Abstract
Purpose
This study presents an analysis of language skills in individuals with Noonan syndrome (NS), an autosomal dominant genetic disorder. We investigated whether the language impairments affecting some individuals arise from deficits specifically within the linguistic system or whether they are associated with cognitive, perceptual, and motor factors. Comparisons of language abilities among the different NS genotypes were also conducted.
Method
Sixty-six children and adolescents with NS were evaluated using standardized speech, language, and literacy assessments. Additional cognitive, perceptual, and motor tasks were administered to examine the relation of these factors to language development. Genotype was noted for those who underwent genetic testing.
Results
Language impairments were more frequent in NS than in the general population and were associated with higher risk for reading and spelling difficulties. Language was significantly correlated with nonverbal cognition, hearing ability, articulation, motor dexterity, and phonological memory. Genotype analyses suggest that the higher performance of SOS1-positive than PTPN11-positive individuals on language tasks was largely mediated by differences in cognitive ability.
Conclusions
Our results indicate that variation in language skill in NS is closely related to cognitive, perceptual, and motor factors. It does not appear that specific aspects of language are selectively affected in this syndrome.
Keywords: Noonan syndrome, language, articulation, literacy, developmental disorders
Noonan syndrome (NS) is a multiple congenital anomaly syndrome first described by Noonan and Ehmke (1963). The incidence is estimated to be 1:1,000 to 1:2,500 live births (Mendez, Opitz, & Allanson, 1985). The phenotype is variable, but common findings include cardiac disease, short stature, facial anomalies, and mild learning disabilities. NS is a single-gene disorder that results from a missense mutation in of one of several different Ras/mitogen-activated protein kinase (MAPK) pathway genes. Mutations in the PTPN11 gene lead to approximately 50% of NS cases (Tartaglia et al., 2001), and mutations in the SOS1, RAF1, or KRAS genes account for an additional 10%–15%, 3%–17%, and <5% of cases, respectively (Pandit et al., 2007; Razzaque et al., 2007; Roberts et al., 2007; Schubbert et al., 2006; Tartaglia et al., 2007). BRAF mutations can also cause a NS phenotype, although mutations in this gene are typically associated with cardiofaciocutaneous syndrome (Nyström et al., 2008; Razzaque et al., 2007). The gene(s) associated with NS in the remaining cases have not yet been identified.
The majority of NS gene mutations result in increased activation of Ras proteins. These proteins regulate cell proliferation, survival, and differentiation, and they are critical for normal growth and development (Schubbert, Bollag, & Shannon, 2007). Much recent research has focused on the pathogenesis of medical features such as congenital heart defects and juvenile cancers in NS (Miyamoto et al., 2008; Nakamura et al., 2007; Sznajer et al., 2007); however, the effects of dysregulation of the Ras/MAPK pathway on brain and behavior are not as well understood. Some research suggests that gain-of-function alterations of this pathway can affect murine neural development (Gauthier et al., 2007). Behavioral studies indicate that cognitive functioning is variable in individuals with NS, ranging from moderate intellectual disability to superior ability (van der Burgt et al., 1999). Generally, children and adolescents with NS are at greater risk for cognitive impairments than their typically developing peers, and they may also have delays in areas such as attention and motor functioning (Horiguchi & Takeshita, 2003; Lee, Portnoy, Hill, Gillberg, & Patton, 2005). A recent study found that some variation in cognitive functioning may be explained by genotype differences, with lower rates of intellectual disability observed in individuals with SOS1 mutations (Pierpont et al., 2009).
Some evidence suggests that speech and language impairments may be a common characteristic of NS, yet this area has received little research attention. Two case reports have documented young children with severe impairments in capacities such as articulation, phonology, grammatical skills, and vocabulary (Hopkins-Acos & Bunker, 1979; Wilson & Dyson, 1982). These reports are an important first step for identifying potential communication impairments, but they provide little information as to whether such impairments are typical in patients with NS. In the current study, we sought to better understand the language phenotype of individuals with NS. Of primary interest was whether speech and language abilities in this population are closely tied to general cognitive, perceptual, and motor factors or whether individuals with NS exhibit impairments that are more specific to the language system.
Relation of Language Impairments to Other Aspects of Development
Whether speech and language impairments arise from deficient language-specific mechanisms or more general developmental or learning problems has been well studied in children with other developmental disabilities (Abbeduto, Evans, & Dolan, 2001; Bishop, 2000; Kjelgaard & Tager-Flusberg, 2001). In some children, the severity of language difficulties cannot be fully explained by general impairments in cognitive functioning or by other neurological or environmental deficiencies. A particularly striking example of this is children with specific language impairment (SLI). This diagnosis is made when a child has difficulties with language development in the absence of obvious explanatory characteristics such as cognitive impairment, hearing loss, physical limitations, or abnormalities in the language learning environment. Children with SLI tend to have particular difficulty with the grammatical structures of language such as morphology and syntax (Leonard, 1998; Tomblin & Zhang, 1999), although the components that are most affected can vary somewhat cross-linguistically (Caselli, Monaco, Trasciani, & Vicari, 2008; Thordardottir, 2008). Expressive language is also usually more severely impaired than receptive language (Laws & Bishop, 2003; Loucas et al., 2008).
In some sense, it is unsurprising that language ability is not commensurate with cognitive ability in SLI because individuals are selected for the diagnosis on this basis. However, some researchers have noted that the pattern of asynchronous language development in relation to cognitive functioning is observed in individuals with other developmental disorders such as Down syndrome (DS; Laws & Bishop, 2003). DS is caused by a chromosomal abnormality (Trisomy 21) that leads to multiple physical and mental disabilities. Although most individuals with DS have intellectual impairments (unlike in SLI), numerous studies have demonstrated that language impairments tend to be more severe than would be predicted on the basis of the intellectual disability (e.g., Abbeduto, Pavetto, et al., 2001; Caselli et al., 2008; Chapman, Schwartz, & Bird, 1991; Kernan & Sabsay, 1996; Price et al., 2008). Further, contributions of other factors such as hearing loss, speech impairments, and socioeconomic factors to variation in language skill in DS tend to be relatively small (Abbeduto et al., 2003; Chapman et al., 1991; Laws, 2004). In DS, impairments are particularly marked in expressive language, and structural aspects of language such as syntax and phonology tend to be more impaired than vocabulary (Abbeduto et al., 2003; Chapman et al., 1991; Laws & Bishop, 2003). Hence, evidence suggests that in some developmental conditions such as DS and SLI, language development is impaired relative to intellectual ability.
In contrast to this profile of more marked difficulties with language in relation to cognitive ability, there are some cases in which physical or cognitive risk factors for language disability are more easily identified. Measurable effects on language learning can occur because of medical or environmental factors such as traumatic brain injuries (Thal, Reilly, Seibert, Jeffries, & Fenson, 2004), various forms of hearing loss (Bennett & Furukawa, 1984; Wake & Poulakis, 2004), or impoverished language input (Hoff, 2003). Additionally, in some populations with intellectual disability, language development may be more consistent with general cognitive development. For example, individuals with intellectual disability of unknown etiology do not tend to show the pattern of more severe language deficits in relation to nonverbal ability that is seen in DS (Chapman, 2006; Kernan & Sabsay, 1996). Similarly, some research indicates that individuals with fragile X syndrome (FXS), a syndrome resulting from a mutation in the FMR1 gene, have grammatical and lexical development that is commensurate with nonverbal abilities. Several studies have demonstrated that some individuals with FXS can perform as well as younger, typically developing control participants on vocabulary and syntax tasks (Abbeduto et al., 2003; Paul et al., 1987; Sudhalter, Scarborough, & Cohen, 1991), although group differences have been observed on some expressive measures (Price et al., 2008). In one study of adolescents and adults with FXS, receptive language skills and nonverbal cognitive ability were found to be highly correlated (Abbeduto et al., 2003). Further, individuals with FXS also perform consistently better on most language tasks (especially grammatical tasks) than mental-age-matched peers with DS (Abbeduto et al., 2003; Abbeduto, Pavetto, et al., 2001; Price et al., 2008). This relative synchrony between different domains in FXS has led some researchers to suggest that language development in this population may have a strong relationship with broader conceptual abilities (Abbeduto et al., 2003). Other identifiable factors that may play a role in accounting for language differences in FXS include gender (FXS is an X chromosome disorder that is generally milder in women; Abbeduto et al., 2003; Fisch et al., 1999) and presence/absence of autistic features (Philofsky, Hepburn, Hayes, Hagerman, & Rogers, 2004). Thus, many of the factors affecting language development in FXS and other individuals with intellectual disability may be general physical or cognitive features rather than specific processing systems.
