Abstract
Although previous studies have established a prominent role for HMGA1 (formerly HMG-I/Y) in aggressive human cancers, the role of HMGA2 (formerly HMGI-C) in malignant transformation has not been clearly defined. The HMGA gene family includes HMGA1, which encodes the HMGA1a and HMGA1b protein isoforms, and HMGA2, which encodes HMGA2. These chromatin binding proteins function in transcriptional regulation and recent studies also suggest a role in cellular senescence. HMGA1 proteins appear to participate in cell cycle regulation and malignant transformation, while HMGA2 has been implicated primarily in the pathogenesis of benign, mesenchymal tumors. Here, we show that overexpression of HMGA2 leads to a transformed phenotype in cultured lung cells derived from normal tissue. Conversely, inhibiting HMGA2 expression blocks the transformed phenotype in metastatic human non-small cell lung cancer cells. Moreover, we show that HMGA2 mRNA and protein are overexpressed in primary human lung cancers compared to normal tissue or indolent tumors. In addition, there is a statistically significant correlation between HMGA2 protein staining by immunohistochemical analysis and tumor grade (p < 0.001). Our results indicate that HMGA2 is an oncogene important in the pathogenesis of human lung cancer. Although additional studies with animal models are needed, these findings suggest that targeting HMGA2 could be beneficial therapeutically in lung and other cancers characterized by increased HMGA2 expression.
Keywords: HMGA2, lung cancer, oncogene, antisense, transformation
Introduction
While previous studies have established a role for high mobility group A2 or HMGA2 gene (formerly HMGI-C) in slow-growing, benign, mesenchymal tumors (1-6), its function in malignant transformation has not been clearly defined. The HMGA genes encode a subfamily of the high mobility group (HMG) proteins, which were discovered over 25 years ago as abundant, nonhistone chromatin binding proteins and named based on their electrophoretic mobility in polyacrylamide gels (6-8). The HMGA protein subfamily (8) includes HMGA1a (formerly HMG-I), HMGA1b (formerly HMG-Y) and HMGA2 (formerly HMGI-C). HMGA1a and HMGA1b protein isoforms result from alternative splicing of the HMGA1 mRNA (9-10), whereas HMGA2 is encoded by the separate but related gene, HMGA2 (1-2). All HMGA proteins contain AT hook DNA binding motifs that mediate binding to the minor groove of chromosomal DNA. After binding to DNA, HMGA proteins induce a conformational change and facilitate DNA-transcription factor interactions. Previous studies indicate that HMGA proteins function in regulating gene expression (reviewed in 11); more recent studies also suggest a role in cellular senescence (12).
Increasing evidence implicates HMGA proteins in normal and neoplastic cell growth. HMGA1 and HMGA2 are expressed as delayed-early genes after growth factor stimulation and their expression is increased in cellular proliferation (13). HMGA1 expression is also increased in neoplastic transformation (11). Several independent laboratories have shown that overexpression of HMGA1a and HMGA1b result in a transformed phenotype in cultured cells (14-18). In addition, two different transgenic mouse models overexpressing HMGA1 develop lymphoid malignancies and other tumors (18-20). Antisense experiments demonstrate that decreasing HMGA1 proteins blocks the transformed phenotype (15-17, 20-21). Moreover, increased levels of HMGA1 mRNA or protein correlate with more advanced disease in some tumors (22).
The role of HMGA2 in normal and neoplastic cell growth is less well established. In vitro studies show that HMGA2 chimeric proteins bind to DNA and alter transcription of reporter genes (23). HMGA2 appears to be involved in the pathogenesis of benign, solid human tumors (1-6). Specifically, the HMGA2 A-T hooks have been identified in chimeric proteins associated with lipomas and other benign, mesenchymal tumors (2-5). These chimeras are thought to function by binding to DNA via the A-T hooks and altering gene expression, possibly through the potential transcriptional regulatory domains acquired in their rearrangement with HMGA2 (3). The HMGA2 chimeras or a truncated HMGA2 with three AT-hook DNA binding domains, induce anchorage-independent cell growth in NIH3T3 murine fibroblasts (24), while full-length HMGA2 confers a transformed phenotype in cultured, rat fibroblasts and human lymphocytes (14). In addition, transgenic mice expressing a truncated HMGA2 transgene develop increased fat, gigantism, and lipomas (25-26). Transgenic mice expressing full length, wild type HMGA2 driven by the cytomegalovirus promoter develop benign epithelial tumors (27), including pituitary adenomas, (85% of females and 40% of males) as well as micropolycystic kidney disease (~ 50% of all mice). At lower frequencies, these mice also develop lipomatosis (20 - 29%), large cell lymphomas (<25%), and lung adenomas (< 25%). In another transgenic model with HMGA2 driven by a differentiated mesenchymal cell (adipocyte)-specific promoter, mice developed mesenchymal tumors, including fibroadenomas of the breast and salivary glands (28). Interestingly, this same group also generated transgenic mice expressing a truncated version of HMGA2 containing only the three DNA-binding domains, which produced similar mesenchymal tumors (28). Both HMGA2 transgenic mouse models resulted in mice with predominantly benign, slow growing tumors, similar to the types of human tumors with mutations leading to expression of abnormal HMGA2 fusion proteins. Interestingly, the HMGA2 (HMGI-C) knock-out mouse displayed a pygmy phenotype (29). More recently, increased expression of HMGA2 was identified in a significant percentage of malignant, primary human breast epithelial tumors (30) and lung cancers (31-32), which indicates a potential role for HMGA2 in the pathogenesis of epithelial cancers in addition to benign mesenchymal tumors.
