Abstract
Objective
In patients with systemic sclerosis (SSc), the relationship between innate immune activation, represented by increased expression of interferon (IFN)-regulated genes, and vascular injury/activation, manifest by increased endothelin-1 (ET-1), endothelin converting enzyme-1 (ECE1) and intercellular adhesion molecule-1 (ICAM1) is uncertain. This study investigates the potential roles of innate immune ligands in both of these pathogenic pathways.
Methods
The effect of known toll-like receptor (TLR) ligands was tested in vitro on dermal microvascular and pulmonary arterial endothelial cells, and on dermal fibroblasts cultured from healthy controls and SSc patients. To test the effect of dsRNA on vascular activation/injury in vivo, poly(I:C) was administered continuously over 7 days by subcutaneous osmotic pump.
Results
Double stranded ribonucleic acid (dsRNA)/poly(I:C) but not other TLR ligands, highly stimulated ET-1 protein and mRNA (EDN1), as well as ICAM-1 and IFN-regulated MX2, by endothelial cells and dermal fibroblasts. Poly(I:C) induced EDN1, (ECE1), and ICAM-1 mRNA expression in poly(I:C) treated skin. Poly(I:C) induced EDN1, ECE-1 and MX2 was not blocked in mice deleted of the type I IFN receptor. However, Poly(I:C)-induced EDN1 and ECE1, but not poly(I:C)-induced ICAM-1 expression was blocked in mice deleted of the TLR3 signaling protein TRIF/TICAM1.
Conclusion
Together these data show that the dsRNA can regulate genes associated with vascular activation, as seen in SSc, that type I IFNs do not mediate these effects, and that EDN1 and ECE1 but not ICAM-1 activation is mediated by TLR3.
Keywords: Toll-like receptor 3, systemic sclerosis, endothelial cells, endothelin-1, poly(I:C)
Endothelin-1 (ET-1 peptide, EDN1 mRNA), has been implicated in the pathogenesis of systemic sclerosis (SSc) vasculopathy through multiple obsevations. ET-1 is increased in SSc skin, particularly localizing to endothelial cells and myofibroblasts [1] and ET-1 levels are increased in the serum, blood vessels, lung, kidney and skin of SSc patients [1-5]. Autocrine ET-1 production by fibroblasts increases fibroblast collagen and fibronectin production and conversion to myofibroblasts [6, 7], and can act as an intermediary in TGF-β induced fibrosis by myofibroblasts [8]. In blood vessels ET-1 activates adhesion molecules such as ICAM-1 on endothelial cells, stimulates smooth muscle cell vasoconstriction, recruits leukocytes to affected tissues and increases leukocyte-fibroblast interactions [9]. Endothelial cell ET-1 over-expression in mice leads to vascular dysregulation, remodeling, and increased macrophage infiltration of tissue and markers of vascular activation [10, 11]. ET-1 is regulated by TGF-β, angiotensin II, hypoxia, and IFN-γ thrombin, adrenalin, insulin, oxyhemoglobin, shear stress, hypoxia, oncostatin M, TNF-α, and IL-1β, and can also induce its own expression [12–15]. However, the stimulus for ET-1 in SSc remains obscure.
The relationship between immune deregulation and vascular changes in SSc is uncertain. Recently, our group and others have shown that peripheral blood mononuclear cells (PBMCs) from SSc patients express increased levels of interferon (IFN)-regulated genes [16, 17]. IFN-regulated genes as well as IFNα are also increased in SSc dermis [18, 19]. and IFN-regulated gene expression in the skin correlates with the modified Rodnan skin score [20], The innate immune, toll-like receptors (TLRs) strongly induce IFNs, suggesting that IFNs in SSc are upregulated by endogenous TLR ligands [17, 21-23].