Pattern of Language Skills
One aim of the current study was to determine whether individuals with NS have an asynchronous pattern of language development or whether language skills are commensurate with nonlinguistic functions. Laws and Bishop (2003) outlined several characteristics of the pattern of abilities that are typically seen in individuals with asynchronous language development, such as people with SLI or DS. The pattern includes the following characteristics: (a) Language abilities are lower than expected on the basis of cognitive ability, and language impairments may occur in individuals with normal IQ; (b) perceptual, motor, and environmental variables have relatively small influences on speech and language; (c) receptive language tends to be stronger than expressive language; and (d) structural aspects of language such as syntax and phonology are more severely affected, whereas vocabulary and pragmatic language skills are relatively spared.
In contrast to this profile, if language impairments in a population occur in the context of more general deficits in intellectual, perceptual, or motor functioning, a more synchronous pattern should emerge. In this case, (a) language development should not be particularly impaired compared with cognitive ability, but performance on language tasks should be correlated with the degree of cognitive deficit/delay; (b) identifiable perceptual or motor deficits such as speech articulation or hearing loss might be expected to account for some variance in language functioning; (c) expressive language skills should not be particularly impaired relative to receptive skills; and (d) performance on different language tasks should not be consistently worse in certain language domains (e.g., grammatical skills).
Investigating the Language Phenotype in NS
In this study, we aimed to analyze the profile of language skills among individuals with NS and to examine the association of language ability to other cognitive, motor, and perceptual factors. To achieve these aims, we conducted behavioral assessments that included both linguistic and nonlinguistic tasks. Our analyses focused on five major research questions:
What is the overall pattern of language abilities in children and adolescents with NS? Because of the dearth of research on communication abilities in NS, we assessed language skills broadly, including both expressive and receptive language measures, pragmatic uses of language, and basic literacy skills (reading decoding and spelling). This allowed us to investigate the performance of individuals with NS compared with the normative population. We also examined performance across different areas of language to see whether a synchronous or an asynchronous pattern emerged.
What is the relationship between language skills and nonverbal cognitive ability? On the basis of previous reports, we hypothesized that at least some individuals with NS would have speech and language impairments. We were interested in the extent to which language difficulties co-occurred with cognitive impairments and, more broadly, whether language and nonverbal cognitive ability had a strong association within this population.
Which cognitive, motor, or perceptual factors are related to language abilities in individuals with NS? To determine which factors place an individual with NS at greater risk for language difficulties, we examined the association between language functioning and several other variables. In addition to nonverbal cognitive functioning, we assessed speech articulation, hearing, manual motor dexterity, and phonological memory. Speech articulation was examined to determine whether deficits in articulatory mechanisms could explain variation in language ability. Hearing ability was examined because of its potential detrimental influence on language development. Hearing loss is more common in individuals with NS than age-matched peers, and both conductive and sensorineural hearing impairments have been documented (Foster & Dyhrkopp, 1998; Qiu, Yin, & Stucker, 1998). We therefore included two basic measures of audiologic functioning. Manual motor skills were assessed to obtain a more global measure of motor functioning. As motor deficits are highly comorbid with language impairments (Hill, 2001) and are common in NS (Lee et al., 2005; Mendez et al., 1985), we aimed to examine whether these skills are related to speech and language skills in affected individuals. Finally, we also examined phonological memory, a processing system known to be consistently impaired in individuals with SLI and DS (Ellis Weismer et al., 2000; Estes, Evans, & Else-Quest, 2007; Hick, Botting, & Conti-Ramsden, 2005; Laws, 2004). Among language-impaired individuals, phonological memory deficits are known to be heritable (Bishop, North, & Donlan, 1996). We hypothesized that participants with NS who had poor phonological memory skills would also be more likely to have language impairments.
Are children and adolescents with NS who have language impairments more likely to have difficulties with literacy? Anecdotal and case reports suggest that reading and spelling skills are an area of weakness in at least some individuals with NS (Teeter, 1999; Troyer & Joschko, 1997). Because reading disabilities are very common in language-impaired populations (Catts, Fey, Tomblin, & Zhang, 2002; McArthur, Hogben, Edwards, Heath, & Mengler, 2000), we suspected children with NS who had language impairments would be at higher risk for literacy difficulties. In particular, we predicted that reading and spelling skills might be related to phonological abilities, as this factor is known to exert independent influence on literacy development (Catts, Adlof, Hogan, & Ellis Weismer, 2005; Fraser & Conti-Ramsden, 2008; Kennedy & Flynn, 2002; Maridaki-Kassotaki & Harakopio, 2002).
Are there genotype differences in language ability in NS? A final aim of our analyses was to investigate whether genetic factors play a role in language outcomes in NS. Our previous work showed a relationship between genotype and cognitive ability in NS, such that individuals with SOS1 mutations were at lower risk for cognitive disabilities than individuals with PTPN11 mutations (Pierpont et al., 2009). Thus, we conducted several analyses to identify whether variation in language could be explained by differences in genotype and, if so, whether this relationship was mediated by the general cognitive differences established previously. Further, we conducted some preliminary analyses to explore whether individuals with rarer genotypes (i.e., RAF1 or BRAF) differed markedly in language ability from the rest of the NS group.
Method
Participants
Sixty-six individuals with NS between the ages of 4 and 18 years (M = 10.0, SD = 4.1) participated in this study. The cohort included 36 male and 30 female participants. Individuals were recruited for the study if they had received a clinical diagnosis of NS from a geneticist. Criteria for inclusion were based on a scoring system by van der Burgt et al. (1994) and were the same as those used in previous studies (e.g., Roberts et al., 2007). These criteria were confirmed by review of medical records requested from the child’s primary physician or geneticist using Health Insurance Portability and Accountability Act (1996) authorizations signed by the families.
Families were recruited from the 2007 meeting of the Noonan Syndrome Support Group and from clinics at the Children’s Hospitals and Clinics of Minnesota, Children’s Hospital Boston, and the Waisman Center at the University of Wisconsin. The study was approved by the Internal Review Board at each of the participating institutions. Participants and their primary caregivers provided written informed consent prior to participation in the research.
Molecular genetic confirmation of NS was available for 41 of 66 (62%) individuals. The cohort included 33 participants with PTPN11 mutations, six with SOS1 mutations, one with a BRAF mutation, and one with a RAF1 mutation. Among the remaining 25 individuals with unknown mutations, 13 had tested negative for a PTPN11 mutation but were untested or had negative results for the remaining genes. The remaining 12 families had chosen not to participate in genetic testing.
Information about the developmental history of participants was obtained through a review of medical records and parental reports. The average age at diagnosis of NS was 40 months. A large majority of parents of individuals with NS (75%) reported that their child did not reach motor milestones at the same rate as their peers. Age of first spoken word ranged from 6 to 60 months (M = 15.7, SD = 9.7). One child (4;6 [years;months]) was not yet producing spoken language at the time of the assessment. A majority of participants in the cohort (70%) had received or were currently receiving speech/language therapy. 48% of parents reported that their child had used simplified sign language as a means of communication or as a supplement to speech at some point in development. All participants were native speakers of English.
Audiologic histories revealed that 26% of participants had a clinical history of hearing loss. Three participants (5%) required a hearing aid for normal daily activity. Parents of nearly half (48%) of individuals in the sample reported that their child had experienced frequent otitis media in childhood. Of the 56 individuals from whom we obtained a valid hearing screening, 15 (27%) failed at least one of the six trials. The majority of the participants who did not pass the hearing screening (86%) had a history of hearing loss or otitis media; however, two participants without a history of these problems failed the screening.
Procedure
All behavioral assessments were administered by the first author. Sessions were conducted in a quiet room and lasted approximately 2.5 hr, with short breaks when needed. Participants completed all assessments in the protocol, unless normative data were unavailable because of chronological age (n = 9 for the Comprehensive Test of Phonological Processing [CTOPP; Wagner, Torgesen, & Rashotte, 1999] assessment and Purdue Pegboard Test [Tiffen, 1968]; n = 12 for the literacy tasks), because of equipment malfunction (n = 3 for the hearing screening and Northwestern University—Children’s Perception of Speech Test [NU–CHIPS; L. L. Elliott & Katz, 1980] tasks), or because the participant was unable to complete the required tasks (n = 7 for the hearing screening and NU–CHIPS tasks; n = 1 for the CTOPP and Goldman Fristoe Test of Articulation—Second Edition [GFTA–2; Goldman & Fristoe, 2000] tasks). Cases were excluded listwise from analyses that contained variables with missing values.