Here, we show that HMGA2 gene and protein expression are increased in human lung cancer cell lines and primary lung tumors compared to benign tumors or normal lung tissue. We also demonstrate that HMGA2 is both necessary and sufficient for transformation in cultured lung cells. Moreover, our findings specifically implicate HMGA2 in the pathogenesis of human lung cancers.
Results
HMGA2 Expression is Increased in Cultured Human Lung Cancer Cells
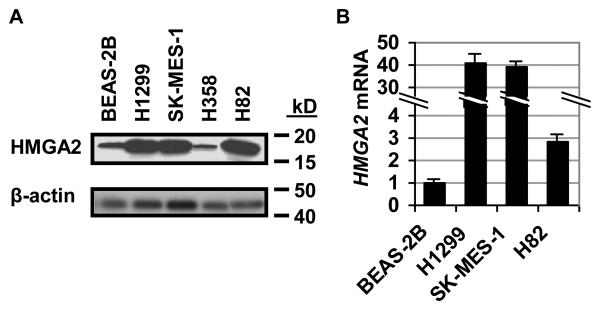
To determine if HMGA2 protein levels are increased in lung cancer cells derived from malignant neoplasms compared to normal lung tissue or benign tumors, we first surveyed cultured human lung cancer cell lines for HMGA2 protein levels and compared them to cultured cells derived from normal lung tissue (Fig. 1A). For control cells, we used BEAS-2B cells, which were isolated from normal human bronchial epithelium (33). We found that HMGA2 protein levels are increased in all metastatic lung cancer cell lines compared to control cells, including H1299 metastatic non-small cell carcinoma cells, SK-MES-1 metastatic squamous cell carcinoma cells, and H82 metastatic small cell carcinoma cells. H358 cells, which were derived from a localized, non-small cell bronchioalveolar carcinoma, had HMGA2 levels similar to the BEAS-2B cells. Interestingly, bronchioloalveolar carcinomas tend to be localized and lacking in parenchymal invasion (34-35). Moreover, patients with bronchioloalveolar carcinomas have a 64.9% one-year survival (34), which is significantly better than most lung cancers (35).
FIGURE 1.
HMGA2 expression is increased in lung cancer cell lines from metastatic disease.
A. Western blot analysis shows increased HMGA2 in the human lung cancer cell lines H1299 (metastatic non-small cell carcinoma), SK-MES-1 (metastatic squamous cell carcinoma), H82 (metastatic small cell carcinoma) compared with the normal human bronchial epithelium cell line BEAS-2B. In H358 cells, derived from non-metastatic, non-small cell bronchioloalveolar carcinoma, HMGA2 protein levels are similar to that of the BEAS-2B immortalized control cells line.
B. Quantitative RT-PCR analysis shows increased HMGA2 mRNA in H1299, SK-MES-1, and H82 metastatic lung cancer cells compared to BEAS-2B cells, consistent with the Western blot analysis (A). Values shown are fold-differences of RNA expression compared to BEAS-2B cells (arbitrarily assigned a value of 1); results represent the mean +/− the standard deviation from two independent experiments performed in triplicate.
We also analyzed HMGA2 expression by quantitative RT-PCR in the H1299, SK-MES-1 and H82 lung cancer cell lines (Fig. 1B). Consistent with our Western results, we found high levels of HMGA2 mRNA in the cells derived from lung cancers compared to normal lung cells (BEAS-2B).
Inhibiting HMGA2 Expression Blocks Transformation in Lung Cancer Cells
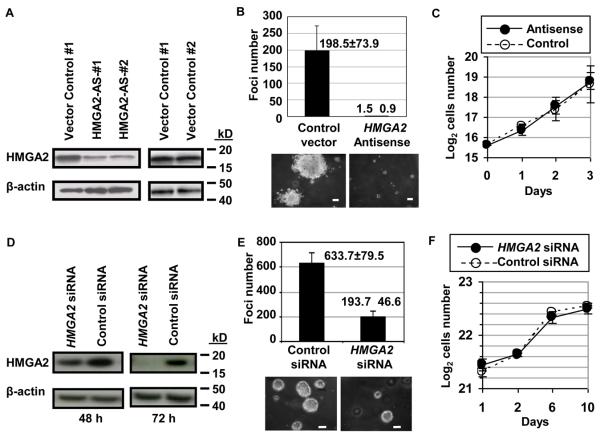
To determine if HMGA2 is required for transformation in lung cancer, HMGA2 protein was decreased in the H1299 lung cancer cells (derived from the metastatic, non-small cell lung cancer) using an RNA interference approach with a ribozyme antisense vector (15). Two polyclonal cell lines (HMGA2-AS-1 and 2) were independently generated by transfecting H1299 with the antisense construct HMGA2-AS. Both cell lines exhibited significantly reduced HMGA2 proteins (Fig. 2A). The related proteins, HMGA1a and HMGA1b were unaffected (not shown), indicating that the antisense vectors caused a specific, significant decrease in HMGA2 proteins. We observed that blocking HMGA2 expression strongly impaired anchorage-independent cell growth on soft agar, demonstrating that HMGA2 is required for this transformed phenotype in these metastatic human lung cancer cells (Fig. 2B). The growth rates of the control and antisense cells were similar, indicating that the difference in foci formation was not due to a general inhibition of cell growth or toxic effect that impairs proliferation (Fig. 2C).