We hypothesized that innate immune receptors might mediate vascular disease characteristic of SSc. In this study we investigated the effect of different innate immune stimuli on vascular endothelial cells and found that dsRNA, the ligand for TLR3, as well as cytoplasmic dsRNA receptors: dsRNA-dependent protein kinase R (PKR), and the RNA helicases, RNA helicase protein retinoic acid-inducible gene I (RIG-I) and melanoma-differentiation-associated gene 5 (MDA5), potently induces ET-1 and ICAM-1, markers of vascular cell activation/injury in SSc. Continuous infusion of this ligand subcutaneously in mice resulted in TRIF/TICAM1-dependent increase in ET-1, but TRIF/TICAM1-independent increase in ICAM-1 expression, indicating that dsRNA regulates these markers of vascular injury through TLR3-dependent (ET-1) and -independent (ICAM-1) pathways.
MATERIAL AND METHODS
Study subjects
All study subjects met the criteria for dcSSc with proximal skin disease as defined previously [24]. The study was conducted under a protocol approved by the Boston University Medical Center, Institutional Review Board and all subjects gave written informed consent. Skin biopsies were performed as previously described [18].
Cell cultures and reagents
Primary human dermal fibroblast explant cultures from patients with diffuse cutaneous SSc (dcSSc) and controls at passages 3–6 were prepared for in vitro studies as previously described [25]. Human dermal microvascular endothelial cells and human pulmonary artery endothelial cells were purchased (Lonza, Basel, Switzerland). Fibroblasts were incubated in the absence of serum for 24h prior to the addition of treatments [25]. Cells were treated with TLR agonists: Pam3CSK4 (TLR2 ligand, 1μg/ml), Poly:I:C: (TLR3 ligand, 2.5μg/ml), LPS (TLR4 ligand, 10μg/ml), imiquimod (TLR7 ligand, 5μg/ml), sspolyU/Lyovec (TLR8 ligand, 100μg/ml), or CpGA (TLR9 ligand, 5mM); rHu-IFNα IFNβ (Biomedical Laboratories, Piscataway, NJ), or IFNγ (R&D System) each 250 U/ml; or TGFβ (R&D System, 5 ng/ml) for 24h in 0.1% FBS.
RNA preparation and real-time polymerase chain reaction (RT-PCR)
Human tissue and fibroblasts were processed and RNAs were purified as described previously [18]. RNA was extracted from murine skin using Trizol reagent (Invitrogen), minced and disrupted using a Polytron homogenizer and processed according to the manufacturer’s protocol. RNA was extracted from endothelial cells using the RNeasy protocol (Qiagen). cDNAs were synthesized from 0.2-μg of total RNA using Superscript II RNase H− reverse transcriptase and random primers (Invitrogen Life Technologies, Rockville, MD). The synthesized cDNAs were used as templates for quantitative real-time PCR (Prism 7300 Sequence Detector, Applied BioSystems). using human MX1, MX2, ICAM1 and EDN1, and mouse EDN1, ECE1 and ICAM-1 TaqMan primers (Applied Biosystems). For human RNAs, expression was normalized to 18S rRNA expression (human 18S TaqMan primer set) and for mouse RNAs expression was normalized to GAPDH expression (mouse GAPDH TaqMan primer set). The change in the relative expression of each gene was calculated using ΔΔCt formula choosing a healthy human subject or wild-type mouse sample as the control [26]. Statistical significance between samples was assessed by t-test.
ET-1 and IP-10/CXCL10 bio-assay
The ET-1 bioassay was performed according to the protocol supplied with the kit from Assay Designs (cat no. 900-020A). ET-1 has identical amino acid sequence in humans and mice and the same kit was used for both in vitro and in vivo experiments. Standards and samples were incubated in supplied pre-coated 96-well plate, washed, incubated with horse radish peroxidase labeled anti-ET-1 antibody and washed again before adding the provided TMB substrate and measuring the absorbance. IP-10/CXCL10 ELISA was conducted according to the supplied protocol (BD Biosciences). Briefly, a 96-well plate was coated with the capture antibody, washed, blocked, standards and samples diluted in PBS/10% FBS incubated on the plate for two hours, and then detection antibody and streptavidin-horseradish peroxidase (SAv-HRP) reagents were added sequentially. Plates were developed using TMB substrate (BD Bioscience) and absorbance measured. The limit of detection for murine and human ET-1 was 0.41pg/ml.
