domain of the receptor and phosphorylation of cofilin
Our findings indicate that P2Y2R activation stimulates the α- and γ-secretase-dependent proteolytic processing of APP to generate the non-amyloidogenic peptide soluble amyloid precursor-α (sAPPα) in both astrocytoma cells expressing the wild type P2Y2R [89] and in primary rat cortical neurons treated overnight with IL-1β [39]. Production of sAPPα from APP would be anticipated to decrease the production of amyloidogenic Aβ peptide, the main component of senile plaques in the AD brain [96, 97]. APP is either proteolytically processed by β- and γ-secretases to release Aβ, or by α- and γ-secretases to produce sAPPα. APP is a transmembrane glycoprotein that is present in a variety of tissues, but predominantly in the brain [98]. APP contains an extracellular N terminus and a short C-terminal region that lies in the cytoplasm. Within APP, a single membrane-spanning region of 39-42 amino acids represents Aβ [99, 100]. Proteolytic cleavage of APP in vivo can occur at the amino terminus of the Aβ domain (by β-secretase), within the Aβ domain (by α-secretase), and at the C-terminus of the Aβ domain (by γ-secretase) [101]. Thus, the ability of the P2Y2R to activate α-secretase and generate sAPPα, the soluble, non-amyloidogenic N-terminal fragment (~100–140 kD) of APP, precludes the potential release of amyloidogenic Aβ1-42 from the same APP molecule. Although not determined in our studies, it has been reported that the membrane-retained fragment resulting from sAPPα release undergoes further cleavage and endocytotic processing [102–104]. The released sAPPα fragment has been shown to have both neurotrophic [105] and neuroprotective [106–109] activities, suggesting that the pro-inflammatory upregulation of P2Y2Rs in neurons may be beneficial.
PKC-dependent and -independent pathways stimulated by several G protein-coupled receptors (GPCRs) have been reported to induce sAPPα release [110–112]. Over-expression of the human M1 and M3 muscarinic receptors in HEK293 cells stimulates sAPPα secretion [113]. Subsequently, thrombin, bradykinin, glutamate, and serotonin (5-HT) receptors have been shown to regulate sAPPα release [114–118]. Other studies indicate that reduction in Aβ42 is associated with receptor-mediated activation of sAPPα release [119–121]. We have found that P2Y2R activation stimulated α-secretase by the furin-dependent activation of two members of the ADAM (for a disintegrin and metalloprotease) family [39, 89], ADAM10, the Kuz enzyme [122] and ADAM17/TACE (tumor necrosis factor-α converting enzyme), the protease responsible for releasing TNF-α from the plasma membrane [123]. The cleavage of pro-IL-1β into mature IL-1β is achieved by a cysteine protease belonging to the caspase family, the IL-1β-converting enzyme (ICE), known to be activated by ATP [124].
P2Y2R-mediated Cytoskeletal Signaling in Primary Rat Neurons
It has been demonstrated that ATP released from the leading edge of the neutrophil surface amplifies chemotactic signals and directs cell orientation by activation of the P2Y2R [38]. Our previous studies indicate that P2Y2R activation in astrocytoma cells promotes the formation of actin stress fibers and induces cell migration [56, 57], although little is known about the effect of P2Y2R activation on cytoskeletal functions in neurons. We found that treatment of primary cortical neurons from mice and rats with IL-1β induced P2Y2R upregulation (39, unpublished data). Subsequent P2Y2R activation with UTP induces Rho and LIM kinase activation that increases the phosphorylation of the actin-depolymerization factor cofilin [56], a response known to promote localized F-actin expansion and the stabilization of dendritic spines [56, 94, 95, 125, 126]. Since we have found that P2Y2R interaction with αvβ3/5 integrins mediates cytoskeletal rearrangements and cell migration in astrocytoma cells via activation of Rho kinase, we postulate that a similar pathway regulates cofilin phosphorylation in neurons (Fig. 5). Previous studies have shown that inhibition of cofilin activation by expressing a phosphomimetic mutant of cofilin (cof-S3D) prevented Aβ-induced spine loss [26]. Activation of the P2Y2R causes dynamic reorganization of the actin cytoskeleton in migratory cell types, and our results indicate that the P2Y2R directly binds FLNa, activates focal adhesion molecules, and induces the phosphorylation of cofilin, suggesting that P2Y2Rs utilize these signaling pathways to regulate actin cytoskeletal rearrangements that promote dendritic spine growth and stabilization in neurons.