Measures
Language and communication
Language abilities were assessed using the Clinical Evaluation of Language Fundamentals—Preschool, Second Edition (CELF–P2; Semel, Wiig, & Secord, 2004) for children 4–5 years of age (n = 14) and the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF–4; Semel, Wiig, & Secord, 2003) for those 6 years of age and older (n = 52). These tests measure both receptive and expressive language, including semantics, morphology, and syntax. For all ages, the core subtests and all subtests in the Receptive Language Index and Expressive Language Index (for both the CELF–P2 and the CELF–4) were administered. Both the CELF–P2 and the CELF–4 have excellent reliability and correlate strongly with other measures of language (Semel et al., 2003, 2004). Parents were also asked to complete the CELF Pragmatics Profile (Semel et al., 2003, 2004). This supplemental measure is a checklist of verbal and nonverbal social communication skills. Scores can be compared with a criterion to identify children who had evidence of difficulties with the pragmatic functions of language.
Nonverbal cognition and literacy
Intellectual abilities were evaluated using the Differential Ability Scales (DAS; C. Elliott, 1990). This measure is standardized for individuals 2.5–18 years of age and provides both a verbal and nonverbal index for all ages (C. Elliott, 1990). In older children (>7 years), the DAS Special Nonverbal Composite can be further divided into spatial and nonverbal reasoning scales. However, in the data analyses, the full composite score was used as the measure of nonverbal cognitive functioning. The supplemental Reading and Spelling subtests were also administered to obtain measures of literacy skills for children more than 6 years of age (n = 52). The Reading subtest measures the child’s ability to decode single words. The Spelling subtest measures the child’s ability to spell single words correctly in written form.
Speech articulation
Speech was evaluated using the GFTA–2 Sounds-in-Words test (Goldman & Fristoe, 2000). The GFTA–2 assesses spontaneous production of English consonant sounds in the initial, medial, and final positions of common words. Words are elicited by asking the examinee to name pictures of objects from a standard test booklet. Participants’ responses were recorded using an iRiver H120 digital recording device so that task scoring could be verified later. This test is standardized for use in participants 2–21 years of age and has excellent reliability.
Motor dexterity
Manual motor dexterity was evaluated using the Purdue Pegboard Test (Tiffen, 1968). Standard scores were obtained using age norms developed for this test (Gardner & Broman, 1979; Yeudall, Fromm, Reddon, & Stefanyk, 1986). A composite score was obtained by averaging each participant’s standard scores for the trial assessing the preferred hand, the trial assessing the nonpreferred hand, and the trial assessing both hands simultaneously.
Audiologic measures
Two brief measures were used to examine audiologic functioning: a standard hearing screening and a speech-in-noise task. The pure-tone hearing screening was performed during the testing session using a portable Beltone audiometer. Pass/fail data were collected for both ears at 20 dB for frequencies of 1000, 2000, and 4000 Hz. A screening score (ranging from 0 to 6) was assigned on the basis of the number of frequencies in which the participant was able to detect the tone.
Auditory processing of speech was assessed with the NU–CHIPS. The NU–CHIPS is a picture pointing word recognition test developed for children 3 years of age and older (L. L. Elliott & Katz, 1980). Words from the Auditec NU–CHIPS CD (female speaker) were delivered by a Sony audio player through the Beltone audiometer. The output was calibrated using the calibration tone (reflecting the average peak level of the speech signal), which was adjusted to 0 on the VU meter. Words were presented at 30-dB sensation level with competing white noise. Participants were administered the 50 items composing Form A. The first five items were presented as practice trials, followed by 15 items each with 30, 60, and 90 dB of white noise presented in the contralateral ear. Accuracy was calculated for each participant across the 45 test trials.
Because the hearing screening and speech-in-noise tasks measured very related constructs and were intercorrelated, a composite measure was calculated by averaging the z scores for these two variables. This hearing score was used as the measure of audiologic functioning in our analyses.
Phonological memory
Phonological memory skills were assessed using the Phonological Memory Index from the CTOPP (Wagner et al., 1999). This index consists of two subtests commonly used to measure short-term recall of phonological information: a digit span task and a non-word repetition task. The digit span task required participants to repeat a set of numbers of increasing length. For the nonword repetition task, participants were asked to repeat nonwords of varying length (e.g., “zid” or “ballop”). Test items from the CTOPP CD were administered through a Sony audio player. This assessment is normed for use with individuals 5–25 years of age and was administered to all participants in our protocol more than 5 years of age (n = 57).
Results
Pattern of Language Ability
Level of functioning and distribution of scores
Descriptive statistics for performance of preschool and school-age individuals with NS on our language assessments and other behavioral tasks are depicted in Table 1. Across the cohort, core language standard scores (as measured by the CELF–P2 and CELF–4) ranged from 40 to 120 (M = 88.92, SD = 20.26). The distribution of language scores is depicted in Figure 1. Most individuals with NS clustered near or slightly below the average range on our language assessments, but the distribution had a long “tail,” with a striking subset of individuals displaying significant language difficulties. Overall, language functioning in individuals with NS was significantly lower than expected on the basis of normative data (M = 100, SD = 15), one-sample t test: t(65) = −4.44, p < .001. Core language abilities did not differ on the basis of the gender of participants, F(1, 64) = 0.183, p = .67, partial η2 = .003. Individuals with NS who passed our hearing screening achieved significantly higher language scores than individuals who failed the screening, F(1, 54) = 5.03, p < .05, partial η2 = .085.
Table 1.
Descriptive statistics for behavioral assessments in preschool and school-age individuals with Noonan syndrome (NS).
| Age | Measure | M | SD | Range |
|---|---|---|---|---|
| Preschool (4–5 years; n = 14) | Language | |||
| CELF–P2 core language score | 90.7 | 22.3 | 45–112 | |
| CELF–P2 Receptive Language Index | 89.6 | 21.7 | 45–109 | |
| CELF–P2 Expressive Language Index | 89.0 | 24.4 | 45–119 | |
| Intellectual functioning | ||||
| DAS Full Scale | 88.1 | 22.1 | 44–123 | |
| DAS Verbal Composite | 90.0 | 22.2 | 44–123 | |
| DAS Nonverbal Composite | 87.6 | 23.3 | 44–122 | |
| Articulation | ||||
| GFTA–2 standard score | 104.7 | 16.5 | 56–120 | |
| School-age (6–18 years; n = 52) | Language | |||
| CELF–4 core language score | 88.4 | 19.9 | 40–120 | |
| CELF–4 Receptive Language Index | 87.4 | 17.7 | 45–116 | |
| CELF–4 Expressive Language Index | 89.5 | 19.5 | 45–124 | |
| Intellectual functioning | ||||
| DAS Full Scale | 85.4 | 17.4 | 44–123 | |
| DAS Verbal IQ | 89.3 | 17.9 | 51–128 | |
| DAS Nonverbal IQ | 85.0 | 16.1 | 48–116 | |
| Articulation | ||||
| GFTA–2 standard score | 97.5 | 13.5 | 40–112 | |
| Academic skills | ||||
| DAS Reading | 89.8 | 16.7 | 55–123 | |
| DAS Spelling | 86.8 | 15.6 | 55–117 | |
| Phonological memory | ||||
| CTOPP Phonological Memory Index | 83.7 | 12.9 | 49–112 | |
| Manual motor skills | ||||
| Purdue Pegboard Test | 73.7 | 22.1 | 10–110 | |
Note. Data are standardized scores (normative M = 100, SD = 15). CELF–P2 = Clinical Evaluation of Language Fundamentals—Preschool, Second Edition; DAS = Differential Ability Scales; GFTA–2 = Goldman Fristoe Test of Articulation—Second Edition; CELF–4 = Clinical Evaluation of Language Fundamentals, Fourth Edition; CTOPP = Comprehensive Test of Phonological Processing.
Figure 1.