In vivo admistration of Poly(I:C)
C57BI/6 WT and C57BI/6 TICAM−/− mice were obtained from The Jackson Laboratory; C57BI/6 IFNAR−/− mice were provided by Dr. John Sprent [27]. Osmotic pumps designed to deliver Poly:(I:C) (0.5 mg/ml in PBS, 0.1mg total dose in 200 μl released over 7 days, Alzet), Pam3CSK4 (1mg/ml in PBS, total dose 0.2mg in 200μl released over 7 days), or PBS were implanted subcutaneously on the back in 6–10 week old mice. After 7 days mice were sacrificed and skin (~1 cm2) surrounding the pump outlet was homogenized in Trizol (Invitrogen) for preparation of RNA as described above.
Results
Increased expression of EDN1 and MX1 in SSc skin
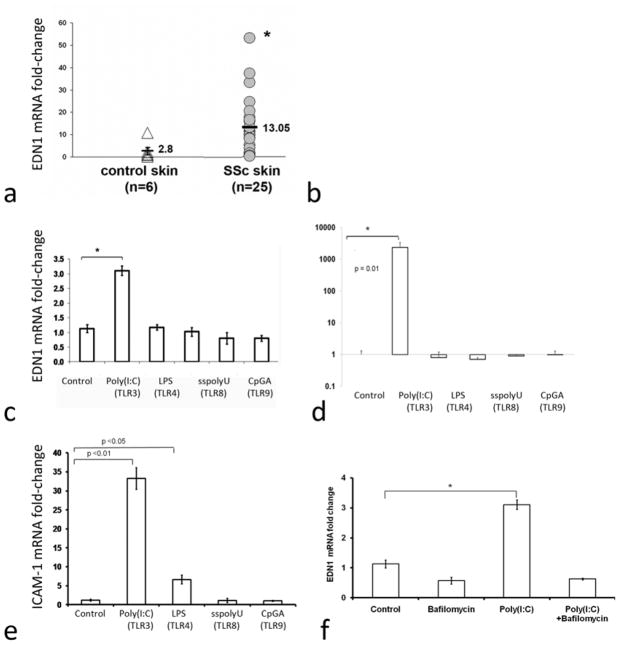
Based on our prior findings of increased inflammatory and pro-fibrotic gene expression in SSc patient skin, we assessed the expression of ET-1 mRNA (EDN1) and the IFN-regulated gene, myxovirus resistance protein-1 (interferon-induced GTP-binding protein MX1, MX1, MxA, IFI78) (Fig. 1, a and b). Average EDN1 expression was 4.98-fold higher and MX1 expression 2.95-fold higher in SSc lesional than healthy control skin (p<0.05 for both).
Figure 1. Regulation of Endothelin 1 and ICAM1 expression in skin from patients with dcSSc and by TLR ligands on human dermal microvascular endothelial cells.
Panels a and b: mRNA expression of EDN1 (n=25) and MX1 (n=36) in lesional skin from dcSSc (SSc) patients and in control skin (n=6). Fold-changes shown on the graph are normalized to mRNA expression by one of the healthy controls. The average fold change of EDN1 and MX1 in SSc skin (13.05 ± SE 2.56 and 2.89 ± SE 0.33, respectively) compared to the average fold-change in control, healthy skin (2.62 ± SE 1.67 and 0.98 ± SE 0.29, respectively) was increased for EDN1 (4.98-fold increase, p<0.05) and MX1 (2.95-fold increase, p<0.05). Panels c-e: Human dermal microvascular endothelial cells (HDMEC) treated with the TLR ligands: poly(I:C), LPS, sspolyU or CpGA in duplicate wells, as described in the methods, were analyzed for EDN1 (panel c), MX2 (panel d) and ICAM-1 (panel e) expression. Expression in each case was normalized to one of the control, untreated wells. Panel f: HDMECs were treated with bafilomycin (30 nM), poly(I:C) or both and analyzed for EDN1 expression.