Conclusion
The neuroprotective mechanisms underlying acute inflammatory responses in the brain become neurodegenerative when sustained [19–21], as occurs in brain pathologies including AD, trauma, and stroke [22]. The ATP and UTP-activated Gq protein-coupled P2Y2R is expressed in glial cells and regulates a variety of intracellular signal transduction pathways via activation of integrins, growth factor receptors, and PLC to promote cytoskeletal rearrangements, cell migration and proliferation, associated with reactive astrogliosis in the AD brain. In neurons, upregulation of P2Y2Rs by IL-1β promotes the nucleotide-induced non-amyloidogenic processing of APP and the phosphorylation of cofilin, responses that are neuroprotective. Thus, the P2Y2R may represent a novel target for the prevention of neuronal damage in AD and related neuroinflammatory diseases.
Acknowledgments
Supported by NIH grants AG18357, DE07389, and DE17591.
Contributor Information
Troy S. Peterson, Email: troy.peterson1@gmail.com, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA
Jean M. Camden, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA
Yanfang Wang, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA.
Cheikh I. Seye, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, Indianapolis, IN 46202, USA
W. G. Wood, Department of Pharmacology, University of Minnesota School of Medicine and Geriatric Research, Education and Clinical Center, Minneapolis, MN 55455, USA
Grace Y. Sun, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA
Laurie Erb, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA.
Michael J. Petris, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA
Gary A. Weisman, Department of Biochemistry, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, USA
References
- 1.Lee RK, Knapp S, Wurtman RJ. Prostaglandin E2 stimulates amyloid precursor protein gene expression: inhibition by immunosuppressants. J Neurosci. 1999;19:940–947. doi: 10.1523/JNEUROSCI.19-03-00940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. Curr Opin Lipidol. 2002;13:289–294. doi: 10.1097/00041433-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. J Neurovirol. 2002;8:539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 5.Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 6.Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Raina AK, Smith MA, Sayre LM, Perry G. Hydroxynonenal, toxic carbonyls, and Alzheimer disease. Mol Aspects Med. 2003;24:305–313. doi: 10.1016/s0098-2997(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004;1000:32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield DA, Boyd-Kimball D. Amyloid beta-peptide (1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14:426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochem. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- 13.Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid-beta induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol Aging. 2004;25:431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45:170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- 18.Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 19.O’Callaghan JP, Jensen KF. Enhanced expression of glial fibrillary acidic protein and the cupric silver degeneration reaction can be used as sensitive and early indicators of neurotoxicity. Neurotoxicol. 1992;13:113–122. [PubMed] [Google Scholar]
- 20.Eddleston M, Mucke L. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neurosci. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menet V, Prieto M, Privat A, Gimenez y Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 23.Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- 24.Rutka JT, Murakami M, Dirks PB, Hubbard SL, Becker LE, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997;87:420–430. doi: 10.3171/jns.1997.87.3.0420. [DOI] [PubMed] [Google Scholar]
- 25.Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- 26.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gahtan E, Overmier JB. Inflammatory pathogenesis in Alzheimer’s disease: biological mechanisms and cognitive sequeli. Neurosci Biobehav Rev. 1999;23:615–633. doi: 10.1016/s0149-7634(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 28.McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001;63:109–115. doi: 10.1002/1097-4547(20010115)63:2<109::AID-JNR1002>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Chan-Ling T, Stone J. Factors determining the migration of astrocytes into the developing retina: migration does not depend on intact axons or patent vessels. J Comp Neurol. 1991;303:375–386. doi: 10.1002/cne.903030304. [DOI] [PubMed] [Google Scholar]