FIGURE 2.
Interfering with HMGA2 expression blocks transformation in metastatic lung cancer cells.
A. Western blot analysis shows decreased HMGA2 in H1299 lung cancer cells transfected with the HMGA2 antisense constructs (HMGA2-AS-1 and HMGA2-AS-2) compared to control cells transfected with vector alone. The blot was probed with the HMGA2 antibody as well as an antibody to β-actin to control for protein loading.
B. Anchorage-independent growth on soft agar is inhibited in H1299 lung cancer cells transfected with the HMGA2 antisense construct compared to control cells transfected with vector alone. The number of foci formed by H1299 cells transfected with the antisense construct is decreased compared to the control cell transfected with vector control. The experiment was performed in duplicate and repeated; results are expressed as an average ± standard deviation from two independent experiments. The photograph shows representative foci. Size bar: 100 μm.
C. The growth rates of the control and antisense cells were similar, indicating that the difference in foci formation was not due to inhibition of growth in general. The graph shows the averages ± standard deviations from duplicate plates. Empty dots and dashed line: control, Solid dots and line: HMGA2 antisense.
D. Western blot analysis shows decreased HMGA2 in SK-MES-1 lung cancer cells transfected with the HMGA2 siRNA compared to cells transfected with the control siRNA (siCONTROL nontargeting siRNA pool; Dharmacon). The blot was probed with the HMGA2 antibody as well as an antibody to β-actin to control for protein loading.
E. Anchorage-independent growth on soft agar is inhibited in SK-MES-1 lung cancer cells transfected with the HMGA2 siRNA compared to cells transfected with the control siRNA. The number of foci formed by SK-MES-1 cells transfected with the HMGA2 siRNA is decreased compared to cells transfected with the control siRNA. The experiment was performed in duplicate and repeated; results are expressed as an average ± standard deviation from two independent experiments. The photograph shows representative foci. Size bar: 100 μm.
F. The growth rates of SK-MES-1 cell transfect with either HMGA2 siRNA or control siRNA were similar, indicating that the difference in foci formation was not due to a generalized inhibition of growth or a toxic effect. The graph shows the averages ± standard deviations from duplicate plates. Empty dots and dashed line: control siRNA, Solid dots and line: HMGA2 siRNA.
To further investigate the role of HMGA2 in advanced-stage, metastatic non-small cell lung cancer cells, we used a short inhibitory RNA interference oligonucleotide (siRNA; Dharmacon) to knock-down HMGA2 expression in another lung cancer cell line. For these experiments, we used SK-MES-1 metastatic squamous cell lung carcinoma cells, which express high levels of HMGA2 mRNA and protein. This siRNA approach effectively silences HMGA2, both at the protein (Fig. 2D) and mRNA level for up to 6 days (Supplementary Fig. S1). Similar to our results with H1299 cells, we found that knocking down HMGA2 expression also blocks foci formation in these lung cancer cells (Fig. 2E). Cellular proliferation was not affected by the siRNAs in standard culture conditions, indicating that the siRNAs are not toxic to cell growth at the concentrations used (Fig. 2F). Taken together, our results demonstrate that inhibiting HMGA2 expression in metastatic lung cancer cells blocks anchorage-independent cell growth and suggests an important role for HMGA2 in this cancer phenotype.
Increased Expression of HMGA2 in Normal Lung Cells Leads to a Transformed Phenotype
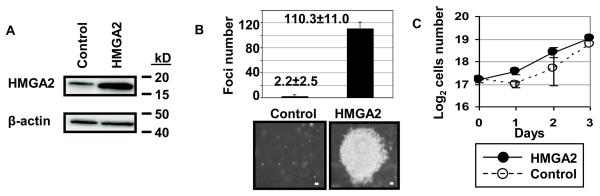
Because our antisense knock-down experiments showed that HMGA2 proteins are necessary for the transformed phenotype in cultured lung cancer cells, we next sought to determine if overexpression of HMGA2 in normal cultured lung cells results in a transformed phenotype. BEAS-2B cells were transfected with an HMGA2 expression construct or the control vector. Western analysis showed that the HMGA2 protein was overexpressed in the cells transfected with HMGA2 compared to control cells transfected with vector alone (Fig. 3A). We found that both BEAS-2B cells exhibited anchorage-independent cell growth and formed foci on soft agar when transfected to overexpress HMGA2 (Fig. 3B). Growth rates in standard tissue culture conditions were not significantly affected by HMGA2 transfection in these cells (Fig. 3C) indicating that the observed phenotypes did not occur simply by enhancing cell growth rates. These studies show that overexpression of HMGA2 leads to a transformed phenotype in cultured cells derived from normal lung tissue and suggest that HMGA2 induces transformation by activating transformation-specific pathways independent of cellular proliferation rates.
FIGURE 3.
Overexpression of HMGA2 in human lung cells derived from normal tissue leads to a transformed phenotype
A. Western blot analysis shows that the BEAS-2B cells transfected with the pSG5-HMG-C (HMGA2) construct overexpress HMGA2 compared to cells transfected with contol vector. All lanes were probed with the HMGA2 antibody as well as the β-actin antibody to control for sample loading.
