Abstract
Purpose
Lower CD4+ T-cell counts are related to increased morbidity and mortality despite virologic suppression. CCR5 antagonists are associated with robust CD4+ T-cell responses. We examined the relationship of CCR5 antagonists to CD4+ T-cell gains.
Design
Meta-regression of recent phase 2–3 trials evaluating new antiretroviral agents in treatment-experienced subjects.
Methods
We analyzed the relationship of CCR5 antagonists to CD4+ T-cell count increase 24 weeks after initiating the new regimen using a linear model with generalized estimating equations controlling for differing rates of virologic suppression. Each treatment group was treated as a data point weighted by sample size.
Results
We included 46 treatment groups from 17 trials (11 groups from 5 trials used CCR5 antagonists). Controlling for average baseline HIV-1 RNA and proportion of subjects achieving HIV-1 RNA <50 copies/mL, use of a CCR5 antagonist was associated with an additional significant CD4+ T-cell gain of +30/μL (95% CI, 19–42) at 24 weeks compared to treatment groups not using a CCR5 antagonist.
Conclusions
Use of a CCR5 antagonist was associated with an enhanced CD4+ T-cell count response independent of virologic suppression. This observation supports further evaluation of CCR5 antagonists in patients with discordant immunologic and virologic responses to ART.
Keywords: antiretroviral therapy, CCR5 antagonist, CD4+ T-cell count, clinical trial, HIV-1 infection, meta-regression
The majority of patients with suppression of HIV-1 on antiretroviral therapy (ART) have marked CD4+ T-cell recovery with a mean increase of 176 cells/μL (95% CI, 170–183) over the first year on a variety of treatment regimens.1 However, a French cohort study found that 17% of patients initiating a protease inhibitor-based regimen had a less than 50 cells/μL increase after 6 months despite having virologic suppression.2 The risk of disease progression was twice as high in this subset as compared to those with an immunologic and virologic response. The ART Cohort Collaboration found that subjects with CD4+ T-cells <200/μL 6 months after initiating ART were at significantly increased risk for AIDS or death as compared with those with >200/μL, even when controlling for plasma HIV-1 RNA level.3 Moreover, recent studies have established that CD4+ T-cell count is related to overall mortality and incidence of non-AIDS-defining cancer even among subjects with CD4+ T-cells over 350/μL.4-6
Several new antiretroviral agents have been approved recently by the US Food and Drug Administration (FDA) on the basis of phase 2 and 3 clinical trials and are available for the treatment of HIV-1 infection for patients with patients with prior antiretroviral experience.7 Maximal virologic suppression, plasma HIV-1 RNA level <50 copies/mL, is now a realistic goal for nearly all HIV-1-infected patients. Subjects receiving CCR5 antagonists have been noted to have robust CD4+ T-cell responses.8 A clinical trial comparing maraviroc to efavirenz in antiretroviral treatment-naïve subjects found a higher CD4+ T-cell increase with maraviroc despite similar rates of virologic suppression.9 We conducted a meta-regression of clinical trials of these newer antiretroviral agents to examine the association of CCR5 antagonists to CD4+ T-cell recovery. Our hypothesis was that CCR5 antagonists would be associated with a greater CD4+ T-cell increase when controlling for differing rates of virologic suppression.