- 30.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller WJ, Leventhal I, Scarsella D, Haydon PG, Janmey P, Meaney DF. Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness. J Neurotrauma. 2009;5:789–797. doi: 10.1089/neu.2008-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franke H, Krugel U, Illes P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- 34.Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, González FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol. 2005;31:169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol. 1996;16:397–400. doi: 10.1111/j.1474-8673.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 36.Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Review. Pharmacol Ther. 2006;109:297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Gendron FP, Newbold NL, Vivas-Mejia PE, Wang M, Neary JT, Sun GW, Gonzalez FA, Weisman GA. Signal transduction pathways for P2Y2 and P2X7 nucleotide receptors that mediate neuroinflammatory responses in astrocytes and microglial cells. Biomed Res. 2003;14:47–61. [Google Scholar]
- 38.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 39.Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY, Weisman GA. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109:1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 41.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 43.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 44.Vitolo OV, Ciotti MT, Galli C, Borsello T, Calissano P. Adenosine and ADP prevent apoptosis in cultured rat cerebellar granule cells. Brain Res. 1998;809:297–301. doi: 10.1016/s0006-8993(98)00713-6. [DOI] [PubMed] [Google Scholar]
- 45.Ostrom RS, Gregorian C, Drenan RM, Gabot K, Rana BK, Insel PA. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol. 2001;281:C524–C531. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- 47.Ciccarelli R, Di Iorio P, Giuliani P, D’Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–98. [PubMed] [Google Scholar]
- 48.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 49.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen SF, Nilius B, Lambert IH, Hoffmann EK. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim Biophys Acta. 1999;1416:271–284. doi: 10.1016/s0005-2736(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 51.Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002;397:131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- 52.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 53.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D’Onofrio M, Caciagli F, Di Iorio P. Involvement of astrocytes in purine mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19:395–414. doi: 10.1016/s0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 54.Weisman GA, Garrad RC, Erb LJ, Santos-Berrios C, Gonzalez FA. P2Y receptors in the nervous system: molecular studies of a P2Y2 receptor subtype from NG108-15 neuroblastoma x glioma hybrid cells. Prog Brain Res. 1999;120:33–43. doi: 10.1016/s0079-6123(08)63544-x. [DOI] [PubMed] [Google Scholar]
- 55.Lustig KD, Erb L, Landis DM, Hicks-Taylor CS, Zhang X, Sportiello MG, Weisman GA. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134:61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- 56.Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. SH3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, Pyk2, and growth factor receptors. J Biol Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 59.Soltoff SP. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J Biol Chem. 1998;273:23110–23117. doi: 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- 60.Soltoff SP, Avraham H, Avraham S, Cantley LC. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem. 1998;273:2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- 61.Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience. 2006;142:411–423. doi: 10.1016/j.neuroscience.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 62.Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24,960–24,965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 63.Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 [corrected] expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol. 2007;45:65–75. doi: 10.1016/j.molimm.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgos M, Neary JT, González FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J Neurochem. 2007;103:1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- 65.Wagner B, Natarajan A, Grünaug S, Kroismayr R, Wagner EF, Sibilia M. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. EMBO J. 2006;25:752–762. doi: 10.1038/sj.emboj.7600988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res. 2008;102:581–588. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, González FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circ. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 68.Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- 69.Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2u purinoceptor. Eur Respir J. 1996;9:542–548. doi: 10.1183/09031936.96.09030542. [DOI] [PubMed] [Google Scholar]