B. Transformed foci in BEAS-2B cells transfected with the pSG5-HMG-C (HMGA2) construct compared to cells transfected with vector alone. The number of transformed foci formed in the cells overexpressing HMGA2 or control cells are indicated. The experiments were performed in triplicate and repeated twice; results are expressed as the mean ± standard deviation from the independent experiments. The photograph shows representative foci. Size bar: 100 μm.
C. The growth rates of the control and antisense cells were similar indicating that HMGA2 overexpression does not affect adherent growth in these cells. The graph shows the averages ± standard deviations from duplicate plates. Empty dots and dashed line: control, Solid dots and line: HMGA2 antisense.
Increased Expression of HMGA2 in Human Lung Cancers
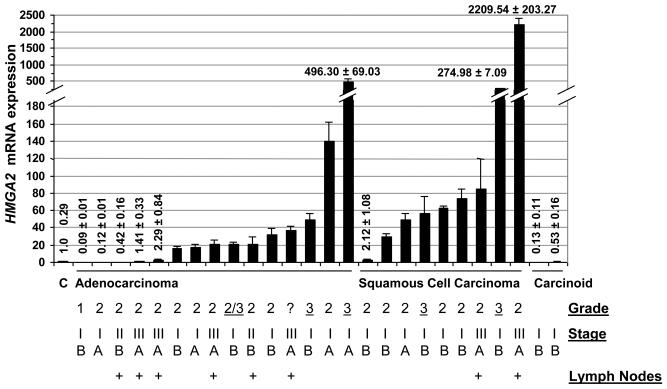
To determine if our studies with cultured cells are relevant to human lung cancers, we evaluated expression of HMGA2 by quantitative RT-PCR in primary human lung tumors, including adenocarcinomas, squamous cell carcinomas, and carcinoid tumors (Fig. 4, Supplementary Table S1). In 20/26 (77%) primary lung tumors, HMGA2 expression was increased by 2-fold or more compared to that of normal controls (Clontech). The control RNA was derived from pooled, lung tissue from 3 previously healthy adults with an age range of 19-50 years who had died from causes other than lung cancer. In most cases, HMGA2 expression was increased by 15-fold or more (Fig. 4). Most of the non-small cell lung cancers had elevated HMGA2 expression, including all squamous cell carcinomas (9/9) and most adenocarcinomas (11/15). Both of these tumor types are associated with smoking and a poor outcome with only a 10% overall 5 year survival rate (35). There was a history of smoking in most of these cases (Supplementary Table S1). Adenocarcinomas are slightly more common lung cancers, accounting for about 40% of all lung cancer, whereas squamous cell carcinomas make up about 20-30% of all lung cancers (35). Of note, the tumor with the lowest HMGA2 mRNA was taken from the only patient with grade 1 histology (well-differentiated). For the squamous cell carcinomas, levels of HMGA2 mRNA were more than 2-fold above pooled, normal control lung RNA for tumors designated as grade 2 (moderately-differentiated) or grade 3 (poorly-differentiated). For adenocarcinoma tumors with HMGA2 mRNA levels ≥ 40-fold above control, 2/3 tumors were grade 3, with the remaining case being grade 2. Interestingly, HMGA2 expression was not increased in the slow-growing, carcinoid tumors. These tumors are typically localized and have a 5-year survival of 96% (35). Thus, HMGA2 expression was increased in most (20/24; 83%) of the malignant lung neoplasms, but not in the slow growing, carcinoid tumors (0/2). These findings suggest that it may be a marker for malignant lung tumors.
FIGURE 4.
HMGA2 mRNA expression is increased in primary lung tumor samples. Quantitative RT-PCR shows that HMGA2 mRNA expression is increased in 20/24 primary lung tumors compared to the control pooled, normal lung tissue or slow-growing carcinoid tumors. Values shown are fold-differences of mRNA expression compared to normal pooled lung mRNA (arbitrarily set at 1); results represent the mean +/− the standard deviation from 2 independent experiments performed in triplicate. Tumor grade and stage are indicated below each bar. Additional clinical data is available (Supplementary Table S1).
To investigate whether HMGA2 overexpression was associated with gene amplification, we performed a qPCR analysis on genomic DNA extracted from 15 of the 26 lung cancer samples for which sufficient frozen material was available. These included adenocarcinomas (n = 10), squamous cell carcinoma (n = 3), and carcinoid tumors (n = 2), and control, normal lung tissue (n = 9). None of these samples showed evidence of amplification.
HMGA2 Protein Expression Correlates with Tumor Grade
To determine if HMGA2 protein levels are increased in lung cancer, we performed immunohistochemical staining of a lung tissue microarray containing 91 primary lung tumors using a commercial HMGA2 antibody (Table 1, Fig. 5). We identified HMGA2 immunoreactivity in 37 of 89 (41.6%) evaluable cases, with scores ranging from 1-9 (median 6). Two cases did not contain sufficient tumor for evaluation and were excluded. Of the positive cases, 4 (10.8%) displayed limited reactivity (score <3), 15 (40.5%) displayed moderate reactivity (score 3-6) and 18 (48.6%) displayed strong reactivity (score >6). There was a significant positive correlation between HMGA2 staining and tumor grade (p < 0.001 by Spearman's rank correlation). The mean HMGA2 score for tumors with a histologic grade > 2 was 4..43 +/− 4.14 compared to 1.52 +/− 2.61 for tumors with a histologic grade ≤ 2 (p = 0.0021; student's t-test). Immunoreactivity for HMGA2 was seen in adjacent non-neoplastic bronchial epithelium, but was not observed in any other normal tissues. These findings demonstrate that HMGA2 protein levels correlate positively with a more advanced histologic tumor grade.