Human dermal microvascular endothelial cells (HDMEC) show increased expression of
EDN1 mRNA in response to TLR3 ligand, but not other TLR ligands. To evaluate whether TLR ligands stimulate gene expression in endothelial cells similar to gene expression found in SSc skin, we studied the effect of various TLR ligands on mRNA expression of EDN1, MX2 and ICAM1 on primary HDMECs. The TLR3 ligand, poly(I:C), but none of the other TLR ligands tested, significantly induced EDN1 mRNA expression (Fig. 1c, 3-fold increase, p<0.05 and see online supplementary Figure S1, 4.3-fold increase, p<0.05). The increase in EDN1 mRNA was dose-dependent and maximal induction of EDN1 mRNA was seen at 18 hours (see online supplementary Figure S2). Poly(I:C) but not other TLR ligands also strongly upregulated IFN-regulated MX2 expression (Fig.1d, 2,350-fold increase, p<0.01, However, unlike EDN1 and MX1, expression of ICAM-1, a marker of endothelial cell activation, was induced by both the TLR4 ligand LPS as well as poly(I:C) (Fig. 1e). The effect of LPS on ICAM-1 mRNA expression has been previously shown to be regulated by NF-kB, a mediator downstream of both TRIF/TICAM-1 and Myd88 [28]. Since TLR3 activation stimulates TRIF/TICAM-1, but not Myd88, these results were consistent with the notion that TLR4 does not activate TRIF/TICAM in HDMECs, but mediates a modest effect on ICAM-1 expression likely through MyD88.
Supporting the potential importance of TRIF-mediated signaling in SSc, another TRIF-regulated gene, the chemokine CXCL10, was significantly increased in SSc patient serum compared to healthy controls (see online supplementary Figure S3). Since CXCL10, ET-1 and ICAM are all regulated by TRIF/TICAM-1, these data support the potential importance of this pathway in SSc vasculopathy [29-31].
As TLR3 is an endosomal ligand and poly(I:C) can also stimulate cytosolic receptors, we also tested whether EDN1 expression could be blocked with inhibitors of endosomal acidification. Indeed, bafilomycin completely blocked poly(I:C)-induced EDN1 expression consistent with the poly(I:C) effect being meditated by TLR3 (Fig. 1f).
DsRNA induces EDN1 by human pulmonary endothelial cells (HPAEC)
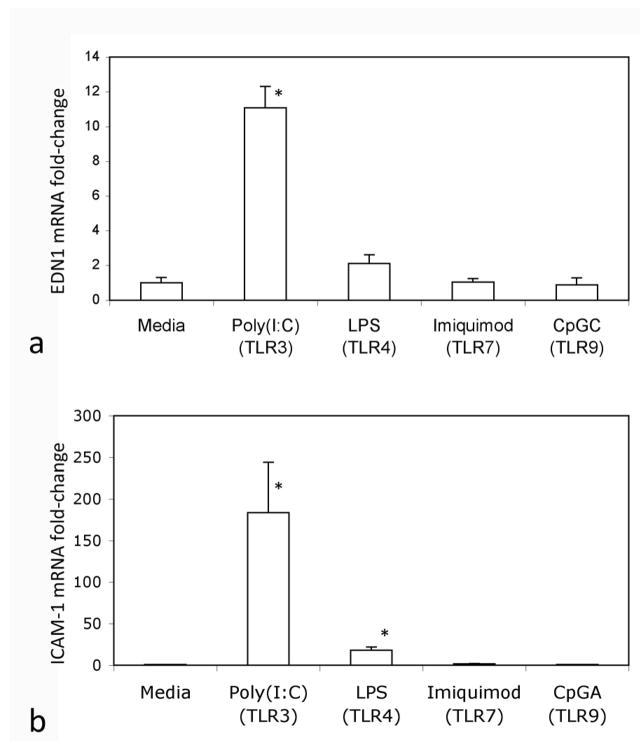
Different vascular beds respond differently to various stimuli and therapeutic agents. We therefore also tested the effect of TLR ligands on human pulmonary endothelial cells (HPAECs). Poly(I:C) stimulated EDN1 expression by (HPAEC), and both poly(I:C) and LPS stimulated ICAM-1 expression by HPAEC (Fig. 2). Likewise, ET-1 bioactive peptide levels were significantly increased by PolyI:C in both HDMECs and HPAECs, although higher levels were induced in HDMECs (see online supplementary Figure S4).Thus, HPAECs responded to TLR ligands similarly to HDMECs although there may be differences in basal level production of ET-1.