METHODS
We reviewed recent phase 2 or 3 clinical trials of investigational agents for treatment of HIV-1 infection in highly treatment-experienced subjects. We did not have access to patient-level data. We included 16 randomized studies and one nonrandomized study that were conducted to support the clinical development of investigational agents beginning in 2003 with the phase 3 trials of enfuvirtide.10,11 Phase 1, phase 4, and postmarketing studies were not included. Only studies using agents that were subsequently approved by the FDA or remain in continued clinical development at the time of analysis were included. The clinical trial design must have consisted of an optimized background regimen (ie, chosen on the basis of treatment history and HIV drug resistance testing) given with an investigational agent, placebo, or active control. The studies of enfuvirtide were chosen as the earliest studies because these were the first studies that used an optimized background regimen with or without the investigational agent. All of the studies included reported the following baseline parameters and results: baseline CD4+ T-cells (mean or median), baseline plasma HIV-1 RNA level (mean or median), proportion that were women, age (mean or median), use of a CCR5 antagonist, sample size per group, and proportion with plasma HIV-1 RNA level <50 copies/mL 24 weeks after entry/randomization and mean change in CD4+ T-cell count at 24 weeks. A phase 2 study of elvitegravir, an investigational integrase inhibitor, was excluded because of a significant design change allowing a change of background therapy during the first 24 weeks and lack of 24-week results.12
The week 24 CD4+ T-cell count gain from each treatment group was a data point within a linear model to obtain an overall estimate of CD4+ T-cell count gain with or without the use of a CCR5 antagonist. The analysis adjusted for treatment group-level information with respect to baseline HIV-1 RNA level and the proportion of patients with HIV-1 RNA level <50 copies/mL at week 24 and was weighted by the sample size of the treatment group. Because studies provided outcomes for multiple treatment groups, a generalized estimating equations approach for estimation was used to appropriately adjust for the covariance structure of the data.13 Analyses were performed using Proc GENMOD using an independence working correlation structure in SAS version 9 (Statistical Analysis Software, Cary, North Carolina, USA).
RESULTS
Forty-six treatment groups from 17 clinical trials that enrolled a total of 6,579 participants were included in the analysis. The clinical trials evaluated CCR5 antagonists (maraviroc14-16 and vicriviroc8,17), a fusion inhibitor (enfuvirtide10,11), an integrase inhibitor (raltegravir18,19), a non-nucleoside reverse transcriptase inhibitor (etravirine20-22), and protease inhibitors (darunavir23-25 and tipranavir26,27) (see Table 1). All trials required prior receipt of antiretroviral drugs from 3 classes with the exception of a phase 2 study of vicriviroc that required virologic failure to 2 prior antiretroviral regimens.8 All were randomized trials except for a large single-group clinical trial of darunavir.25 Eleven of 17 were placebo-controlled, and 5 of 17 had an active control group. Eleven treatment groups from 5 clinical trials enrolling a total of 1,133 subjects used a CCR5 antagonist. Summary statistics of baseline characteristics and week 24 outcomes of the treatment groups are presented in Table 2.
Table 1.
Description of clinical trial arms included in the analysis
| Investigational agent (drug class) [ref] |
No. of arms (no. using CCR5 antagonist ) |
Total sample size (no. receiving CCR5 antagonist) |
Regimens compared, each given with OBR |
|---|---|---|---|
| Darunavir (protease inhibitor) | |||
| POWER 1 [23] | 5 (0) | 318 (0) | Phase 2 dose-finding study of darunavir vs best available PI |
| POWER 2 [24] | 5 (0) | 212 (0) | Phase 2 dose-finding study of darunavir vs best available PI |
| POWER 3 [25] | 1 (0) | 327 (0) | Single arm trial of darunavir |
| Enfuvirtide (fusion inhibitor) | |||
| TORO-1 [10] | 2 (0) | 491 (0) | Phase 3 trial of enfuvirtide vs placebo |
| TORO-2 [11] | 2 (0) | 504 (0) | Phase 3 trial of enfuvirtide vs placebo |
| Etravirine (NNRTI) | |||
| DUET-1 [20] | 2 (0) | 612 (0) | Phase 3 trial of etravirine vs placebo |
| DUET-2 [21] | 2 (0) | 591 (0) | Phase 3 trial of etravirine vs placebo |
| TMC125-C223 [22] | 3 (0) | 199 (0) | Phase 2 dose-finding of etravirine vs. control (OBT alone) |
| Maraviroc (CCR5 antagonist) | |||
| MOTIVATE 1 [14] | 3 (2) | 565 (467) | Phase 3 trial of maraviroc (once-daily, twice daily) vs placebo |
| MOTIVATE 2 [16] | 3 (2) | 464 (373) | Phase 3 trial of maraviroc (once-daily, twice daily) vs placebo |
| Pfizer 1029 [15] | 3 (2) | 186 (124) | Phase 2 trial of maraviroc (once-daily, twice daily) vs placebo |
| Raltegravir (integrase inhibitor) | |||
| BENCHMRK-1 [18] | 2 (0) | 350 (0) | Phase 3 trial of raltegravir vs placebo |
| BENCHMRK-2 [19] | 2 (0) | 349 (0) | Phase 3 trial of raltegravir vs placebo |
| Tipranavir (protease inhibitor) | |||
| RESIST-1 | 2 (0) | 620 (0) | Phase 3 trial of tipranavir vs best available PI |
| RESIST-2 | 2 (0) | 539 (0) | Phase 3 trial of tipranavir vs best available PI |
| Vicriviroc (CCR5 antagonist) | |||
| A5211 [8] | 4 (3) | 118 (90) | Phase 2 dose-finding trial of vicriviroc vs placebo |
| VICTOR-E1 [17] | 3 (2) | 114 (79) | Phase 2 dose-finding trial of vicriviroc vs placebo |
Note: OBR = optimized background regimen; OBT = optimized background therapy; PI = protease inhibitor.
