Abstract
Anticoagulation therapy significantly reduces the incidence of thromboembolic events in patients with atrial fibrillation (AF), and warfarin therapy at discharge is a class I–indicated drug in patients with ischemic stroke with persistent or paroxysmal AF without contraindications. The aim was to determine whether participation in the Get With The Guidelines-Stroke (GWTG-S) quality improvement program would be associated with improved adherence to anticoagulation guidelines for patients with all types of AF. Adherence to warfarin treatment at hospital discharge was assessed in eligible patients with AF who presented with stroke or transient ischemic attack, based on type of AF. Of patients with stroke, 10.5% presented with some form of AF. When AF was documented using electrocardiography or telemetry (ECG) during the present admission, eligible patients were more likely to receive warfarin compared with patients for whom AF was reported using medical history only (78.8% vs 49.4%; p <0.0001). Improvement after GWTG-S participation in warfarin use was observed in patients with ECG-documented AF (73.8% at baseline vs 88.5% after the intervention; p <0.0001), but not patients using history only. Women and elderly patients were less likely to receive warfarin, and these gaps in treatment did not narrow during the quality improvement program for patients with ECG-documented AF and those with history only. In conclusion, anticoagulation for stroke prevention was underused in general for patients with AF, even in such high-risk groups as patients with stroke. GWTG-S was associated with improved adherence for patients with ECG-documented AF, but patients with a history of AF alone were largely untreated.
Quality improvement programs can increase adherence to published guidelines. Get With The Guidelines (GWTG) is a hospital-based voluntary national performance improvement program sponsored by the American Heart Association associated with improvements in guideline adherence over time.1 This study used data obtained from the GWTG-Stroke (GWTG-S) module. Because these patients presented with transient ischemic attack (TIA) or stroke, they were by definition at high risk of recurrent embolic events (CHADS2 score ≥2). We sought to determine whether participation in GWTG-S would be associated with improved adherence to anticoagulation guidelines compared with the preintervention baseline for patients with all types of atrial fibrillation (AF).
Methods
The GWTG-S database was queried for demographic, clinical, and treatment data for consecutive patients presenting with ischemic stroke or TIA (International Classification of Diseases, Ninth Revision codes 433 to 436) and AF. Hospitals were recruited to participate during the course of the study. Mean follow-up in participating hospitals was 5.72 quarters. There were 562 participating hospitals from October 1, 2001, to when the data were analyzed as of December 31, 2005. GWTG-S uses preprinted orders, an Internet-based Patient Management Tool (Outcome Science, Inc., Cambridge, Massachusetts), educational conferences, and quality improvement review processes to improve guideline adherence. Methods for the GWTG-S quality improvement program have been described in detail previously.2 Trained abstractors at each site reviewed the medical records and electrocardiographic and telemetry tracings and reports for evidence of AF. In GWTG-S, specific data elements include “history of AF” and “AF documented using ECG during current admission.” A history of AF was defined as any medical history of AF. In this case, no electrocardiographic documentation of AF was required. AF during the present admission was defined as an electrocardiogram showing AF during the index hospitalization. Prescription of anticoagulation therapy on discharge (e.g., warfarin or heparin bridging to warfarin) was recorded in the Patient Management Tool. Patients were excluded if the clinician determined there was a contraindication to anticoagulation that was documented. Patients were also excluded if they died, left against medical advice, or were discharged to hospice or transferred to another acute-care facility. At the time of this analysis, the performance measure and decision support used in GWTG-S for anticoagulation in patients with AF included only patients with AF documented using electrocardiography and telemetry (ECG) during the present admission and excluded those with AF documented using history only without electrocardiographic documentation. Thus, the 2 study groups identified based on the presence or absence of electrocardiographic and telemetry documentation were AF documented using ECG during the present admission (group 1) and AF documented using medical history only (group 2). Percentages of patients prescribed warfarin recorded for patients in groups 1 and 2 were analyzed separately.