Figure 2. Regulation of Endothelin-1 and ICAM1 expression by TLR ligands in human pulmonary artery endothelial cells.
Human pulmonary endothelial cells treated with the TLR ligands: poly(I:C), LPS, and CpGC or CpGA as indicated in duplicate wells as described in the methods were analyzed for EDN1 (panel a) and ICAM-1 (panel b) expression. Results shown are from two independent experiments. Expression in each case was normalized to one of the control, untreated wells, * indicate p<0.05 compared to media control.
DsRNA potently induces EDN1 expression by human dermal fibroblasts
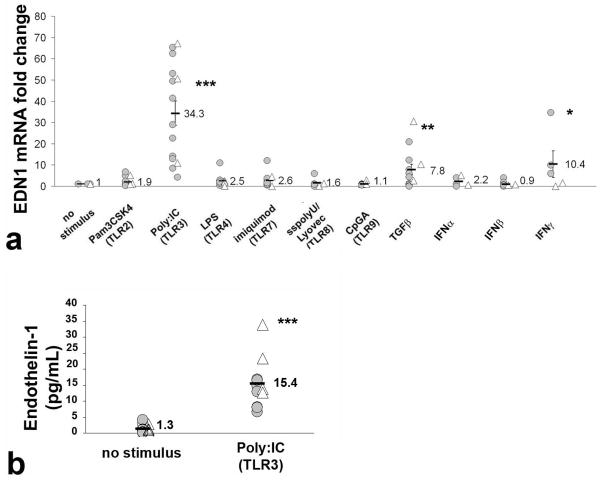
Dermal fibroblasts mediate fibrosis and also express END1 in SSc skin [1]. Thus, we investigated the effects of TLR ligands on EDN1 gene expression by dermal fibroblasts isolated from SSc patients and healthy controls. Poly(I:C) strongly upregulated EDN1 mRNA expression (Fig. 3a, average 34.3-fold increase, p<0.005), but other TLR ligands had no effect on EDN1 expression. Previously described ligands, TGF-β and IFN-γ also stimulated EDN1 (7.8-fold, p<0.01 and 10.4-fold, p<0.05, respectively). To verify that the increase in EDN1 mRNA corresponded to an increase in the bioactive 21-aa ET-1 peptide, we measured levels of ET-1 in supernatants of TLR ligand-treated fibroblasts. ET-1 was significantly increased in the supernatant of poly(I:C) treated dermal fibroblasts (Fig. 3b, average 11.8-fold increase, p<0.005), but not the other TLR ligands tested (not shown). Additionally, poly(I:C) induced ET-1 protein more highly than previously identified inducers of ET-1, namely TGF-β, IFN-γ , and angiotensin II (data not shown). We also tested the effect of TLR ligands on primary explant fibroblast cultures from patients with dcSSc and limited systemic sclerosis (lSSc) and found no significant difference in EDN1 stimulation by poly(I:C) (see online supplementary Figure S5).
Figure 3. Induction of Endothelin-1 by TLR ligands in dermal fibroblasts from patients with dcSSc and healthy controls.