Table 1.
HMGA2 expression by Histologic Type and Clinical Characteristics in tissue microarray
| Histologic Type* |
Age, years (mean +/− SD) |
Gender M/F |
Smoking History^ |
Stage | Death before 5 years |
HMGA2 3 to |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | <3 | 6 | >6 | |||||
| AC (n = 29) | 66.3 +/−11.4 | 9/20 | 22/25 | 17 | 6 | 1 | 5 | 13 | 24 | 4 | 1 |
| SCC (n = 30) | 68.1 +/− 8.0 | 25/5 | 25/27 | 18 | 3 | 5 | 4 | 16 | 12 | 10 | 8 |
| BAL (n = 8) | 66.4 +/− 9.7 | 2/6 | 5/6 | 8 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| CA, NOS (n = 8) | 66.3 +/− 8.4 | 4/4 | 7/7 | 4 | 1 | 1 | 2 | 4 | 2 | 0 | 6 |
| Carcinoid (n = 3) | 61.3 +/−17.1 | 1/2 | 1/3 | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 0 |
| LCLC (n = 2) | 76.5 +/− 5.0 | 1/1 | 2/2 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| AGC (n = 1) | 68 | 1/0 | 1/1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| PSC (n = 1) | 63 | 0/1 | 1/1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| SCLC (n = 3) | 64 +/− 3.0 | 2/1 | 3/3 | NA | NA | NA | NA | 1 | 3 | 0 | 0 |
| Undiff (n = 3) | 60 +/− 13.1 | 1/2 | 1/3 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 2 |
AC – adenocarcinoma; SCC – squamous cell carcinoma; BAL – bronchoalveolar carcinoma; CA, NOS – Carcinoma, not otherwise specified; LCLC – large cell lung cancer, AGC – anaplastic giant cell carcinoma; PSC – pleomorphic sarcomatoid carcinoma; SCLC – small cell lung carcinoma; Undiff – undifferentiated carcinoma
Smoking history where available
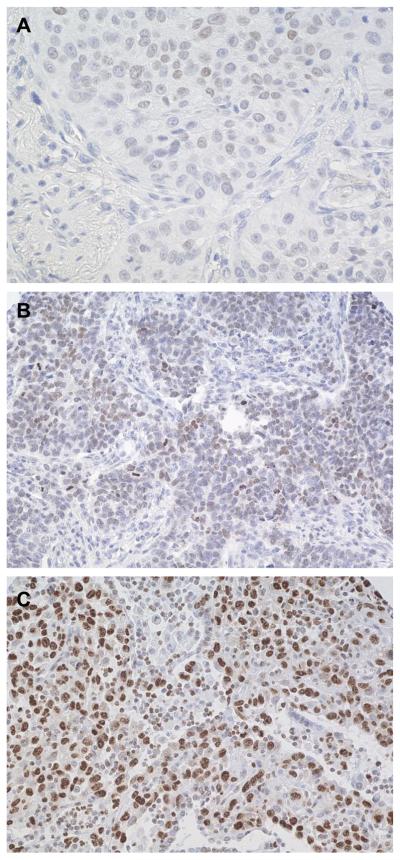
FIGURE 5.
Immunohistochemical analysis of the HMGA2 protein expression in lung squamous cell carcinoma.
A. Grade 2, moderately-differentiated squamous cell carcinoma with weak staining (score <3, magnification × 100).
B. Grade 2, moderately-differentiated squamous cell carcinoma with moderate staining (score 3-6, magnification × 64).
C. Grade 3, poorly-differentiated SCC with strong staining (score >6, magnification × 64)
Discussion
Lung cancer is the leading cause of cancer death worldwide, striking nearly 175,000 individuals with over 160,000 deaths each year in the United States alone (35). About 1 in 13 men and 1 in 17 women will develop lung cancer over the course of a lifetime and the incidence is rising, particularly in developing nations (35). The overall 5-year survival for all lung cancers is only 15%, and it is only 10% for squamous cell carcinoma and adenocarcinoma (35). Thus, research is urgently needed to identify the molecular pathways that lead to lung carcinogenesis. The inactivation of tumor suppressor genes, such as p53, Rb, and p16 is a frequent molecular abnormality in lung cancer, as well as the activation or amplification of proto-oncogenes, including K-ras, c-myc, and HER2/neu, EGFR, cyclin D1, and BCL2 (36-37). Telomerase RNA and the hTERT catalytic component are also expressed in most lung cancers, and may provide a mechanism for unlimited cellular replication (36). These genetic alterations are believed to lead to aberrant cell growth and ultimately contribute to the malignant phenotype. Interestingly, HMGA2 has been shown to bind to and inactivate pRB, which could play a role in lung carcinogenesis (38).