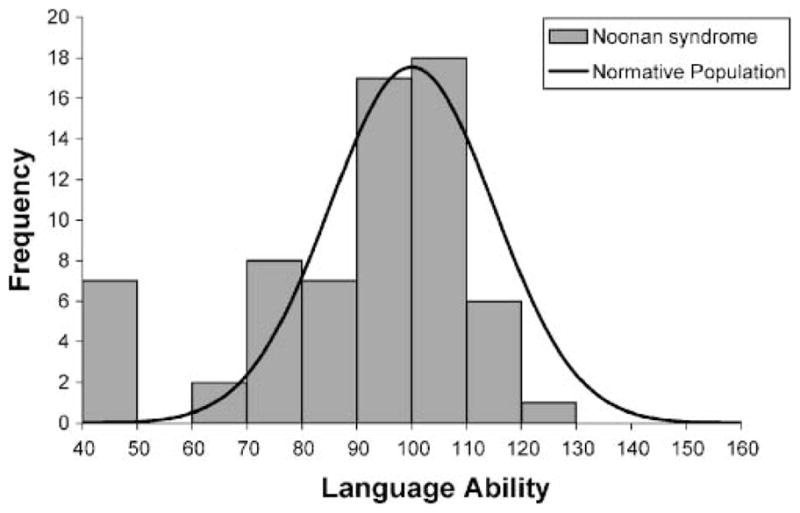
Distribution of language scores (core language scores on the Clinical Evaluation of Language Fundamentals—Preschool, Second Edition [CELF–P2] and the Clinical Evaluation of Language Fundamentals, Fourth Edition [CELF–4]) among children and adolescents with Noonan syndrome (n = 66), with a normative population curve displayed.
To determine how many individuals in our cohort showed evidence of language impairment, a criterion of >1.25 SDs below the mean (10th percentile or less) in receptive and/or expressive language ability was applied to identify individuals with significant language difficulties. This cutoff has been used in numerous studies to identify individuals with SLI (e.g., Mainela-Arnold, Evans, & Coady, 2008; Tomblin, Records, & Zhang, 1996; Webster, Majnemer, Platt, & Shevell, 2004) and has been shown to align with a level that experienced clinicians agree constitutes language impairment (Records & Tomblin, 1994). According to this criterion, 20 individuals in our sample (30%) qualified as having language impairment. The rates of language impairment were similar among preschool (29%) and school-age (31%) individuals with NS.
Pragmatic language skills based on the CELF Pragmatics Profile were compared with criterion scores for each individual’s chronological age. Criterion scores are based on raw score frequency distributions for individuals with and without pragmatic language impairment (Semel et al., 2003). Of the 65 participants, 39 (60%) for whom scores were available scored above criterion, indicating age-appropriate social-pragmatic language use. The remaining 40% of participants did not meet criterion. Of the female participants, 21% had significant pragmatic language deficits, whereas 56% of the male participants had significant pragmatic language deficits. Male participants with NS were significantly more likely to have marked difficulties in pragmatic language use than female participants, F(1, 63) = 9.01, p < .01, partial η2 = .125.
In the area of speech, standard scores on the GFTA–2 indicate that the majority of individuals with NS do not have significant difficulties articulating consonant sounds in single words. However, when a cutoff of −1.25 SDs (10th percentile or lower) was applied, 13 individuals in the sample (20%) fell in the range of significant articulation impairment. Articulation impairment was more common in NS than in the normative sample (χ2 = 6.90, p < .01). Individuals with NS who failed the hearing screening performed more poorly on the articulation test than those who passed the screening, F(1, 54) = 19.10, p < .001, partial η2 = .261. The mean age of participants who had articulation impairments did not differ significantly from the mean age of those without articulation impairments, F(1, 64) = 0.001, p = .98, partial η2 = .000, indicating that speech difficulties may persist across different stages of development in NS. The most difficult sounds (i.e., those in which greater than 15% of participants produced errors) included word-initial consonant clusters (/br/, /fr/, /gr/, /kr/, and /sl/), word-initial liquids (/r/), word-medial fricatives (/δ/ and /θ/), and word-final fricatives (/s/). In contrast, stops (/p/, /b/, /t/, /d/, /k/, and /g/) and nasals (/m/ and /n/) were produced relatively more accurately, with fewer than 6% of participants producing errors in these sounds at any position in the word. In general, this pattern of errors is not unusual; fricatives, liquids, and consonant clusters are also among the most difficult consonant sounds for typically developing children to produce and are mastered later in development (Kelley, Jones, & Fein, 2004; McLeod, van Doorn, & Reed, 2001). Thus, although some individuals with NS did exhibit deficits in articulation relative to their peers, the pattern of errors within the group as a whole was not deviant.
Receptive–expressive language profiles
Across the cohort, receptive language skills (M = 87.83, SD = 18.49) were slightly lower than expressive language skills (M = 89.42, SD = 20.40), but the difference between these areas was not significant, paired-samples t test: t(65) = −1.21, p = .23. A difference score was calculated for each participant to measure his or her expressive language ability relative to receptive ability. Discrepancies between expressive and receptive language were compared with significance tables for the CELF–P2 and CELF–4 norms at the p < .05 level. The majority of individuals (76%) did not show a significant difference between receptive and expressive language ability (receptive language = expressive language). Six participants (9%) had significantly better receptive than expressive skills (receptive language > expressive language), and 10 participants (15%) had significantly better expressive than receptive skills (receptive language < expressive language). Difference scores measuring the discrepancy between receptive and expressive language (receptive language minus expressive language) were correlated with overall core language ability, r(64) = −.245, p < .05, such that individuals with relatively strong expressive skills (receptive language < expressive language) scored higher overall on the language assessments than those with the opposite pattern.
Lexical–grammatical profiles
Scores of individuals with NS on different domains within language were also compared. To determine whether participants with NS (like individuals with SLI or DS) have particular difficulty with grammatical tasks, CELF subtests examining lexical/conceptual abilities versus grammatical abilities were contrasted (see Table 2). Because subtests differed across the age range studied, comparisons were made separately for preschool (4–5 years of age), early school-age (6–8 years of age), and late school-age (9–17 years of age) groups. For each age range examined, standardized scores on grammatical tasks were compared with scores on conceptual/lexical tasks. Comparisons were made in both receptive and expressive domains; however, because no receptive subtest was available for grammatical skill in the late school-age range, this comparison was omitted. Results indicate that performance on grammatical language tasks did not differ significantly from those on conceptual language tasks in four of the five comparisons. In two of those comparisons, the trend was toward stronger grammatical abilities, whereas in two other comparisons, the trend was toward stronger lexical abilities. Among 9–17-year-olds, performance on an expressive syntax task (Formulating Sentences) was significantly better than performance on an expressive vocabulary task (Word Classes–Expressive). Overall, these analyses indicate that individuals with NS do not perform consistently better in a specific area of language and do not tend to have particular difficulty with grammatical aspects of language.
Table 2.
Comparison of lexical/conceptual tasks versus grammatical subtests on the CELF–P2 and CELF–4 in 66 individuals with NS.
| Task | Expressive tasks |
Receptive tasks |
|||
|---|---|---|---|---|---|
| Age 4–5 years (n = 14) | Age 6–8 years (n = 14) | Age 9–18 years (n = 38) | Age 4–5 years (n = 14) | Age 6–8 years (n = 14) | |
| Lexical/conceptual | |||||
| Expressive Vocabulary | 9.2 (3.7) | ||||
| Basic Concepts | 10.3 (4.4) | ||||
| Word Classes–Expressive | 7.1 (3.7) | 8.0 (3.2) | |||
| Word Classes–Receptive | 6.7 (4.1) | ||||
| Grammatical | |||||
| Word Structure | 9.0 (4.1) | 7.3 (3.5) | |||
| Sentence Structure | 8.8 (3.3) | 8.2 (4.7) | |||
| Formulating Sentences | 9.2 (3.7) | ||||
| Comparison (t test) | 0.20 | −0.38 | −3.2 | 1.3 | −1.3 |
| Significance (p value) | ns | ns | <.01 | ns | ns |
Note. Descriptive statistics for each subtest (normative M = 10, SD = 2) are reported in the following format: M (SD).
The Relationship Between Language and Nonverbal Intellectual Functioning
Several analyses were performed to assess whether language ability was commensurate with nonverbal cognitive ability in NS. We first examined whether individuals with language impairments also had difficulties on nonverbal cognitive tasks. Among the 20 individuals identified with language impairment in the sample, 17 (85%) also scored below the average range (standard score < 85) on the nonverbal cognition index. Thus, the large majority of participants with language impairments also had cognitive delays. The remaining three individuals, who exhibited an “SLI-like” profile of significant language deficits despite average-range intellectual functioning, represented 5% of the entire sample of participants with NS. This rate of SLI among individuals with NS does not differ significantly from the expected base rate of SLI in the general population (~7%; Tomblin et al., 1997; χ2 = 0.61, p = .43). This indicates that a profile of average-range intellectual functioning with severe language difficulties is not particularly common in NS. Moreover, as a group, individuals with NS scored significantly higher, on average, on the verbal scale of the DAS than on the nonverbal scale, paired-sample t test: t(65) = 2.77, p < .01 (see Pierpont et al., 2009, for further discussion of cognitive profiles).