- 71.Berti-Mattera LN, Wilkins PL, Madhun Z, Suchovsky D. P2-purigenic receptors regulate phospholipase C and adenylate cyclase activities in immortalized Schwann cells. Biochem J. 1996;314:555–561. doi: 10.1042/bj3140555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3- sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boucsein C, Zacharias R, Färber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;11:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 75.Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci USA. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou M, Moller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20:2064–2069. doi: 10.1161/01.atv.20.9.2064. [DOI] [PubMed] [Google Scholar]
- 77.Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjögren’s syndrome. Arch Oral Biol. 2005;50:533–540. doi: 10.1016/j.archoralbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Ventura MA, Thomopoulos P. ADP and ATP activate distinct signaling pathways in human promonocytic U-937 cells differentiated with 1, 25-dihydroxy-vitamin D3. Mol Pharmacol. 1995;47:104–114. [PubMed] [Google Scholar]
- 79.Parker AL, Likar LL, Dawicki DD, Rounds S. Mechanism of ATP-induced leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol. 1996;270:L695–L703. doi: 10.1152/ajplung.1996.270.5.L695. [DOI] [PubMed] [Google Scholar]
- 80.Weisman GA, Garrad RC, Erb LJ, Otero M, Gonzalez FA, Clarke LL. Structure and function of P2Y2 nucleotide receptors in cystic fibrosis (CF) epithelium. Adv Exp Med Biol. 1998;431:417–424. doi: 10.1007/978-1-4615-5381-6_82. [DOI] [PubMed] [Google Scholar]
- 81.Denlinger LC, Fisette PL, Garis KA, Kwon G, Vazquez-Torres A, Simon AD, Nguyen B, Proctor RA, Bertics PJ, Corbett JA. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- 82.Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J Cell Sci. 2009;122:2524–2533. doi: 10.1242/jcs.044891. [DOI] [PubMed] [Google Scholar]
- 83.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- 84.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circ. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 85.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ballerini P, Di Iorio P, Caciagli F, Rathbone MP, Jiang S, Nargi E, Buccella S, Giuliani P, D’Alimonte I, Fischione G, Masciulli A, Romano S, Ciccarelli R. P2Y2 receptor up-regulation induced by guanosine or UTP in rat brain cultured astrocytes. Int J Immunopathol Pharmacol. 2006;19:293–308. doi: 10.1177/039463200601900207. [DOI] [PubMed] [Google Scholar]
- 87.Hou M, Moller S, Edvinsson L, Erlinge D. MAPKK-dependent growth factor induced upregulation of P2Y2 receptors in vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;258:648–652. doi: 10.1006/bbrc.1999.0676. [DOI] [PubMed] [Google Scholar]
- 88.Morigiwa K, Fukuda Y, Yamashita M. Neurotransmitter ATP and cytokine release. Nippon Yakurigaku Zasshi Review. 2000;115:185–192. doi: 10.1254/fpj.115.185. Japanese. [DOI] [PubMed] [Google Scholar]
- 89.Camden JM, Schrader AM, Camden RE, González FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- 90.Colangelo J, Schurr MJ, Ball RP, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 91.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 92.Degagné E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sévigny J, Gendron FP. P2Y2 receptor transcription is increased by NF-κB and stimulates cyclooxygenase-2 expression and PGE2 release by intestinal epithelial cells. J Immunol. 2009;183:4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng LH, Colman DR, Miki N. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer precursor protein in neuronal PC12 cells. Neurosci Lett. 1991;128:126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- 97.Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 98.Mattson MP. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 99.Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- 100.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 101.Mills J, Reiner PB. Regulation of amyloid precursor protein cleavage. J Neurochem. 1999;72:443–460. doi: 10.1046/j.1471-4159.1999.0720443.x. [DOI] [PubMed] [Google Scholar]