Table 2.
Summary statistics of 46 clinical trial groups
| Parameter median (range) | Trial groups using CCR5 antagonists (n=11) |
Trials groups not using CCR5 antagonists (n=35) |
Total (n=46) |
|---|---|---|---|
| Number of study subjects | 61 (30–235) | 118 (28–335) | 86 (28–335) |
| Proportion of women | 11% (7%–22%) | 11% (6%–30%) | 11% (6%–30%) |
| Age, years | 45 (43–48) | 45 (41–48) | 45 (41–48) |
| Baseline HIV-1 RNA, log10 copies/mL |
4.8 (4.5–5.1) | 4.7 (4.4–5.2) | 4.7 (4.4–5.2) |
| Baseline CD4+ T-cell, cells/μL | 155 (40–202) | 109 (42–226) | 121 (40–226) |
| Proportion of subjects with HIV-1 RNA <50 copies/mL at week 24 |
41% (21%–64%) | 25% (5%–65%) | 27% (5%–66%) |
| CD4+ T-cell change from baseline at week 24, cells/μL |
102 (60–142) | 59 (−9 to 124) | 65 (−9 to 142) |
| Proportion of subjects discontinuing prior to week 24 |
33% (15%–90%) | 23% (8%–95%) | 27% (8%–95%) |
The proportion of subjects discontinuing prior to week 24 varied widely across study groups from 8% to 95%. In general, the main reason for early discontinuation was for virologic failure rather than toxicity. Overall, treatment groups receiving the investigational agents had lower rates of discontinuation than did control groups (21% vs 60%). Across the clinical trials, 2 methods were used for imputing CD4+ T-cell values at week 24 for subjects discontinuing prior to week 24. Thirteen clinical trials imputed the last observed CD4+ T-cell count while remaining on study. Four clinical trials imputed a CD4+ T-cell change of zero for such patients.
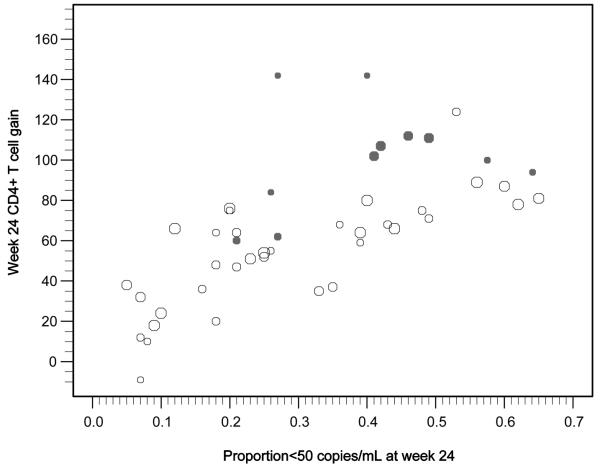
The change in CD4+ T-cell count for each treatment group plotted against observed suppression of HIV-1 RNA (proportion achieving plasma HIV-1 RNA level <50 copies/mL 24 weeks after initiating new ART regimen) is shown in Figure 1. Controlling for observed virologic suppression and baseline plasma HIV-1 RNA in each study group, use of a CCR5 antagonist was associated with an additional gain of 30 cells/μL (95% CI, 19–42) compared to not using a CCR5 antagonist. Multiple sensitivity analyses were performed to examine the robustness of these results. These are presented in Table 3. All of these models controlled for baseline plasma HIV-1 RNA level and observed virologic suppression in addition to the parameters listed.