HMGA2 has been implicated primarily in benign tumors derived from mesenchymal tissue (2-5), although there is an emerging body of evidence indicating that HMGA2 may also play a role in the pathogenesis of aggressive tumors (30-32). We first showed that overexpression of HMGA2 leads to anchorage-independent cell growth in both rat and human cultured cells (14). There have been limited studies to suggest that HMGA2 is required for transformation in cancer. An earlier study showed that down-regulating HMGA2 by an antisense approach prevented the transformation of thyroid cells by v-mos or v-ras-Ki (39). In this study, it was not clear if the antisense vector was specific for HMGA2 alone, or also led to the down-regulation of HMGA1 proteins. It was later shown that repressing HMGA2 expression in retinoblastoma cells decreases proliferation in these cells, although anchorage-independent cell growth, tumorigenicity, or other transformation phenotypes were not assessed (39). It was later shown that suppressing HMGA2 protein synthesis also blocks proliferation and induces apoptosis in well-differentiated liposarcoma cells (40). There have been no prior studies to show that inhibiting HMGA2 expression in metastatic or advanced-stage cancer cell lines blocks anchorage-independent cell growth. More recently, nuclear staining for both HMGA1 and HMGA2 proteins was found to be elevated in varied lung cancer subtypes, including adenocarcinoma, adenosquamous carcinoma, squamous cell carcinoma, large-cell lung cancer, large-cell neuroendocrine carcinoma, small-cell lung cancer, and pleomorphic carcinoma (31). In that study, increased levels of HMGA2 protein using a semiquantitative approach with immunohistochemical analysis also correlated with patient death in primary lung adenocarcinomas from 49 cases. Quantitative RT-PCR assay was used to assess HMGA2 mRNA in a small subset of these tumors. A more recent study showed similar results with increased HMGA2 mRNA in lung cancers samples compared to adjacent lung tissue (32). In our study, we extend these studies and demonstrate a functional role for HMGA2 in lung cancer. We show that HMGA2 mRNA expression (assessed by quantitative RT-PCR) from primary tumors is increased in most primary lung cancers (21/24 or 88%), including 12/15 adenocarcinoma samples and 9/9 squamous cell carcinomas compared to control lung tissue or indolent, carcinoid tumors (2/2). We also found that HMGA2 protein levels correlate with increasing tumor grade in a large tissue microarray of primary lung tumors. Our studies also demonstrate that ectopic expression of HMGA2 is sufficient for anchorage-independent cell growth and transformation in soft agar in cells derived from normal lung tissue. Moreover, HMGA2 is required for the transformed phenotype in two different, metastatic non-small cell lung cancer cells (H1299 and SK-MES-1). Taken together, our results indicate that HMGA2 plays an important role in lung carcinogenesis. Although additional studies are needed, our studies also suggest that HMGA2 could be a rational therapeutic target in lung and other aggressive cancers characterized by increased HMGA2 expression.
Materials and methods
Cell Culture and Transfection
BEAS-2B, H1299, SK-MES-1, H358, and H82 cells were obtained from the American Type Culture Collection (ATCC) and grown according to ATCC guidelines. Cells were transfected using Lipofectin as described by the manufacturer (GIBCO/BRL). Polyclonal, pooled, resistant cell lines overexpressing HMGA2 or control vector were selected in media containing puromycin (0.75 ug/ml). H1299 cells transfected with the HMGA2 ribozyme antisense or control constructs were selected in media containing Zeocin (75 ug/ml). Two polyclonal, pooled, resistant cell lines generated from two independent transfection experiments, both with decreased HMGA2 proteins by Western analysis, were used for soft agar analysis.
Plasmids
The HMGA2 antisense construct was derived from the vector pU1/RIBOZYME (15) by inserting a synthetic HMGA2 specific dsDNA sequence in the EcoRI/SpeI cloning site (5′-GAATTCTTTAGAGGGACCTGATGAGTCCGTGAGGACGAAACTCTTGTTTTTCTGCCTAG-3′). The cloned DNA fragment incorporates an autocatalytic hammerhead ribozyme sequence (underlined). The parent vector pU1/RIBOZYME was used as a control. The plasmids pSG5-HMG-C (HMGA2) has been previously described (14).
HMGA2 siRNA Knock-Down Experiments
SK-MES-1 cells were transiently transfected with the HMGA2 specific siRNA or siCONTROL non-targeting pooled siRNA ((20 μmol/L, Dharmacon) using Lipofectamine 2000 (Invitrogen) according to manufacturer instructions. HMGA2 and β-actin protein levels were assessed at 2, 3 and 6 days after transfection with the siRNA by Western analysis. mRNA levels were assessed at 1, 2, 3, and 6 days as described under quantitative RT-PCR methods.
Western Analysis
Western blot analysis of HMGA proteins was performed as described (14, 15).
Cellular Growth Rate Determinations
Cellular growth rates were determined as previously described (14, 15), with the following exceptions. Cells were seeded at 5 × 104 into 6 separate 5 cm tissue culture dishes. Duplicate dishes were harvested every 24 hrs for 3 days and the cells were counted.
Soft Agar Assays
The soft agar assay was performed as previously described (14, 15) except that cells were suspended in 3 ml of 0.4% agarose and poured onto a 5 ml 0.7% agarose bed in 100 mm tissue culture dishes. Colonies greater than 0.1 mm were counted after 3-4 weeks.
Quantitative RT-PCR
Quantitative RT-PCR was performed by Taqman chemistry as previously described (19, 20) except that the HMGA2 specific primers 5′-AAAGCAGCTCAAAAGAAAGCA and 5′-TGTTGTGGCCATTTCCTAGGT and the HMGA2 specific probe 5′-CACTGGAGAAAAACGGCCAAG were used. Gene expression indicated as fold-changes compared to control samples was calculated with the Applied Biosystem software using the absolute quantification (standard curve) option and beta-actin as housekeeping internal standard as we previously described (20). Control samples were arbitrarily assigned a value of 1.0.