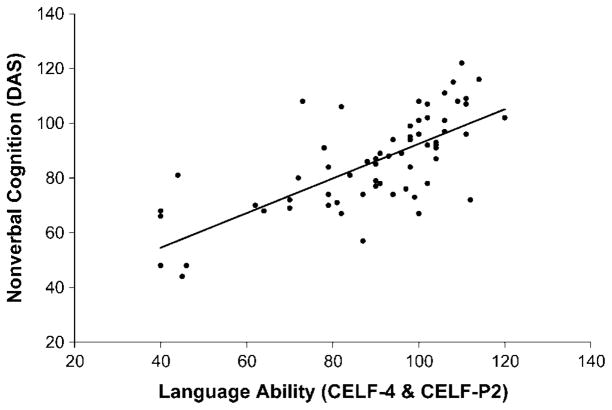
To further examine the relationship between language and cognitive functioning, the correlation between core language scores on the CELF–P2 and CELF–4 tests and the DAS nonverbal IQ scores was computed (see Figure 2). Scores on these measures were highly correlated (r = .725, p < .001), indicating a strong relationship between intellectual functioning and language ability in individuals with NS.
Figure 2.
Correlation between language and nonverbal cognitive ability in individuals with Noonan syndrome. DAS = Differential Ability Scales.
Correlates of Language Ability in NS
In addition to nonverbal cognitive abilities, we also examined the relationships between language ability and audiologic functioning, articulation, manual motor skills, and phonological memory. Table 3 displays the intercorrelations among these variables. Nonverbal cognition and phonological memory had the highest correlations with language ability. Moderate associations were seen between language and perceptual-motor factors such as hearing score, articulation, and motor skill. All five factors were significantly correlated with language ability at the p < .01 level.
Table 3.
Intercorrelations of language abilities and other cognitive, perceptual, and motor tasks in individuals with NS.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Language (CELF–P2 and CELF–4) | — | .73** | .47** | .46** | .37* | .72** |
| 2. Nonverbal cognition (DAS) | — | .48** | .18 | .58** | .49** | |
| 3. Articulation (GFTA–2) | — | .41* | .40* | .61** | ||
| 4. Hearing score | — | .11 | .64** | |||
| 5. Manual motor skill (Purdue Pegboard Test) | — | .21 | ||||
| 6. Phonological memory (CTOPP) | — |
p < .01.
p < .001.
Relationship of Literacy Skills to Language Ability
Scores for single-word Reading (i.e., reading decoding) and Spelling subtests of the DAS were available for the 52 school-age participants in our sample (6–18 years of age). Descriptive statistics for these assessments are reported in Table 1. Eighteen of the school-age participants in the sample (35%) scored in the impaired range (>1.25 SDs below the mean for their age) on at least one of the literacy assessments. To determine whether literacy scores lagged behind intellectual ability, scores on the Reading and Spelling subtests were compared with predicted scores on the basis of each child’s nonverbal cognitive skill. Using significance tables in the DAS manual, it was determined whether the difference between actual and predicted scores was statistically significant for each participant (p < .05; C. Elliott, 1990). Twenty participants (38%) performed within the expected range (on the basis of their cognitive abilities) on the Reading sub-test. Sixteen (31%) participants performed higher than expected, and 16 (31%) performed more poorly than expected. For the Spelling subtest, nine participants (17%) scored higher than the expected level, 21 (41%) performed in the expected range, and 22 (42%) performed below the expected level.
Scores on the Reading and Spelling subtests were very highly correlated: r(50) = .824, p < .001. Therefore, the scores on these subtests were averaged to obtain a single measure of literacy that could be examined in relation to language and cognitive skills. We hypothesized that literacy skills would be related to overall language skills as well as to phonological memory skills. Further, we expected that literacy skills may be related to overall cognitive ability. To control for Type I error associated with multiple tests, a Bonferroni correction was applied so that only tests with ps < .016 would be considered significant. Literacy skills were significantly correlated with overall language ability on the CELF assessments, r(52) = .51, p < .001, with phonological memory scores on the CTOPP, r(48) = .51, p < .001, and with nonverbal cognitive ability, r(52) = .41, p < .01. Taken together, these analyses suggest that individuals with NS are at greater risk for academic difficulties in reading and spelling if they have poor language and/or cognitive abilities, particularly if phonological memory skills are weak.
Genotype Analysis
A final set of analyses investigated whether variation in language functioning in NS could be explained by genotype differences. Table 4 displays the scores for individuals with PTPN11, SOS1, and unknown mutations on the language and nonverbal cognitive assessments. It is worth noting that very wide variation was observed even among individuals with mutations in the same gene; in just the PTPN11 group, language skills ranged from the very low range to the high range of ability. Further, large differences could be found even among individuals with the same NS genotype. In two siblings with an identical PTPN11 mutation (exon 13, amino acid change P491S), one sibling received a standard score of 44 (severely impaired), and the other scored 84 (just below the average range). This striking difference (>2.5 SDs) between individuals with the same genotype highlights the tremendous variability in language outcomes in NS that is attributable to factors other than genotype.
Table 4.
Descriptive statistics for language (CELF–P2 and CELF–4) and nonverbal cognitive functioning (DAS) in individuals with NS, grouped by the gene in which a mutation is located.
| Measure | Statistic | Genotype |
||
|---|---|---|---|---|
| PTPN11 (n = 33) | SOS1 (n = 6) | Unknown (n = 25) | ||
| Language ability | M | 89.2 | 106.2 | 84.5 |
| SD | 19.1 | 5.4 | 23.0 | |
| Range | 40–112 | 100–114 | 40–120 | |
| Nonverbal cognition | M | 85.7 | 101.8 | 82.3 |
| SD | 12.8 | 10.8 | 22.8 | |
| Range | 66–115 | 87–116 | 44–122 | |
A genotype–phenotype analysis was conducted to address the question of whether there was evidence of genetic differences in language ability that could not be accounted for by differences in cognitive functioning. For this analysis, we focused on the two groups with identified mutations and a large enough sample to make comparisons: those with PTPN11 mutations and those with SOS1 mutations. In a previous analysis of a nearly identical cohort (Pierpont et al., 2009), individuals with SOS1 mutations were found to have significantly higher scores on both verbal and nonverbal cognitive tests than individuals with PTPN11 mutations. Here, we examined whether these groups differed also on language functioning.
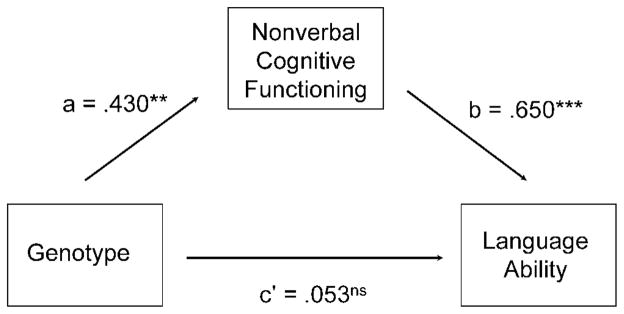
Standard scores for the PTPN11-positive and the SOS1-positive groups in language ability were compared. The SOS1 group (M = 106.17, SD = 5.38) scored significantly higher in core language ability on the CELF assessments than the PTPN11 group (M = 89.18, SD = 19.08), F(1, 37) = 4.59, p < .05, partial η2 = .110. To determine whether this genotype difference was specific to language or whether it was mediated by variation in nonverbal intellectual functioning, a mediation analysis was conducted (see Figure 3). On the basis of the steps recommended by Baron and Kenny (1986), we first examined the effect of genotype as a predictor of language functioning. Using dummy coding for the genotypes (PTPN11 = 0, SOS1 = 1), the regression analysis confirmed a significant total effect of genotype on language ability (β = .33, p < .05), with the direction of the effect indicating higher scores for the SOS1 group than the PTPN11 group. A second regression analysis examined the effect of genotype on nonverbal cognitive functioning (the mediator); this analysis revealed a significant relationship (Path a; β = .430, p < .01). Finally, when genotype and nonverbal cognitive functioning were added concurrently into a regression model predicting language ability, the effect of nonverbal cognitive functioning on language ability was significant (Path b; β = .650, p < .001). However, the direct effect of genotype on language ability was not significant when controlling for nonverbal cognitive functioning (Path c′; β = .053, p = .70), indicating a significant mediation effect. Thus, differences between SOS1-positive and PTPN11-positive individuals in language ability were largely mediated by differences in general cognitive ability. This mediation effect was confirmed by the Sobel test (z = 2.47, p < .05).