Panel a: Dermal fibroblasts from healthy controls (△) or SSc patients (
 ) were treated with TLR ligands, TGFβ, IFN α, IFN β or IFNγ as described in the methods and END1 mRNA analyzed by qRT-PCR. Fold-change shown is normalized to mRNA expression by the corresponding unstimulated cells. The average fold-change is represented by a horizontal line ± SE. Compared to untreated cells, poly(I:C) (TLR3 ligand) stimulated 34.3-fold increase in EDN1 expression, (p<0.0001). TGFβ, and IFNγ also induced, respectively, 7.8 (p<0.01) and 10.4-fold (p<0.05) increase in EDN1 expression. Panel b: Bioactive 21-aa ET-1 peptide in normal (△) and in SSc (
) were treated with TLR ligands, TGFβ, IFN α, IFN β or IFNγ as described in the methods and END1 mRNA analyzed by qRT-PCR. Fold-change shown is normalized to mRNA expression by the corresponding unstimulated cells. The average fold-change is represented by a horizontal line ± SE. Compared to untreated cells, poly(I:C) (TLR3 ligand) stimulated 34.3-fold increase in EDN1 expression, (p<0.0001). TGFβ, and IFNγ also induced, respectively, 7.8 (p<0.01) and 10.4-fold (p<0.05) increase in EDN1 expression. Panel b: Bioactive 21-aa ET-1 peptide in normal (△) and in SSc (
 ) dermal fibroblasts by Poly(I:C) (p<0.0001) stimulation for 24h treatment. ET-1 protein was measured by ELISA in the supernatants from SSc and normal fibroblast cultures. The average protein concentration for each group is represented as a bar ± SE.
) dermal fibroblasts by Poly(I:C) (p<0.0001) stimulation for 24h treatment. ET-1 protein was measured by ELISA in the supernatants from SSc and normal fibroblast cultures. The average protein concentration for each group is represented as a bar ± SE.
DsRNA induces markers of vascular activation in murine skin
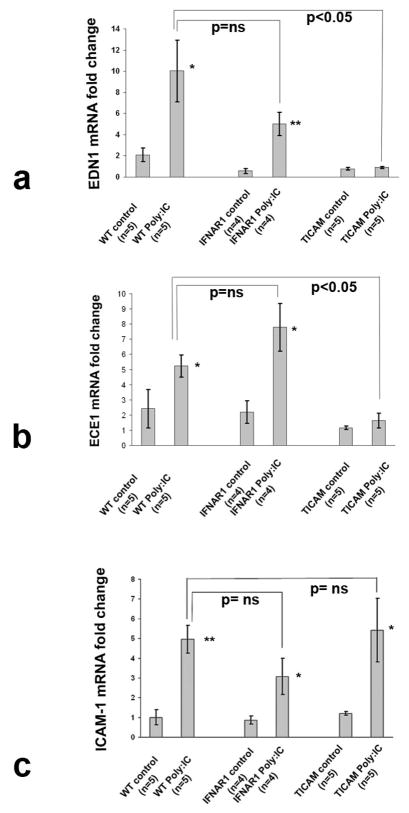
In order to assess the potential importance of TLR3 activation in vivo on the vasculopathy associated with SSc, we tested the effect of poly(I:C) delivered subcutaneously by osmotic pump on expression of EDN1, endothelin converting enzyme (ECE1) and ICAM-1 on mouse skin. We have shown that in this model mice develop inflammation, increased expression of IFN-regulated genes and skin fibrosis similar to that seen in the skin of SSc patients [32]. Mice receiving 7-days of continuous poly(I:C) showed highly increased expression of EDN1, ECE1 and ICAM-1 (Fig. 4, a-c). Increased levels of bioactive ET-1 peptide were also present in the serum of mice treated with poly(I:C) but not with Pam3CSK4 (TLR2 ligand) (see online supplementary Figure S6).
Figure 4. In vivo effect of poly(I:C) on EDN1, ECE1 and ICAM-1 expression.