To assess possible gene amplification, quantitative RT-PCR was performed on DNA from frozen specimens as previously described (41, 42) using SYBR green chemistry. The primers were: 5′-ACCAACCGGTGAGCCCTCTC and 5′-TTGAGCTGCTTTAGAGGGACTCTTG (for HMGA2 exon 2), 5′-AAAGCAGAAGCCACTGGAG and 5′-CATTTCCTAGGTCTGCCTC (for HMGA2 exon 3), and 5′-ACAGCATTCGGGCCGAGATG and 5′-GATAGCCTCCAGGCCAGAAAG for beta-2 microglobulin exon 1 as an internal control. Standard curves from serial dilutions of cDNA were calculated for each run and primer/probe set.
Immunohistochemical analysis
Tissue microarray blocks were created using 1 mm cores taken from 91 archival, routinely-processed, paraffin-embedded, lung cancer specimens retrieved from the surgical pathology archives of the Johns Hopkins Medical Institutions, Baltimore, MD. The specimens had been accessioned between 1997 and 2001 and were selected solely based on availability of tumor tissue in the block. Each case was represented by three cores on the tissue microarray. Additionally, each array contained cores from routinely-processed, paraffin-embedded normal tissues also taken from the archives, including artery, brain, breast, cecum, duodenum, gallbladder, intestine, kidney, liver, lung, lymph node, ovary, muscle, pancreas, placenta, prostate, salivary gland, skin, stomach, testis, thyroid and tonsil. Tissue microarrays were sectioned at 4 μm intervals, deparaffinized in xylene and rehydrated with graded alcohols. The sections were then heated in antigen retrieval solution (Dako, Carpinteria, CA) and were immunohistochemically stained for HMGA2 protein following manufacturer directions (HMGA2, 0.004 mg/ml, Biocheck, Inc.). Positive staining was identified as nuclear immunoreactivity and was scored using a 9 point scale based on the product of staining intensity (weak =1, moderate =2, strong =3) and staining extent (% of positive cells; 5-30% =1, 30-60% =2, >60% =3). The highest score per case was used for subsequent analyses. Tissue microarrays were analyzed microscopically in a blinded fashion by a single investigator (AH).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by grants from the NIH (L.M.S.R., M.M., and L.J.W.), the American Cancer Society (L.M.S.R.), the Leukemia & Lymphoma Society, and Alex's Lemonade Stand Foundation (L.M.S.R.), the Flight Attendant Medical Research Institute (F.d.C. and R.B.), the Gynecologic Cancer Foundation, and the Prevent Cancer Foundation (J.H.). We acknowledge the Lung SPORE for providing the tissue microarrays. We also thank Dr. Joseph Maher for the HMGA2 antibody and Dr. Abeba Tesfaye for the HMGA2 primer design. We apologize to the many authors whose important work could not be cited as a result of space limitations.
REFERENCES
- 1.Giancotti V, Bandiera A, Buratti E, et al. Comparison of multiple forms of the high mobility group I proteins in rodent and human cells. identification of the human high mobility group I-C protein. Eur J Biochem. 1991;198:211–6. doi: 10.1111/j.1432-1033.1991.tb16003.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishwad CS, Shriver MD, Lassige DM, Ferrell RE. The high mobility group I-C gene (HMGI-C): Polymorphism and genetic localization. Hum Genet. 1997;99:103–5. doi: 10.1007/s004390050320. [DOI] [PubMed] [Google Scholar]
- 3.Schoenmakers EF, Wanschura S, Mols R, Bullerdiek J, Van den Berghe H, Van de Ven WJ. Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet. 1995;10:436–44. doi: 10.1038/ng0895-436. [DOI] [PubMed] [Google Scholar]
- 4.Rogalla P, Drechsler K, Frey G, et al. HMGI-C expression patterns in human tissues. implications for the genesis of frequent mesenchymal tumors. Am J Pathol. 1996;149:775–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Hess JL. Chromosomal translocations in benign tumors: The HMGI proteins. Am J Clin Pathol. 1998;109:251–61. doi: 10.1093/ajcp/109.3.251. [DOI] [PubMed] [Google Scholar]
- 6.Lund T, Holtlund J, Fredriksen M, Laland SG. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 152:163–7. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–9. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 8.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci. 2001;26:152–3. doi: 10.1016/s0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KR, Cook SA, Davisson MT. Chromosomal localization of the murine gene and two related sequences encoding high-mobility-group I and Y proteins. Genomics. 1992;12:503–9. doi: 10.1016/0888-7543(92)90441-t. [DOI] [PubMed] [Google Scholar]
- 10.Friedmann M, Holth LT, Zoghbi HY, Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 1993;21:4259–67. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves R. Molecular biology of HMGA proteins: Hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 12.Narita M, Narita M, Krizhanovsky V, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–14. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Lanahan A, Williams JB, Sanders LK, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919–29. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood LJ, Maher JF, Bunton TE, Resar LM. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–61. [PubMed] [Google Scholar]