Comparisons and trend analyses of warfarin adherence were made during 12 quarters of each individual hospital participating in the program. Comparisons were made using chi-square test or Wilcoxon's rank-sum test (for ordinal and continuous variables). Trend analysis was performed using the Cochran-Mantel-Haenszel method. A generalized estimating equation method explored disparities in the use of anticoagulation for patients with AF, adjusted for site factors and common risk factors (age, gender, race, body mass index, previous stroke/TIA, coronary artery disease/previous myocardial infarction, carotid stenosis, diabetes, peripheral vascular disease, hypertension, and dyslipidemia). A p value <0.05 was considered significant for all tests. All analyses were performed using SAS software (version 8.2; SAS Institute, Cary, North Carolina). The data analysis for this study was performed by the Duke Clinical Research Institute and funded by the American Heart Association. The American Heart Association had no role in the design, analysis, or interpretation of the data. Because data were deidentified, the Institutional Review Board from Metro-Health Medical Center, Cleveland, Ohio, granted a waiver of full review.
Results
A total of 147,128 patients with stroke were entered into the GWTG-S database from October 1, 2001, to December 31, 2005, at 562 hospitals with an average of 355 beds/institution. Characteristics of these hospitals are listed in Table 1. A total of 1,970 patients had documented contraindications to anticoagulation therapy in the database and were excluded. Contraindications were more frequently documented in group 1 compared with group 2 (1,940 of 9,575 vs 100 of 7,926 patients; p <0.0001). This was expected because the decision support and performance measures focused on patients with AF documented using ECG. In total, 10.5% of all patients had AF without contraindications to anticoagulation therapy. The breakdown of study groups is shown in Figure 1.
Table 1.
Hospital characteristics
| No. of GWTG-S hospitals | 562 |
| No. of hospital beds/hospital | 355 ± 215 |
| Hospital teaching hospital status | 43% |
| No. of strokes/yr (ischemic stroke discharge frequency) | |
| 0–100 | 23% |
| 101–300 | 48% |
| ≥301 | 29% |
| US hospital region | |
| Northeast | 25% |
| Midwest | 21% |
| South | 37% |
| West | 17% |
Figure 1.
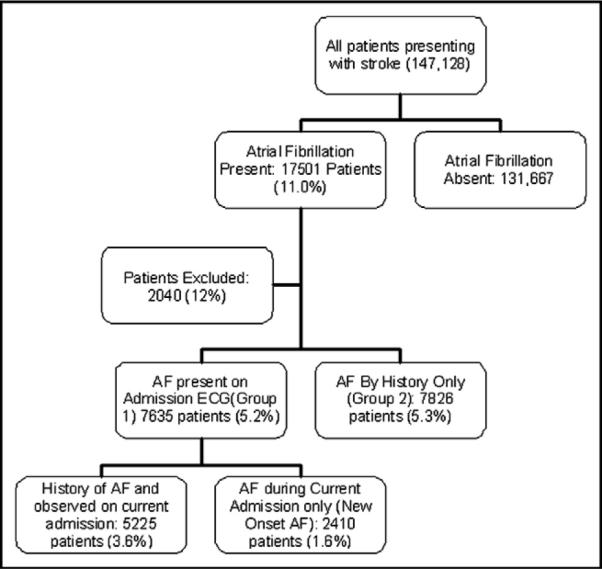
Flow diagram shows the subgroups for analysis in the study and their incidence.
For 7,826 patients (5.3% of the total patient population), the only evidence of AF was in the history (group 2). For 7,635 patients (5.2% of the total patient population), AF was documented electrocardiographically during the present admission (group 1). Of these patients, 5,225 (68%) had a history of AF and 2,410 (32%) had AF newly documented during the hospitalization with no history of AF. Thus, 16% of all patients with AF presented with a stroke and new-onset AF. Demographic characteristics of the 2 study groups are listed in Table 2.
Table 2.