Figure 3.
Model depicting the relationship between Noonan syndrome genotype (PTPN11 vs. SOS1 mutation) and language ability, with nonverbal cognitive ability as a mediator. a = effect of genotype on cognitive ability (mediator); b = effect of nonverbal cognitive ability on language ability (outcome variable); c′ = effect of genotype on language ability, controlling for nonverbal cognition. ns = not significant. **p < .01. ***p < .001.
Additional analyses explored communication abilities in individuals with rarer mutations. Our cohort included one individual with a BRAF mutation (exon 15, amino acid change L597V) and one individual with a RAF1 mutation (exon 7, amino acid change P261S). To determine whether performance of these individuals differed from other individuals with NS, modified t tests that were developed to compare a single case with a sample of controls were conducted (Crawford & Howell, 1998). The two single cases were compared with a control group composed of the individuals with NS with identified mutations in PTPN11 or SOS1 (n = 39). The level of language ability shown by the BRAF-positive individual (CELF–4 standard score = 94; 34th percentile) did not differ significantly from other NS individuals with identified mutations, t(38) = 0.117, p = .91, and was in the average range on the basis of normative data. This individual (14;4) demonstrated average-range articulation skills and low average/borderline cognitive and literacy skills (see also Pierpont et al., 2009). The participant with a RAF1 mutation (9;9) also scored within the average range in language abilities (CELF–4 standard score = 90; 25th percentile). This child’s language ability did not differ significantly from NS participants with PTPN11 and SOS1 mutations, t(38) = −0.100, p = .93. The participant exhibited low average cognitive ability and scored in the 4th percentile (impaired range) on the articulation test but scored in the high average range on the Reading and Spelling subtests.
Discussion
Profile of Language Abilities in NS
This research study is the first to investigate speech and language functioning in a large cohort of individuals with NS. Scores on standardized assessments indicate that the majority of participants performed within the average range relative to normative data on language tests. However, significant impairments in expressive and/or receptive language were present among 30% of individuals in our cohort, and severe difficulties with articulation affected approximately 20% of participants. Further, parental reports indicate that difficulties with social-pragmatic aspects of language were also common; roughly two in five participants with NS did not reach criterion for their age on the pragmatics checklist that was administered. Pragmatic language difficulties were particularly frequent among male participants with NS. Finally, basic literacy skills in reading decoding and spelling were variable. Although these skills were a relative strength for some participants, approximately one third of participants with NS scored lower than expected based on their age and cognitive abilities.
By examining whether different aspects of language and nonlinguistic cognition develop in tandem or asynchronously in NS, we sought to better understand the nature of language difficulties in this interesting population. Results suggest that individuals with NS do not tend to show a pattern of selective or asynchronous impairments in language processing mechanisms. Rather, language functioning in NS appears to be strongly associated with other factors, many of which are nonlinguistic. A first piece of evidence supporting this claim is that language development in NS was typically found to be commensurate with nonverbal cognition. On average, verbal skills were significantly higher than nonverbal skills, and scores on our language assessment were highly correlated with nonverbal cognition. Further, the majority (85%) of those individuals in our sample who demonstrated significant speech and language impairments also exhibited cognitive delays. Thus, results from this study indicate that language impairments in NS may be strongly related to the cognitive disabilities occurring in some affected individuals. This relatively “synchronous” relation between language and nonverbal cognition is similar to what has been reported in some other groups of individuals with cognitive impairments, such as FXS or intellectual disability of unknown etiology (Abbeduto et al., 2003; Kernan & Sabsay, 1996).
Further support for the idea that language difficulties in NS are closely tied to more general, nonlinguistic factors comes from the finding that significant relationships between language and perceptual and motor factors were observed. The association between language and hearing abilities may be of particular importance. This finding has significant clinical implications, highlighting the need for frequent evaluation of hearing abilities in all individuals with NS. Hearing loss is more common in NS than in the general population (Qiu et al., 1998) and is a significant predictor of performance on tests of verbal intelligence (Pierpont et al., 2009) as well as the speech and language skills described in this article.
Examining the profile of language abilities in NS also revealed that patterns observed in other populations in which language is a relative weakness (Laws & Bishop, 2003) were not seen in NS. For example, grammatical skills were not an area of unusual difficulty in our cohort of NS participants and were at least as strong as vocabulary abilities in most comparisons across the age ranges tested. Further, unlike in individuals with SLI or DS, expressive language did not consistently lag behind receptive language in NS individuals. Rather, the majority of participants (91%) had expressive skills that were statistically equal to or stronger than their receptive language abilities. It is interesting to note, however, that those individuals who did display relatively weak expressive skills tended to have poorer overall language abilities than those with the opposite pattern.
Achieving a better understanding of communication impairments in NS is an important part of improving care for individuals with this condition. It has been known for some time that individuals with speech or language impairments are at greater risk for academic and social difficulties (Bashir & Scavuzzo, 1992). In our sample of individuals with NS, reading and spelling skills were strongly related to language ability as well as nonverbal cognitive ability. Difficulties in phonological memory skills were particularly indicative of literacy problems. Thus, our results suggest that deficits in phonological and language skills might indicate risk for basic written language difficulties in NS.
Genotype Analysis
The genotype analyses conducted in this study also lend support to the idea that the NS gene mutations are unlikely to target language mechanisms specifically. Rather, the effects of the different mutations appear to be more general. Observed differences between the PTPN11-positive and SOS1-positive participants in language skills were mediated by differences in nonverbal cognitive ability. Thus, although our results suggest that the severity of effects on brain/cognitive functioning may differ according to genotype, it appears that specific language functions are not selectively affected.
Although the sample was not large enough to test for other group differences directly, single-case comparisons indicate that the scores seen in the BRAF-positive and RAF1-positive participants do not differ significantly from those of individuals with PTPN11 and SOS1 mutations. However, comparison of this finding with information from previous reports suggests that our participant with a BRAF mutation, who had language functioning in the average range on the basis of normative data, had unusually high language ability compared with other individuals with mutations in this gene. Individuals with BRAF mutations typically exhibit significant intellectual and language disabilities and a clinical diagnosis of cardiofaciocutaneous syndrome (Armour & Allanson, 2008; Yoon, Rosenberg, Blaser, & Rauen, 2007). Larger groups of participants with SOS1, RAF1, and BRAF mutations are needed to determine whether language profiles differ among these different genotypes. However, the unusual abilities demonstrated by the BRAF-positive individual in our cohort serve to highlight the enormous within-gene variability seen among disorders associated with the Ras/MAPK pathway.
Limitations
The primary limitation of this study is the lack of a control group. For this initial investigation of language abilities in NS, our analyses focused on comparisons with normative data and understanding within-group variation. This same approach has proven to be useful in establishing an initial summary of language functioning in other special populations. For instance, Charman, Drew, Baird, and Baird (2003) characterized early language abilities in young children on the autism spectrum (without benefit of a control group) by assessing their performance on the MacArthur Communicative Development Inventory (Fenson et al., 1993) relative to normative data for that measure. In the present study, we have drawn general conclusions regarding the pattern of language abilities in NS and attempted to make some comparisons with other populations with language disorders on the basis of prior research with those groups. However, further studies are needed to confirm these speculations by directly comparing language profiles in NS with those seen in other developmental syndromes such as DS and FXS or in children with SLI.
A second limitation of this study is that our main measurement of language ability, the CELF–4/CELF–P2, is a relatively broad indicator of language functioning. Although our analyses did not identify any specific areas of strength or weakness in NS when comparing broadly across language domains, the high demands that some CELF subtests place on attention and/or memory may limit our ability to isolate specific deficits within the language system (e.g., morphosyntax). Therefore, further research is needed to examine more isolated language processes in NS as well as to explore more comprehensively some of the potentially important areas such as pragmatic language use.
Conclusions
Results from this study indicate that communication impairments occur more frequently in NS than in the general population. Although difficulties in language skills were largely associated with cognitive, perceptual, and motor factors (rather than selective deficits in specific language domains), this only highlights the need for early identification of communication impairments. If potential risk factors for speech or language impairment can be identified early, more appropriate interventions can be implemented. For example, interventions for hearing loss could potentially ameliorate some language impairments in this population.