Figure 1.
Week 24 HIV-1 RNA level and CD4+ T-cell count responses in recent studies of antiretroviral agents. Each data point represents a single treatment group. The size of the bubble indicates the relative sample size (ie, larger bubbles indicate larger studies). The solid bubbles represent groups using a CCR5 antagonist and open bubbles are groups not using a CCR5 antagonist.
Table 3.
Sensitivity analyses
| Sensitivity analysis | Additional CD4+ T-cell gain associated with CCR5 antagonist |
No. of arms (no. using CCR5 antagonist ) |
Total sample size (no. receiving CCR5 antagonist) |
|---|---|---|---|
| Control for baseline CD4+ T-cell count | 30 cells/μL (95% CI, 15–45) | Full data set | Full data set |
| Observed virologic suppression as a quadratic variable |
26 cells/μL (95% CI, 15–38) | Full data set | Full data set |
| Including only the groups receiving the investigational agent (ie, excluding control groups) |
27 cells/μL (95% CI, 16–39) | 30 (11) | 4,337 (1,133) |
| Include only those groups using LOCF |
27 cells/μL (95% CI, 14–40) | 36 (9) | 5,063 (1,054) |
| Include only those groups with <50% discontinuing prior to week 24 |
34 cells/μL (95% CI, 23–45) | 33 (8) | 5,601 (979) |
| Include only those groups with ≥30% achieving plasma HIV-1 RNA level <50 copies/mL at week 24 |
37 cells/μL (95% CI, 27–47) | 22 (7) | 3,511 (949) |
| Maraviroc only (vicriviroc arms excluded) |
27 cells/μL (95% CI, 16–38) | 39 (6) | 6,347 (964) |
| Vicriviroc only (maraviroc arms excluded) |
46 cells/μL (95% CI, 9–83) | 37 (5) | 5,344 (169) |
Note: LOCF = last observation carried forward.
DISCUSSION
Treatment groups using CCR5 antagonists exhibited a significantly better CD4+ T-cell increase over other treatment groups in treatment-experienced HIV-1–infected subjects. This was not explained by an improved degree of virologic suppression of HIV-1 because of their use. Multiple sensitivity analyses found similar estimates confirming the robustness of this effect.
A similar enhanced CD4+ T-cell effect also was seen in a phase 3 trial comparing combination regimens with maraviroc, a CCR5 antagonist, and efavirenz, a non-nucleoside reverse transcriptase inhibitor, for initial ART of HIV-1–infected patients.9 Although maraviroc did not meet prespecified criteria for noninferiority of virologic suppression to efavirenz in that study, subjects receiving maraviroc had a significantly greater CD4+ T-cell rise 48 weeks after randomization (+26 cells/μL; 95% CI, 7–46).
Although an absolute increase of 25–35 CD4+ T-cells/μL may not be clinically significant for subjects with higher CD4+ T-cell counts, the additional CD4+ T-cell gain experienced may be clinically important for subjects with low CD4+ T-cells (eg, <200/μL) to avoid clinical complications. There is certainly a rationale to improve CD4+ T-cells to over 200/μL, but one could theorize that raising CD4+ T-cells to greater than 350 or 500/μL may become an important therapeutic goal because of the relationship of CD4+ T-cell count to overall mortality and the risk of other serious non-AIDS-defining events such as certain malignancies, liver disease, and cardiovascular disease.4-6 There are limited options for the treatment for suboptimal CD4+ T-cell recovery on effective ART. Parenteral interleukin-2 was evaluated in 2 very large clinical trials and was found to have no impact on the occurrence of opportunistic disease or death despite significantly raising CD4+ T-cell counts.28,29 This suggests that new therapies that increase CD4+ counts by mechanisms other than suppression of HIV replication will need to be confirmed with clinical endpoint studies.