Patient characteristics
| Variable | Group 2 (n = 7,826) | Group 1 (n = 7,635) | p Value |
|---|---|---|---|
| Age (yrs) | 79 ± 10 | 77.5 ± 10 | <0.0001 |
| Men | 3,256 (42%) | 3,279 (43%) | 0.0892 |
| Caucasian | 6,766 (87%) | 6,508 (85%) | 0.0003 |
| Previous stroke/TIA | 2,990 (38%) | 2,475 (32%) | <0.0001 |
| Coronary artery disease/myocardial infarction | 2,791 (36%) | 2,445 (32%) | <0.0001 |
| Diabetes | 2,057 (26%) | 1,923 (25%) | 0.1186 |
| Hypertension | 5,745 (73%) | 5,636 (74%) | 0.5642 |
| Dyslipidemia | 2,392 (31%) | 2,381 (31%) | 0.4037 |
| Tobacco use (current or past year) | 557 (7%) | 582 (8%) | 0.2290 |
| Stroke subtype: ischemic | 5,849 (75%) | 6,208 (81%) | <0.0001 |
Group 1, AF documented using ECG; group 2, AF documented using history only.
Overall, for patients with AF without contraindications, warfarin was prescribed in 9,882 of 15,461 patients (63.9%). In patients with AF documented during the hospitalization (group 1), warfarin was more often administered in comparison to patients with a history of AF who did not have evidence of AF during the index hospitalization (group 2); (78.8% vs 49.4%; p <0.0001; Figure 2). In all patients with AF, warfarin use increased from baseline to the final quarter of hospital participation (61.7% vs 69.4%). However, improvement was confined to patients in group 1 (p trend <0.0001; Figure 2). In contrast, a slight but statistically significant decrease in adherence was observed in group 2 patients (p trend = 0.006; Figure 2). The increase in warfarin use in group 1 was not explained by an increase in documentation of contraindications to warfarin from baseline to the final quarter of hospital participation (18.7% vs 20.3%; p trend = 0.43).
Figure 2.
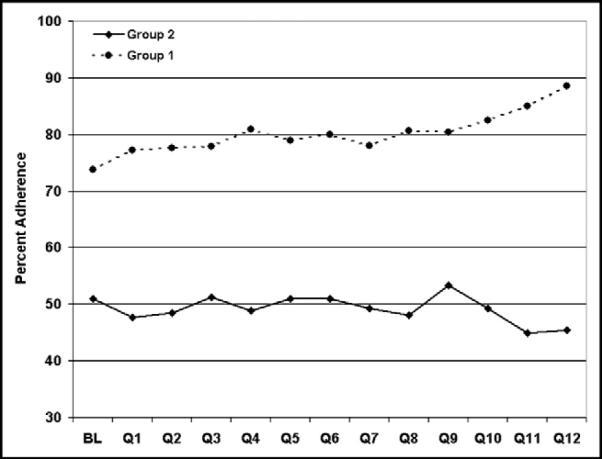
Percentage of eligible patients with AF discharged with warfarin therapy prescribed at baseline (BL) and over 12 quarters (Q) of participation in the GWTG program. Solid line, percentage of adherence in patients with only a history of AF documented (group 2); dashed line, adherence in patients with electrocardiographically documented AF (group 1). Patients in group 1 had a higher adherence rate compared with patients in group 2. Adherence to anticoagulation guidelines improved over time in group 1, but not in group 2.
In univariate analysis, men were more likely to be treated with warfarin compared with women for both group 2 (52.2% vs 47.4%; p <0.0001) and group 1 (81.1% vs 77.1%; p <0.0001). Overall, women were less likely to receive warfarin. However, this difference was small. The adjusted odds ratios of adherence on a quarterly basis in women compared with men for groups 1 and 2 were of borderline statistical significance. In univariate analysis, elderly patients (>65 years) were less likely to be treated with warfarin for AF compared with patients <65 years for both group 2 (59.3% vs 48.3%; p <0.0001) and group 1 (85.2% vs 77.9%; p <0.0001; Figure 3). The adjusted odds ratios suggested that elderly patients were treated less often than younger patients (Figure 4), and this ratio did not improve over time. These disparities in gender and age were present and significant after controlling for the confounding factors of race, body mass index, previous stroke/TIA, coronary artery disease, carotid stenosis, diabetes, peripheral vascular disease, hypertension, and dyslipidemia.