Our results also suggest that individuals with cognitive impairments should be considered for language interventions, as these children are at greater risk for severe communicative and literacy difficulties. It is worth noting, however, that a few participants in our cohort did have communication difficulties in the context of normal cognitive ability. Hence, there does not appear to be a single language profile for the group as a whole. Rather, our findings support the need for comprehensive speech and language evaluations of all patients with NS. Finally, this study has revealed that among individuals with mutations in the same gene, and even those having the same amino acid substitution, language abilities can vary widely. This variability suggests that behavioral interventions have enormous potential to contribute to outcomes in individuals with NS and other related genetic disorders.
Acknowledgments
This work was supported by the 2007 Jeanette Anderson Hoffman Memorial Wisconsin Distinguished Graduate Fellowship award to the first author and by National Institutes of Health Grant T32 DC005459-07, “Interdisciplinary Research Training in Speech-Language Pathology” (Susan Ellis Weismer, Principal Investigator). This project was also funded, in part, by Grant UL1 RR025758-01 from the National Center for Research Resources, National Institutes of Health, to the Harvard Catalyst Clinical & Translational Science Center. We thank Andrea Nett and Rebecca Rozek for their valuable assistance in our research efforts as well as Richard Pauli, David Wargowski, and Jody Haun at the Waisman Center (Madison, Wisconsin) for their aid in recruiting participants. We express our gratitude to all of the families that participated in this research and to the Noonan Syndrome Support Group.
Contributor Information
Elizabeth I. Pierpont, University of Wisconsin—Madison
Susan Ellis Weismer, University of Wisconsin—Madison.
Amy E. Roberts, Children’s Hospital Boston, MA
Erica Tworog-Dube, Brigham and Women’s Hospital, Boston, MA.
Mary Ella Pierpont, Children’s Hospitals and Clinics of Minnesota, Minneapolis and St. Paul, and University of Minnesota, Minneapolis.
Nancy J. Mendelsohn, Children’s Hospitals and Clinics of Minnesota, Minneapolis and St. Paul, and University of Minnesota, Minneapolis
Mark S. Seidenberg, University of Wisconsin—Madison
References
- Abbeduto L, Evans J, Dolan T. Theoretical perspectives on language and communication problems in mental retardation and developmental disabilities. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7:45–55. doi: 10.1002/1098-2779(200102)7:1<45::AID-MRDD1007>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Abbeduto L, Murphy MM, Cawthon SW, Richmond EK, Weissman MD, Karadottir S, O’Brien A. Receptive language skills of adolescents and young adults with Down syndrome or Fragile X syndrome. American Journal on Mental Retardation. 2003;108:149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Abbeduto L, Pavetto M, Kesin E, Weissman M, Karadottir S, O’Brien A, Cawthon S. The linguistic and cognitive profile of Down syndrome: Evidence from a comparison with Fragile X syndrome. Down Syndrome: Research & Practice. 2001;7:9–15. doi: 10.3104/reports.109. [DOI] [PubMed] [Google Scholar]
- Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: Clinical features of 38 individuals with proven mutations. Journal of Medical Genetics. 2008;45:249–254. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bashir AS, Scavuzzo A. Children with language disorders: Natural history and academic success. Journal of Learning Disabilities. 1992;25:53–65. doi: 10.1177/002221949202500109. [DOI] [PubMed] [Google Scholar]
- Bennett FC, Furukawa CT. Effects of conductive hearing loss on speech, language and learning development. Clinical Reviews in Allergy. 1984;2:377–385. [PubMed] [Google Scholar]
- Bishop DVM. How does the brain learn language? Insights from the study of children with and without language impairment. Developmental Medicine and Child Neurology. 2000;42:133–142. doi: 10.1017/s0012162200000244. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: Evidence from a twin study. Journal of Child Psychology and Psychiatry. 1996;37:391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Caselli MC, Monaco L, Trasciani M, Vicari S. Language in Italian children with Down syndrome and with specific language impairment. Neuropsychology. 2008;22:27–35. doi: 10.1037/0894-4105.22.1.27. [DOI] [PubMed] [Google Scholar]
- Catts HW, Adlof SM, Hogan TP, Ellis Weismer S. Are specific language impairment and dyslexia distinct disorders? Journal of Speech, Language, and Hearing Research. 2005;48:1378–1396. doi: 10.1044/1092-4388(2005/096). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts HW, Fey ME, Tomblin JB, Zhang X. A longitudinal investigation of reading outcomes in children with language impairments. Journal of Speech, Language, and Hearing Research. 2002;45:1142–1157. doi: 10.1044/1092-4388(2002/093). [DOI] [PubMed] [Google Scholar]
- Chapman RS. Language learning in Down syndrome: The speech and language profile compared to adolescents with cognitive impairment of unknown origin. Down Syndrome: Research & Practice. 2006;10:61–66. doi: 10.3104/reports.306. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Schwartz SE, Bird EK. Language skills of children and adolescents with Down syndrome: I. Comprehension. Journal of Speech and Hearing Research. 1991;34:1106–1120. doi: 10.1044/jshr.3405.1106. [DOI] [PubMed] [Google Scholar]
- Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) Journal of Child Language. 2003;30:213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- Elliott C. Differential Ability Scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Elliott LL, Katz D. The Northwestern University—Children’s Perception of Speech Test (NU–CHIPS) St. Louis, MO: Auditec; 1980. [Google Scholar]
- Ellis Weismer S, Tomblin JB, Zhang X, Buckwalter P, Chynoweth JG, Jones M. Nonword repetition performance in school-age children with and without language impairment. Journal of Speech, Language, and Hearing Research. 2000;43:865–878. doi: 10.1044/jslhr.4304.865. [DOI] [PubMed] [Google Scholar]
- Estes KG, Evans JL, Else-Quest NM. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. Journal of Speech, Language, and Hearing Research. 2007;50:177–195. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, …Reilly JS. The MacArthur Communicative Development Inventories: User’s guide and technical manual. San Diego, CA: Singular; 1993. [Google Scholar]
- Fisch GS, Holden JJ, Carpenter NJ, Howard-Peebles PN, Maddalena A, Pandya A, Nance W. Age-related language characteristics of children and adolescents with Fragile X syndrome. American Journal of Medical Genetics. 1999;83:253–256. [PubMed] [Google Scholar]
- Foster CA, Dyhrkopp PJ. Noonan’s syndrome with sensorineural hearing loss and vestibular abnormalities. Otolaryngology—Head and Neck Surgery. 1998;119:508–511. doi: 10.1016/S0194-5998(98)70111-1. [DOI] [PubMed] [Google Scholar]
- Fraser J, Conti-Ramsden G. Contribution of phonological and broader language skills to literacy. International Journal of Language & Communication Disorders. 2008;43:552–569. doi: 10.1080/13682820701778069. [DOI] [PubMed] [Google Scholar]
- Gardner RA, Broman M. The Purdue Pegboard Test: Normative data on 1,334 school children. Journal of Clinical Psychology. 1979;1:156–162. [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe Test of Articulation. 2. Circle Pines, MN: AGS; 2000. [Google Scholar]
- Health Insurance Portability and Accountability Act, Pub. L. No. 104–191, 110, Stat. 1936 (1996).