- 15.Wood LJ, Mukherjee M, Dolde CE, et al. HMG-I/Y, a new c-myc target gene and potential oncogene. Mol Cell Biol. 2000;20:5490–502. doi: 10.1128/mcb.20.15.5490-5502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves R, Edberg DD, Li Y. Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol. 2001;21:575–94. doi: 10.1128/MCB.21.2.575-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolde CE, Mukherjee M, Cho C, Resar LM. HMG-I/Y in human breast cancer cell lines. Breast Cancer Res Treat. 2002;71:181–91. doi: 10.1023/a:1014444114804. [DOI] [PubMed] [Google Scholar]
- 18.Fedele M, Pentimalli F, Baldassarre G, et al. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene. 2005;24:3427–35. doi: 10.1038/sj.onc.1208501. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Sumter TF, Bhattacharya R, et al. The HMG-I oncogene causes highly penetrant, aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res. 2004;64:3371–5. doi: 10.1158/0008-5472.CAN-04-0044. [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye A, Di Cello F, Hillion J, et al. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer Res. 2007;67:3998–4004. doi: 10.1158/0008-5472.CAN-05-1684. [DOI] [PubMed] [Google Scholar]
- 21.Scala S, Portella G, Fedele M, Chiappetta G, Fusco A. Adenovirus-mediated suppression of HMGI(Y) protein synthesis as potential therapy of human malignant neoplasias. Proc Natl Acad Sci U S A. 2000;97:4256–61. doi: 10.1073/pnas.070029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002 Jan 24;415(6870):436–42. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 23.Kubo T, Matsui Y, Goto T, Yukata K, Yasui N. Overexpression of HMGA2-LPP fusion transcripts promotes expression of the alpha 2 type XI collagen gene. Biochem Biophys Res Commun. 2006;340:476–81. doi: 10.1016/j.bbrc.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Fedele M, Berlingieri MT, Scala S, et al. Truncated and chimeric HMGI-C genes induce neoplastic transformation of NIH3T3 murine fibroblasts. Oncogene. 1998;17:413–8. doi: 10.1038/sj.onc.1201952. [DOI] [PubMed] [Google Scholar]
- 25.Battista S, Fidanza V, Fedele M, et al. The expression of a truncated HMGI-C gene induces gigantism associated with lipomatosis. Cancer Res. 1999;59:4793–7. [PubMed] [Google Scholar]
- 26.Arlotta P, Tai AK, Manfioletti G, Clifford C, Jay G, Ono SJ. Transgenic mice expressing a truncated form of the high mobility group I-C protein develop adiposity and an abnormally high prevalence of lipomas. J Biol Chem. 2000;275:14394–400. doi: 10.1074/jbc.m000564200. [DOI] [PubMed] [Google Scholar]
- 27.Fedele M, Battista S, Kenyon L, et al. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21:3190–8. doi: 10.1038/sj.onc.1205428. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi MR, Okada Y, Chada KK. Misexpression of full-length HMGA2 induces benign mesenchymal tumors in mice. Cancer Res. 2006;66:7453–9. doi: 10.1158/0008-5472.CAN-06-0931. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376:771–4. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- 30.Rogalla P, Drechsler K, Kazmierczak B, Rippe V, Bonk U, Bullerdiek J. Expression of HMGI-C, a member of the high mobility group protein family, in a subset of breast cancers: Relationship to histologic grade. Mol Carcinog. 1997;19:153–6. doi: 10.1002/(sici)1098-2744(199707)19:3<153::aid-mc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Sarhadi VK, Wikman H, Salmenkivi K, et al. Increased expression of high mobility group A proteins in lung cancer. J Pathol. 2006;209:206–12. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 32.Meyer B, Loeschke S, Schultze A, et al. HMGA2 overexpression in non-small cell lung cancer. Mol Carcinog. 2007;46:503–11. doi: 10.1002/mc.20235. [DOI] [PubMed] [Google Scholar]
- 33.Didier ES, Rogers LB, Orenstein JM, et al. Characterization of encephalitozoon (septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 34.Read WL, Page NC, Tierney RM, Piccirillo JF, Govindan R. The epidemiology of bronchioloalveolar carcinoma over the past two decades: Analysis of the SEER database. Lung Cancer. 2004;45:137–42. doi: 10.1016/j.lungcan.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 35.American Cancer Society . Cancer facts and figures. American Cancer Society; 2006. [Google Scholar]
- 36.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 37.Gazdar AF. The molecular and cellular basis of human lung cancer. Anticancer Res. 1994;14:261–7. [PubMed] [Google Scholar]
- 38.Fedele M, Visone R, De Martino I, et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–71. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Berlingieri MT, Manfioletti G, Santoro M, et al. Inhibition of HMGI-C protein synthesis suppresses retrovirally induced neoplastic transformation of rat thyroid cells. Mol Cell Biol. 1995;15:1545–3. doi: 10.1128/mcb.15.3.1545. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Pentimalli F, Dentice M, Fedele M, et al. Suppression of HMGA2 protein synthesis could be a tool for the therapy of well differentiated liposarcomas overexpressing HMGA2. Cancer Res. 2003;63:7423–7. [PubMed] [Google Scholar]
- 41.Hostein I, Pelmus M, Aurias A, Pedeutour F, Mathoulin-Pelissier S, Coindre JM. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: A potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol. 2004;202:95–102. doi: 10.1002/path.1495. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann U, Glockner S, Kleeberger W, von Wasielewski HF, Kreipe H. Detection of gene amplification in archival breast cancer specimens by laser-assisted microdissection and quantitative real-time polymerase chain reaction. Am J Pathol. 2000;156:1855–64. doi: 10.1016/S0002-9440(10)65059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.