Expression of EDN1 (a) and ECE1 (b), and ICAM-1 (c) by RT-PCR analysis of skin mRNA from C57BI/6 WT (n=10), C57BI/6/IFNAR−/− (n=8) and C57BI/6 TICAM−/− (n=10) mice one week after subcutaneous insertion of osmotic pumps containing poly(I:C) as described in methods. Fold-change shown in the graphs is normalized to mRNA expression by one of the control mice. Results presented are means ± SE and are representative of four independent experiments; * p<0.05; ** p<0.01.
As IFN-regulated genes are increased in this model and IFNs have been associated with vascular injury and regulate expression of END1 and ICAM-1, we tested the effect of poly(I:C) in mice deleted of the type I IFN receptor (IFNAR1). Deletion of IFNAR1 had no significant effect on expression of END1, ECE1 or ICAM-1 (Fig. 4, a-c).
Several receptors for dsRNA exist in eukaryotic cells including TLR3 and cytosolic receptors. Therefore, we next investigated whether the observed regulation of EDN1, ECE1 and ICAM-1 in poly(I:C)-treated mice was through TLR3 or cytosolic dsRNA receptors, RIG-I, MDA5 and PKR that also recognize poly(I:C) [33–36]. Although TLR3 is expressed on the surface of some human vascular endothelial cells, poly(I:C) can spontaneously enter the cell through an as yet unidentified pathway, potentially allowing poly(I:C) to activate cytoplasmic receptors [37, 38]. Since cytosolic receptors activate IRF3 through the mitochondrial adaptor protein (IPS-1 or MAVS) and not TICAM-1, signaling through these receptors is unaffected in TICAM-1 (−/−) mice [39]. Poly(I:C)-induced EDN1 and ECE1 mRNA levels were abrogated in TICAM-1 (−/−) mice, indicating that EDN1 and ECE1 induction following poly(I:C) stimulation is mediated by TLR3 (Fig. 4a and b). In contrast to the striking effect of TICAM-1 deletion on EDN1 and ECE1 expression, ICAM1 expression was not affected in TICAM-1-deleted mice, suggesting that a TLR3-independent mechanism at least in part regulates its expression in vivo, likely through activation of cytosolic dsRNA receptors (discussed below).
Discussion
We show in these studies that dsRNA and not other innate immune ligands have potent effects on vascular endothelial cells, stimulating expression of markers of vascular activation that are also upregulated in SSc skin: EDN1 and ICAM1. Endothelin is known to play a particularly important role in vascular pathogenesis in that endothelin receptor blockade ameliorates important vascular complications seen in SSc patients, pulmonary arterial hypertension and digital ulcers [40–42]. Our data show that this effect of dsRNA extends to fibroblasts, markedly stimulating both EDN1 mRNA and ET-1 peptide secretion. Further, these studies show that dsRNA stimulates similar altered gene expression in mice and that the effect on EDN1 and ECE1 is blocked by deletion of TICAM-1, but not IFNAR1, strongly implicating the TLR3/TICAM-1 pathway in the pathogenesis of innate immune mediated vascular disease and potentially in SSc vascular disease.
The lack of complete inhibition of ICAM-1 induction by dsRNA in TICAM-1 deficient mice suggests that redundancy exists in dsRNA signaling, likely mediated through other intracellular dsRNA receptors such as RIG-I and MDA5. Prior in vivo studies have shown redundancy of intracellular signaling in poly(I:C) induction of certain cytokines such as IL-6, IFN- β, and IL-12p40, as increased levels of these cytokines was only abrogated in mice deleted of both TICAM-1 and IPS-1 [39]. In intestinal epithelial cells, poly(I:C) induction of ICAM-1 was attenuated by inhibitors of NFκB as well as with a TLR3-blocking antibody [43, 44], suggesting that NFκB might mediate the effect of both TICAM-1 and IPS-1 on poly(I:C)-induced ICAM-1.