Figure 3.
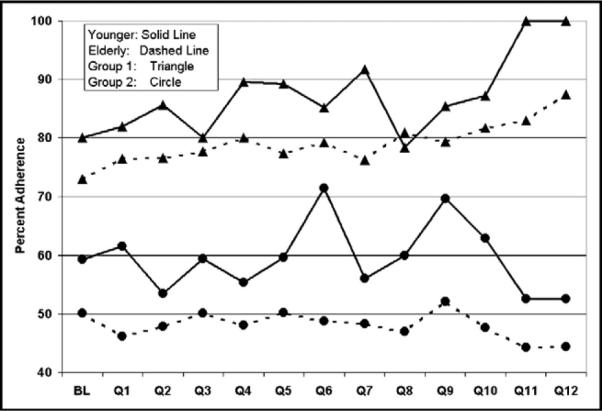
Percentage of younger versus elderly patients with AF documented using ECG (group 1) and history only (group 2) discharged with warfarin therapy prescribed at baseline (BL) and over 12 quarters (Q) of participation in the GWTG program. Younger patients were more likely to be discharged with anticoagulation treatment for both groups.
Figure 4.
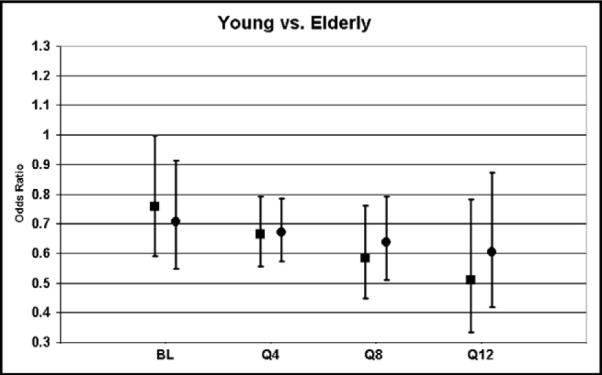
Odds ratios of warfarin therapy in elderly compared with younger patients. Black squares, patients with AF documented using ECG (group 1); black circles, patients with AF documented using history only (group 2). Elderly patients were less likely than younger patients to receive warfarin therapy regardless of how AF was documented. The treatment gap did not become narrower, shown by the decrease in odds ratios over 12 quarters of participation in the program.
Discussion
This was the first study to show that a comprehensive quality improvement program such as GWTG-S was associated with significantly improved adherence to anticoagulation guidelines in patients with stroke and electrocardiographic and telemetry evidence of AF. The observed success of this program may be caused by its comprehensive nature using an Internet-based concurrent Patient Management Tool, preprinted orders, educational conferences, and quality improvement review processes. This study also showed that warfarin was prescribed at hospital discharge with greater frequency in eligible patients with stroke when AF was documented using ECG during the index stroke hospitalization. Furthermore, adherence to this guideline recommendation in eligible stroke patients increased over time in association with GWTG-S. In contrast, adherence was lower and did not improve over time when AF was documented only within the medical history.
The finding that rates of treatment with anticoagulation in eligible patients with stroke differed by type of AF has not been previously reported. There were a number of potential explanations for this finding. Electrocardiographic and telemetry documentation of AF provided physical “proof” that this abnormal rhythm existed. Physicians may be reluctant to prescribe a higher risk drug, such as warfarin, in the absence of such indisputable evidence. At the time, the GWTG-S program performance measure and decision support included only patients with AF using a current electrocardiogram. Because decision support and performance measure feedback are important elements of improving the delivery of evidence-based care, one could argue that the finding of a larger degree of improvement in adherence to warfarin therapy in group 1 (AF documented using ECG) versus group 2 (AF documented using history only) was not surprising. This failure to include patients with medical history of AF without current electrocardiographic and telemetry evidence of AF in the performance measure denominator could have unintentionally contributed to lower conformity with guideline recommendations. However, it was unlikely that this difference accounted for such large disparity in adherence between the 2 groups and the lack of improvement over time in group 2. This suggested a knowledge gap relating to the need for warfarin in this subgroup of patients with medical history of AF without current electrocardiographic and telemetry evidence of AF.