- Hick RF, Botting N, Conti-Ramsden G. Short-term memory and vocabulary development in children with Down syndrome and children with specific language impairment. Developmental Medicine and Child Neurology. 2005;47:532–538. doi: 10.1017/s0012162205001040. [DOI] [PubMed] [Google Scholar]
- Hill E. Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Hopkins-Acos P, Bunker K. A child with Noonan syndrome. Journal of Speech and Hearing Disorders. 1979;44:494–503. doi: 10.1044/jshd.4404.494. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Takeshita K. Neuropsychological developmental change in a case with Noonan syndrome: Longitudinal assessment. Brain Development. 2003;25:291–293. doi: 10.1016/s0387-7604(02)00227-9. [DOI] [PubMed] [Google Scholar]
- Kelley E, Jones G, Fein D. Language assessment in children. In: Goldstein G, Beers SR, Hersen M, editors. Comprehensive handbook of psychological assessment: Intellectual and neuropsychological assessment. Vol. 1. Hoboken, NJ: Wiley; 2004. pp. 191–215. [Google Scholar]
- Kennedy EJ, Flynn MC. Early phonological awareness and reading skills in children with Down syndrome. Down Syndrome: Research & Practice. 2002;8:100–109. doi: 10.3104/reports.136. [DOI] [PubMed] [Google Scholar]
- Kernan KT, Sabsay S. Linguistic and cognitive ability of adults with Down syndrome and mental retardation of unknown etiology. Journal of Communication Disorders. 1996;29:401–422. doi: 10.1016/0021-9924(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws G. Contributions of phonological memory, language comprehension and hearing to the expressive language of adolescents and young adults with Down syndrome. Journal of Child Psychology and Psychiatry. 2004;45:1085–1095. doi: 10.1111/j.1469-7610.2004.t01-1-00301.x. [DOI] [PubMed] [Google Scholar]
- Laws G, Bishop DVM. A comparison of language abilities in adolescents with Down syndrome and children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2003;46:1324–1339. doi: 10.1044/1092-4388(2003/103). [DOI] [PubMed] [Google Scholar]
- Lee DA, Portnoy S, Hill P, Gillberg C, Patton MA. Psychological profile of children with Noonan syndrome. Developmental Medicine and Child Neurology. 2005;47:35–38. doi: 10.1017/s001216220500006x. [DOI] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Loucas T, Charman T, Pickles A, Chandler S, Meldrum D, Baird G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. Journal of Child Psychology and Psychiatry. 2008;49:1184–1192. doi: 10.1111/j.1469-7610.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- Mainela-Arnold E, Evans JL, Coady JA. Lexical representations in children with SLI: Evidence from a frequency-manipulated gating task. Journal of Speech, Language, and Hearing Research. 2008;51:381–393. doi: 10.1044/1092-4388(2008/028). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridaki-Kassotaki K, Harakopio U. The relation between phonological memory skills and reading ability in Greek-speaking children: Can training of phonological memory contribute to reading development? European Journal of Psychology of Education. 2002;17:63–75. [Google Scholar]
- McArthur GM, Hogben JH, Edwards VT, Heath SM, Mengler ED. On the “specifics” of specific reading disability and specific language impairment. Journal of Child Psychology and Psychiatry. 2000;41:869–874. [PubMed] [Google Scholar]
- McLeod S, van Doorn J, Reed VA. Normal acquisition of consonant clusters. American Journal of Speech-Language Pathology. 2001;10:99–110. [Google Scholar]
- Mendez HM, Opitz JM, Allanson JE. Noonan syndrome: A review. American Journal of Medical Genetics. 1985;21:493–506. doi: 10.1002/ajmg.1320210312. [DOI] [PubMed] [Google Scholar]
- Miyamoto D, Miyamoto M, Takahashi A, Yomogita Y, Higashi H, Kondo S, Hatakeyama M. Isolation of a distinct class of gain-of-function SHP-2 mutants with oncogenic RAS-like transforming activity from solid tumors. Oncogene. 2008;27:3508–3515. doi: 10.1038/sj.onc.1211019. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW, II, Robbins J. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. Journal of Clinical Investigation. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan JA, Ehmke DA. Associated noncardiac malformations in children with congenital heart disease. Journal of Pediatrics. 1963;63:468–470. [Google Scholar]
- Nyström AM, Ekvall S, Berglund E, Björkqvist M, Braathen G, Duchen K, Bondeson ML. Noonan and cardio-facio-cutaneous syndromes: Two clinically and genetically overlapping disorders. Journal of Medical Genetics. 2008;45:500–506. doi: 10.1136/jmg.2008.057653. [DOI] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature Genetics. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Paul R, Dykens E, Leckman JF, Watson M, Breg WR, Cohen DJ. A comparison of language characteristics of mentally retarded adults with Fragile X syndrome and those with nonspecific mental retardation and autism. Journal of Autism and Developmental Disorders. 1987;17:457–468. doi: 10.1007/BF01486963. [DOI] [PubMed] [Google Scholar]
- Philofsky A, Hepburn S, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with Fragile X syndrome. American Journal on Mental Retardation. 2004;109:208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Seidenberg MS. Genotype differences in cognitive functioning in Noonan syndrome. Genes, Brain, and Behavior. 2009;8:275–282. doi: 10.1111/j.1601-183X.2008.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JR, Roberts JE, Hennon EA, Berni MC, Anderson KL, Sideris J. Syntactic complexity during conversation of boys with Fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2008;51:3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- Qiu W, Yin S, Stucker F. Audiologic manifestations of Noonan syndrome. Otolaryngology—Head and Neck Surgery. 1998;118:319–323. doi: 10.1016/S0194-59989870308-0. [DOI] [PubMed] [Google Scholar]
- Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, Matsuoka R. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nature Genetics. 2007;39:1013–1017. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- Records NL, Tomblin JB. Clinical decision making: Describing the decision rules of practicing speech-language pathologists. Journal of Speech and Hearing Research. 1994;37:144–156. [PubMed] [Google Scholar]
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Kucherlapati RS. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nature Genetics. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: New tricks for an old dog. Current Opinion in Genetics and Development. 2007;17:15–22. doi: 10.1016/j.gde.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Zenker M, Rowe S, Boll S, Klein C, Bollag G, Kratz CP. Germline KRAS mutations cause Noonan syndrome. Nature Genetics. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals—Preschool. 2. San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- Sudhalter V, Scarborough HS, Cohen IL. Syntactic delay and pragmatic deviance in the language of fragile X males. American Journal of Medical Genetics. 1991;38:493–497. doi: 10.1002/ajmg.1320380270. [DOI] [PubMed] [Google Scholar]
- Sznajer Y, Keren B, Baumann C, Pereira S, Alberti C, Elion J, Verloes A. The spectrum of cardiac anomalies in Noonan syndrome as a result of mutations in the PTPN11 gene. Pediatrics. 2007;119:e1325–e1331. doi: 10.1542/peds.2006-0211. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nature Genetics. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, Gelb BD. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature Genetics. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- Teeter PA. Noonan syndrome. In: Goldstein S, Reynolds C, editors. Handbook of neurodevelopmental and genetic disorders in children. New York, NY: Guilford Press; 1999. pp. 337–349. [Google Scholar]
- Thal DJ, Reilly J, Seibert L, Jeffries R, Fenson J. Language development in children at risk for language impairment: Cross-population comparisons. Brain and Language. 2004;88:167–179. doi: 10.1016/S0093-934X(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Thordardottir E. Language-specific effects of task demands on the manifestation of specific language impairment: A comparison of English and Icelandic. Journal of Speech, Language, and Hearing Research. 2008;51:922–937. doi: 10.1044/1092-4388(2008/068). [DOI] [PubMed] [Google Scholar]
- Tiffen J. Purdue Pegboard Test. Chicago, IL: Science Research Associates; 1968. [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Zhang X. A system for the diagnosis of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1996;39:1284–1294. doi: 10.1044/jshr.3906.1284. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X. Language patterns and etiology in children with specific language impairment. In: Tager-Flusberg H, editor. Neurodevelopmental disorders. Cambridge, MA: MIT Press; 1999. pp. 361–382. [Google Scholar]
- Troyer A, Joschko M. Cognitive characteristics associated with Noonan syndrome: Two case reports. Child Neuropsychology. 1997;3:199–205. [Google Scholar]
- van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. American Journal of Medical Genetics. 1994;53:187–191. doi: 10.1002/ajmg.1320530213. [DOI] [PubMed] [Google Scholar]
- van der Burgt I, Thoonen G, Roosenboom N, Assman-Hulsmans C, Gabreels F, Otten B, Brunner HG. Patterns of cognitive functioning in school-aged children with Noonan syndrome associated with variability in phenotypic expression. Journal of Pediatrics. 1999;135:707–713. doi: 10.1016/s0022-3476(99)70089-2. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen J, Rashotte C. Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wake M, Poulakis Z. Slight and mild hearing loss in primary school children. Journal of Pediatrics and Child Health. 2004;40:11–13. doi: 10.1111/j.1440-1754.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- Webster RI, Majnemer A, Platt RW, Shevell MI. The predictive value of a preschool diagnosis of developmental language impairment. Neurology. 2004;63:2327–2331. doi: 10.1212/01.wnl.0000147472.33670.b6. [DOI] [PubMed] [Google Scholar]
- Wilson M, Dyson A. Noonan syndrome: Speech and language characteristics. Journal of Communication Disorders. 1982;15:347–352. doi: 10.1016/0021-9924(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Yeudall LT, Fromm D, Reddon JR, Stefanyk WO. Normative data stratified by age and sex for 12 neuropsychological tests. Journal of Clinical Psychology. 1986;42:918–946. doi: 10.1002/1097-4679(198705)43:3<346::aid-jclp2270430308>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Developmental Medicine and Child Neurology. 2007;49:894–899. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]