Even though the basis for the variability in CD4+ T-cell reconstitution with ART is not known, the importance of CCR5-mediated pathways is highlighted by the observation that greater CD4+ T-cell recovery on effective ART is associated with certain CCL3L1-CCR5 genotypes.30 The mechanism of the enhanced CD4+ T-cell gain associated with CCR5 antagonists is not clear. In addition to enhanced virologic suppression, there are several processes that may be affected by CCR5 antagonists. In vitro, the binding of free or infected cell-bound gp120 to uninfected CCR5-expressing CD4+ T-cells induces the apoptosis of uninfected CD4+ T-cells.31,32 The interaction of CCR5 and its cognate β-chemokine ligand, RANTES (regulated on activation normal T cell expressed and secreted; CCL5), can induce the apoptosis of uninfected CD4+ T-cells.33 Blocking RANTES-CCR5 interactions may also decrease levels of immune activation, which has been associated with a smaller CD4+ T-cell recovery after the initiation of ART.34,35 Also, blocking the RANTES-CCR5 interaction may cause CD4+ T-cell redistribution from the lymphoid tissue to the peripheral blood, as suggested by the temporal pattern of CD4+ T-cell reconstitution with concomitant increases in CD8+ T-cells after the use of maraviroc in treatment-naïve subjects.36 A CCR5 antagonist could block apoptosis, reduce immune activation, and/or promote redistribution of CD4+ T-cells. If this last mechanism is solely responsible for the observed CD4+ T-cell increase, then this increase may not be associated with a clinical benefit.
This study had several limitations. This analysis relies on combining data from clinical trials conducted in varying populations and in differing time periods, and we did not have access to patient-level data. We could not control for differences in background ART regimens, which may be associated with CD4+ T-cell responses. These studies reported a wide range for the proportion discontinuing randomized treatment prior to week 24. The clinical trials of CCR5 antagonists included in this analysis required subjects to have CCR5-using HIV-1 for eligibility with one exception.15 The clinical trials of other agents included in this analysis had no restriction on viral tropism at entry. Subjects with CCR5-using HIV-1 may be prone to larger CD4+ T-cell increases than those with dual- or mixedtropic infections. However, a similar effect size was seen in the one study of a CCR5 antagonist in subjects with dual- or mixed-tropic HIV-1, suggesting that the mechanism of CD4+ T-cell gain may not be dependent on having HIV-1 exclusively using CCR5.15 Coreceptor tropism was not related to CD4+ T-cell recovery among participants in the phase 3 trials of enfuvirtide.37 We are unable to assess which patient factors are associated with CD4+ T-cell gain. In particular, we could not assess the relationship of enfuvirtide, another HIV-1 entry inhibitor, to CD4+ T-cell gain. Subjects initiating a new antiretroviral regimen with lower CD4+ T-cells are more in need of a robust CD4+ T-cell gain. However, this meta-regression was unable to establish whether the enhanced CD4+ T-cell gain associated with CCR5 antagonists is concentrated among subjects in a particular baseline CD4+ T-cell stratum.
In summary, this meta-regression of phase 2 and 3 trials of investigational antiretroviral agents found that use of a CCR5 antagonist was associated with a significantly larger CD4+ T-cell count gain compared to groups that did not use CCR5 antagonists independent of the degree of observed virologic suppression. The mechanism of this increase is unclear and should be investigated further. Potentially, this effect could be exploited to enhance CD4+ T-cell recovery among subjects with suboptimal immunologic responses to ART despite sustained virologic suppression. However, use of CCR5 antagonists for enhancing CD4+ T-cell responses cannot be recommended until clinical trials specifically examining this effect have been conducted.
ACKNOWLEDGMENTS
This material was presented in part at the 15th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2008; Boston, MA, USA.
Support
This study was supported by grants K24 AI-51966 (Dr. Gulick.), U01 AI068634 (Dr. Ribaudo), U01-AI069471 (Dr. Tenorio).