- 102.Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 103.Oltersdorf T, Ward PJ, Henriksson T, Beattie EC, Neve R, Lieberburg I, Fritz LC. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990;265:4492–4497. [PubMed] [Google Scholar]
- 104.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 105.Wallace WC, Akar CA, Lyons WE. Amyloid precursor protein potentiates the neurotrophic activity of NGF. Brain Res Mol Brain Res. 1997;52:201–212. doi: 10.1016/s0169-328x(97)00258-1. [DOI] [PubMed] [Google Scholar]
- 106.Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 107.Bowes MP, Masliah E, Otero DA, Zivin JA, Saitoh T. Reduction of neurological damage by a peptide segment of the amyloid β/A4 protein precursor in a rabbit spinal cord ischemia model. Exp Neurol. 1994;129:112–119. doi: 10.1006/exnr.1994.1152. [DOI] [PubMed] [Google Scholar]
- 108.Smith-Swintosky VL, Pettigrew LC, Craddock SD, Culwell AR, Rydel RE, Mattson MP. Secreted forms of β-amyloid precursor protein protect against ischemic brain injury. J Neurochem. 1994;63:781–784. doi: 10.1046/j.1471-4159.1994.63020781.x. [DOI] [PubMed] [Google Scholar]
- 109.Barger SW, Van Eldik LJ, Mattson MP. S100β protects hippocampal neurons from damage induced by glucose deprivation. Brain Res. 1995;677:167–170. doi: 10.1016/0006-8993(95)00160-r. [DOI] [PubMed] [Google Scholar]
- 110.Nitsch RM, Slack BE, Farber SA, Schulz JG, Deng M, Kim C, Borghesani PR, Korver W, Wurtman RJ, Growdon JH. Regulation of proteolytic processing of the amyloid β-protein precursor of Alzheimer’s disease in transfected cell lines and in brain slices. J Neural Transmiss Suppl. 1994;44:21–27. doi: 10.1007/978-3-7091-9350-1_2. [DOI] [PubMed] [Google Scholar]
- 111.Nitsch RM, Wurtman RJ, Growdon JH. Regulation of proteolytic processing of the amyloid β-protein precursor by first messengers. A novel potential approach for the treatment of Alzheimer’s disease. Arzneimittel-Forschung. 1995;45:435–438. [PubMed] [Google Scholar]
- 112.Checler F. Processing of the β-amyloid precursor protein and its regulation in Alzheimer’s disease. J Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- 113.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 114.Nitsch RM, Slack BE, Farber SA, Borghesani PR, Schulz JG, Kim C, Felder CC, Growdon JH, Wurtman RJ. Receptor-coupled amyloid precursor protein processing. Ann NY Acad Sci. 1993;695:122–127. doi: 10.1111/j.1749-6632.1993.tb23039.x. [DOI] [PubMed] [Google Scholar]
- 115.Nitsch RM, Deng M, Growdon JH, Wurtman RJ. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J Biol Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 116.Nitsch RM, Deng A, Wurtman RJ, Growdon JH. Metabotropic glutamate receptor subtype mGluR1α stimulates the secretion of the amyloid β-protein precursor ectodomain. J Neurochem. 1997;69:704–712. doi: 10.1046/j.1471-4159.1997.69020704.x. [DOI] [PubMed] [Google Scholar]
- 117.Davis-Salinas J, Saporito-Irwin SM, Donovan FM, Cunningham DD, Van Nostrand WE. Thrombin receptor activation induces secretion and nonamyloidogenic processing of amyloid β-protein precursor. J Biol Chem. 1994;269:22623–22627. [PubMed] [Google Scholar]
- 118.Lee RK, Wurtman RJ, Cox AJ, Nitsch RM. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc Natl Acad Sci USA. 1995;92:8083–8087. doi: 10.1073/pnas.92.17.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hung AY, Haass C, Nitsch RM, Qiu WQ, Citron M, Wurtman RJ, Growdon JH, Selkoe DJ. Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J Biol Chem. 1993;268:22959–22962. [PubMed] [Google Scholar]
- 120.Buxbaum JD, Koo EH, Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid β/A4 peptide. Proc Natl Acad Sci USA. 1993;90:9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolf BA, Wertkin AM, Jolly YC, Yasuda RP, Wolfe BB, Konrad RJ, Manning D, Ravi S, Williamson JR, Lee VM. Alzheimer’s disease amyloid precursor protein secretion and amyloid β-protein production in human neuronal NT-2 cells. J Biol Chem. 1995;270:4916–4922. doi: 10.1074/jbc.270.9.4916. [DOI] [PubMed] [Google Scholar]
- 122.Rooke J, Pan D, Xu T, Rubin GM. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273:1227–1231. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- 123.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 124.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1β by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 125.Takemura M, Mishima T, Wang Y, Kasahara J, Fukunaga K, Ohashi K, Mizuno K. Ca2+/calmodulin-dependent protein kinase IV-mediated LIM kinase activation is critical for calcium signal-induced neurite outgrowth. J Biol Chem. 2009;284:28554–28562. doi: 10.1074/jbc.M109.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. Review. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]