The 2 groups most vulnerable for recurrent stroke in this setting were patients with paroxysmal AF and patients with apparent successful treatment of AF with antiarrhythmic drugs. It was well documented that patients with paroxysmal AF were at nearly as high a risk of stroke as patients with persistent AF. However, it may be that this information was not well known in the community of physicians treating hospitalized patients with stroke.3 Warfarin therapy has been shown to lower this risk.4 Despite these findings, studies have shown that patients with paroxysmal AF were less likely to receive oral anticoagulation.5,6 The diagnosis of paroxysmal AF in patients with stroke depended entirely on the rigor used in looking for electrocardiographic documentation. In a study by Barthélémy et al,7 20% of consecutive patients with stroke had sustained AF. Thirty-five percent of these patients had a diagnosis with AF using only monitoring with ambulatory ECG. The paroxysmal nature of AF explains why, in the present study, a large cohort of patients had AF documented using only history and not using ECG. More frequent monitoring of patients, as well as investigation of the validity of their cardiac history, will likely result in more patients identified with AF requiring anticoagulant therapy.
Additionally, patients with AF treated using a strategy of rhythm control alone were at increased risk of stroke without anticoagulation.8,9 In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Study, 69% of strokes observed in the rhythm control strategy occurred in patients in sinus rhythm at presentation, and most strokes occurred when anticoagulation had been discontinued or was subtherapeutic.9 Restoration of sinus rhythm did not confer protection against emboli. Nevertheless, there is a belief that persists that maintenance of sinus rhythm obviates the need for warfarin. Nieuwlaat et al10 observed that patients with AF treated using a rhythm control strategy were 43% less likely to receive oral anticoagulation.
Our study population consisted of a cohort of patients who presented with stroke or TIA. Thus, treatment with warfarin was not for primary stroke prevention, but secondary stroke prevention, and therefore warfarin was indisputably indicated. Despite this, only half the patients with a history of AF and without current AF (group 2) were prescribed warfarin.
One explanation for this discrepancy was that most patients with stroke were cared for by a neurologist, hospitalist, or internist, who may be less knowledgeable about the need for long-term anticoagulation in patients with a history of AF who are now in sinus rhythm. This is in contrast to patients admitted with a primary diagnosis of AF, usually to a cardiology service, where the focus is on the cardiac arrhythmia. Patients were more likely to receive warfarin when AF was coded as a primary rather than a secondary diagnosis.11 Additionally, patients treated by a cardiologist or in collaboration with a cardiologist for AF were more likely to receive oral anticoagulation compared with patients treated by an internist alone.6,12 Therefore, strategies must be developed to enhance awareness of the value of long-term anticoagulation to reduce the risk of stroke in patients with paroxysmal AF or those with AF suppressed using antiarrhythmic drug therapy. Even within the context of GWTG-S, an effective quality improvement program, no improvement was observed in patients without AF electrocardiographically documented during the index hospitalization. This study also confirmed the previously reported treatment gaps associated with age and gender. Although differences between men and women were statistically different, they were not clinically meaningful.
As our population ages, combined with the increase in frequency of AF with age,13 the burden of AF is likely to increase in the future. It is clear that better data definitions and enhanced provider education are required to improve adherence in this category. Other barriers to enhanced adherence to anticoagulation guidelines in patients with AF include the difficulty administering and monitoring warfarin and the lack of such structured programs as anticoagulation clinics.