Footnotes
Conflicts of Interest
Dr. Wilkin received research grant support from Tibotec. He has served as a consultant for Pfizer and Quest Diagnoistics. Dr. Gulick received research grant support from Merck, Pfizer, Schering, and Tibotec and served as an ad hoc consultant to Bristol-Myers, Gilead, GlaxoSmithKline, Merck, Monogram, Pathway, Pfizer, Progenics, Schering, Tibotec, and Virostatics. Drs. Ribaudo and Tenorio have no relevant financial disclosures.
REFERENCES
- 1.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviralnaive HIV-infected adults. AIDS. 2006;20:2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 2.Grabar S, Le Moing V, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Chene G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: Analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 4.Lodwick R, Porter K, Sabin C, et al. Age- and sex-specific death rates in ART-naïve patients with CD4 count above 350 cells/mm3 compared with the general population; Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. February 3–6, 2008; Abstract 141. [Google Scholar]
- 5.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Immunodeficiency and risk of AIDS-defining and non-AIDS-defining cancers: ANRS CO3 Aquitaine Cohort, 1998 to 2006; Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. February 3–6, 2008; Abstract 15. [Google Scholar]
- 6.Marin B, Thiebaut R, Rondeau V, et al. Association between CD4 and HIV RNA with non AIDS-related causes of death in the era of combination antiretroviral therapy (cART); Program and abstracts from the 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. July 22–25, 2007; Abstract WEPEB019. [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Dec 19, 2008. Updated November 3, 2008. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 8.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 9.Saag M, Prudence I, Jayvant H, et al. A multicenter, randomized, double-blind, comparative trial of a novel CCR5 antagonist, maraviroc versus efavirenz, both in combination with Combivir (zidovudine [ZDV] / lamivudine [3TC]), for the treatment of antiretroviral naïve patients infected with R5 HIV-1: week 48 results of the MERIT study; Program and abstracts of the 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. July 22–25, 2007; Abstract WESS104. [Google Scholar]
- 10.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–2195. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 11.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 12.Zolopa AR, Mullen M, Berger D, et al. The HIV integrase inhibitor GS-9137 demonstrates potent antiretroviral activity in treatment-experienced patients; Program and abstracts from the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. February 25–28, 2007; Abstract 143LB. [Google Scholar]
- 13.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 14.Nelson M, Fatkenheuer G, Konourina I, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic, ART-experienced patients infected with CCR5-tropic HIV-1 in Europe, Australia, and North America: 24-week results; Program and abstracts from the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. February 25–28, 2007; Abstract 104aLB. [Google Scholar]
- 15.Mayer H, Van der Ryst E, Saag M, et al. Safety and efficacy of maraviroc, a novel CCR5 antagonist, when used in combination with optimized background therapy for the treatment of antiretroviral-experienced subjects infected with dual/mixed-tropic HIV-1: 24-week results of a phase 2b exploratory trial; Program and abstracts from the XVI International AIDS Conference; Toronto, Canada. August13–18, 2006; Abstract THLB0215. [Google Scholar]
- 16.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [104bLB]; Program and abstracts from the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. February 25–28, 2007; Abstract 104bLB. [Google Scholar]
- 17.Zingman B, Suleiman J, DeJesus E, et al. Vicriviroc, a next generation CCR5 antagonist, exhibits potent, sustained suppression of viral replication in treatment-experienced adults: VICTOR-E1 48-week results [39LB]; Program and abstracts from the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. February 3–6, 2008; Abstract 39LB. [Google Scholar]
- 18.Cooper D, Gatell J, Rockstroh J, et al. 48-week results from BENCHMRK-1, a phase III study of raltegravir in patients failing ART with triple-class resistant HIV-1; Program and abstracts from the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. February 3–6, 2008; Abstract 788. [Google Scholar]
- 19.Steigbigel R, Kumar P, Eron J, et al. 48-week results from BENCHMRK-2, a phase III study of raltegravir in patients failing ART with triple-class resistant HIV; Program and abstracts from the 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. February 3–6, 2008; Abstract 789. [Google Scholar]
- 20.Madruga JV, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 21.Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 22.TMC125–C223 Writing Group. Nadler JP, Berger DS, Blick G, et al. Efficacy and safety of etravirine (TMC125) in patients with highly resistant HIV-1: primary 24-week analysis. AIDS. 2007;21:F1–10. doi: 10.1097/QAD.0b013e32805e8776. [DOI] [PubMed] [Google Scholar]
- 23.Haubrich R, Berger D, Chiliade P, et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS. 2007;21:F11–8. doi: 10.1097/QAD.0b013e3280b07b47. [DOI] [PubMed] [Google Scholar]
- 24.Katlama C, Esposito R, Gatell JM, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007;21:395–402. doi: 10.1097/QAD.0b013e328013d9d7. [DOI] [PubMed] [Google Scholar]
- 25.Molina JM, Cohen C, Katlama C, et al. Safety and efficacy of darunavir (TMC114) with low-dose ritonavir in treatment-experienced patients: 24-week results of POWER 3. J Acquir Immune Defic Syndr. 2007;46:24–31. doi: 10.1097/QAI.0b013e3181359cfb. [DOI] [PubMed] [Google Scholar]
- 26.Cahn P, Villacian J, Lazzarin A, et al. Ritonavir-boosted tipranavir demonstrates superior efficacy to ritonavir-boosted protease inhibitors in treatment-experienced HIV-infected patients: 24-week results of the RESIST-2 trial. Clin Infect Dis. 2006;43:1347–1356. doi: 10.1086/508352. [DOI] [PubMed] [Google Scholar]
- 27.Gathe J, Cooper DA, Farthing C, et al. Efficacy of the protease inhibitors tipranavir plus ritonavir in treatment-experienced patients: 24-week analysis from the RESIST-1 trial. Clin Infect Dis. 2006;43:1337–4136. doi: 10.1086/508353. [DOI] [PubMed] [Google Scholar]
- 28.Losso M, Abrams D, the INSIGHT ESPRIT Study Group Effect of interleukin-2 on clinical outcomes in patients with a CD4+ count >300/mm3; Program and abstracts from the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. February 8–11, 2009; Abstract 90aLB. [Google Scholar]
- 29.Levy Y, the SILCAAT Scientific Committee Effect of interleukin-2 on clinical outcomes in patients with a CD4+ count 50 to 299/mm3; Program and abstracts from the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. February 8–11, 2009; Abstract 90bLB. [Google Scholar]
- 30.Ahuja SK, Kulkarni H, Catano G, et al. CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1 infected individuals. Nature Med. 2008;14:413–420. doi: 10.1038/nm1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Algeciras-Schimnich A, Vlahakis SR, et al. CCR5 mediates Fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells. AIDS. 2002;16:1467–1478. doi: 10.1097/00002030-200207260-00003. [DOI] [PubMed] [Google Scholar]
- 32.LaBonte JA, Madani N, Sodroski J. Cytolysis by CCR5-using human immunodeficiency virus type 1 envelope glycoproteins is dependent on membrane fusion and can be inhibited by high levels of CD4 expression. J Virol. 2003;77:6645–6659. doi: 10.1128/JVI.77.12.6645-6659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murooka TT, Wong MM, Rahbar R, Majchrzak-Kita B, Proudfoot AE, Fish EN. CCL5-CCR5-mediated apoptosis in T cells: requirement for glycosaminoglycan binding and CCL5 aggregation. J Biol Chem. 2006;281:25184–25194. doi: 10.1074/jbc.M603912200. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 35.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodefi ciency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 36.Lazzarin A, Battegay M, Cooper DA, et al. CD4+ cell restoration at 48 weeks in the maraviroc treatment-naive MERIT trial; Program and abstracts from the Joint Infectious Disease Society of America/International Conference of Antimicrobial Agents and Chemotherapy; Washington, DC, USA. October 25–28, 2008; Abstract H-1248. [Google Scholar]
- 37.Melby T, Despirito M, Demasi R, Heilek-Snyder G, Greenberg ML, Graham N. HIV-1 coreceptor use in triple-class treatment-experienced patients: baseline prevalence, correlates, and relationship to enfuvirtide response. J Infect Dis. 2006;194:238–246. doi: 10.1086/504693. [DOI] [PubMed] [Google Scholar]