There were several limitations to this report. The registry data analyzed in this study were self-reported by hospitals rather than collected by independent medical abstraction. There was no separate verification of complete case ascertainment or data quality or accuracy. This analysis of GWTG-S was not a randomized clinical trial with concurrent control, and improvements in the performance measure may have been influenced by secular trends and concurrent factors other than participation in the study. However, the finding that there was an increase in anticoagulation use in group 1, but not group 2, made this less likely to be caused by secular trends. Contraindications to warfarin therapy were more frequently documented in group 1. This was expected because the decision support was targeted at this group. However, there was no significant increase in documentation of contraindications over time in this group, which thus was unlikely as an important contributor to the increased adherence. There could be instances in which AF was transient and reversible and thus would not require warfarin therapy and were not documented as reasons for nontreatment in the medical record. These reversible causes would represent only a very small number of patients. The observed treatment gap disparity affecting women and the elderly might be a function of site-specific poor adherence rather than broad-based disparity if these populations are unequally distributed across sites. This bias was less likely to be observed in women and elderly patients, for whom the distribution between hospitals was likely to be more uniform than race or ethnicity.
Acknowledgment
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health. Dr. Lewis had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
The data analysis of this study was performed by the Duke Clinical Research Institute, Durham, North Carolina, and funded by the American Heart Association, Dallas Texas. Dr. Super was supported by Grant No. M01 RR000080 from the National Center for Research Resources, National Institutes of Health, Bethesda, Maryland. Dr. Lewis has received speaker honoraria from Reliant Pharmaceuticals. Dr. Fonarow has received research grants from GlaxoSmithKline and honoraria from GlaxoSmith-Kline, Merck, Schering-Plough, and Bristol Myers Squibb and is or has been a consultant for Glaxo-Smith-Kline, Schering-Plough, Merck, and Bristol Myers Squibb. Dr. Cannon has received research grants from Accumetrics, AstraZeneca, Bristol-Myers Squibb, Glaxo Smith Kline, Merck, Sanofi-Aventis, and Schering Plough.
References
- 1.Roe MT. Success stories: how hospitals are improving care. Am Heart J. 2004;148(suppl):S52–S55. doi: 10.1016/j.ahj.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 2.LaBresh KA, Reeves MJ, Frankel MR, Albright D, Schwamm LH. Hospital treatment of patients with ischemic stroke or transient ischemic attack using the “Get With The Guidelines” program. Arch Intern Med. 2008;168:411–417. doi: 10.1001/archinternmed.2007.101. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000;35:183–187. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 4.Atrial Fibrillation, Aspirin, Anticoagulation Study. Boston Area Anticoagulation Trial for Atrial Fibrillation Study. Canadian Atrial Fibrillation Anticoagulation Study. Stroke Prevention in Atrial Fibrillation Study. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Study Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 5.Marcu CB, Ghantous AE, Caracciolo EA, Donohue TJ. Patterns of anticoagulation in patients hospitalized with atrial fibrillation: warfarin is underused in paroxysmal atrial fibrillation. Conn Med. 2003;67:595–598. [PubMed] [Google Scholar]
- 6.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-Study on Atrial Fibrillation (SCAF-Study) Eur Heart J. 2006;27:1954–1964. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 7.Barthélémy JC, Feasson-Gerard S, Garnier P, Gaspoz JM, Da Costa A, Michel D, Roche F. Automatic cardiac event recorders reveal paroxysmal atrial fibrillation after unexplained strokes or transient ischemic attacks. Ann Noninvasive Electrocardiol. 2003;8:194–199. doi: 10.1046/j.1542-474X.2003.08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 9.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, Lopez-Sendon J, Vardas PE, Aliot E, Santini M, Crijns HJ. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–3026. doi: 10.1093/eurheartj/ehl015. [DOI] [PubMed] [Google Scholar]
- 11.Lim MJ, Roychoudhury C, Baker PL, Bossone E, Mehta RH. Differences in quality of care among patients hospitalized with atrial fibrillation as primary or secondary cause for admission. Int J Qual Health Care. 2005;17:255–258. doi: 10.1093/intqhc/mzi026. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry NK, Soumerai SB, Normand SL, Ross-Degnan D, Laupacis A, Anderson GM. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. Am J Med. 2006;119:607–615. doi: 10.1016/j.amjmed.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]