Even though the TLRs share similar features, TLR3 is unique in many ways potentially relevant to SSc pathogenesis and autoimmunity. Although the TLR family members signal through four adaptor proteins: MyD88, TIRAP/Mal, TRIF/TICAM-1 and TIRP/TRAM/TICAM-2, TLR3 is the only member to signal exclusively through TICAM-1. TLR4 can activate both MyD88 and TICAM-1, but requires the adaptor molecule TRAM (TRIF-related adaptor molecule) to recruit the TICAM-1 complex, the other members of the TLR family signaling only through MyD88. Both TICAM-1 and MyD88 activate NFκB, but TICAM-1 additionally activates IRF3 and IFN-β leading to increased expression of CXCL10 (IP10) [45, 46]. Vascular endothelial cells lack expression of TRAM and therefore, LPS stimulation of TLR4 does not activate TICAM-1 [47]. However, TLR3 signals through TICAM-1 directly, without utilizing TRAM, and therefore TICAM-1 activation by TLR3 is preserved in endothelial cells. Cells of the immune system typically express TRAM and therefore LPS and other TLR4 agonists can induce MyD88-independent ET-1 production by cells such as conventional dendritic cells [48].
TLR3 expression on human endothelial cells is both intracellular in endosomes, as well as on the cell surface. This has also been shown to be the case in epithelial cells, but not in resting macrophages, CD8+ T-cells, NK cells, and CD11c+ DCs. TLR3 stimulation occurs within intracellular endosomal compartments [38, 49–53]. Thus, our data showing that bafilomycin blocks poly(I:C)-induced EDN1 supports a role for TLR3, as other receptors for dsRNA, such as MDA5, RIG-I, PKR, are cytosolic rather than endosomal and are not affected by bafilomycin or chloroquine. These results also suggest an intriguing possible role for anti-malarials for inhibiting SSc vascular disease.
In this work we show that dsRNA increases EDN1 expression by dermal fibroblasts. Fibroblasts have not previously been shown to be prominent producers of ET-1, but they are strongly implicated in SSc pathogenesis as mediators of fibrosis. TLR3 stimulation provokes a gene expression and cytokine pattern that mimics that found in many SSc patients and thus, TLR3 activation of fibroblasts may provide a link between inflammation, vascular injury/activation and fibrosis seen in SSc.
Speculating as to a possible cause of TLR3 activation in SSc, viral infections are one leading candidate, but TLR3 activation has also been recently identified following tissue injury. TLR3 recognizes endogenous RNA molecules expressed on the surface of dead cells and therefore, TLR3 activation of resident fibroblasts, endothelial cells and potentially other mesenchymal and epithelial cells may play a heretofore unrecognized role as first-responders to tissue injury, infection or necrosis [54].
We hypothesize that signaling from extracellular dsRNA stimulates a different program of gene expression than intracellular dsRNA, with the former stimulating a response to injury or tissue necrosis, and that the uncoupling of TICAM-1 from TLR4 is due to an inherent difference in the function of TLR3 in endothelial, epithelial and mesenchymal cells compared to cells of the immune system. In SSc, increased cell death from endothelial apoptosis, a key feature of early SSc also found in the avian model of SSc, is one potential source for this exposure to dsRNA. SSc is also characterized by autoantibodies directed against known RNA-binding proteins such as fibrillarin and anti-RNA polymerase III, which may contain RNA in the form of circulating immune complexes. Recent work has shown that immune complexes from SSc patients with anti-DNA topoisomerase-I (Scl-70) or anti-RNA polymerase III can stimulate interferonα in PBMC, an effect inhibited by the addition of RNase, bafilomycin, anti-FcgRIII or anti-BCDA2, a receptor on pDCs [21]. Interestingly, pDCs do not express TLR3 and therefore, the TLR involved in IFN-production following incubation with SSc immune complexes is most likely TLR7 and/or 8, which recognize ssRNA. However, as we have shown that neither TLR7 nor 8 are functional in human EC or fibroblasts, TLR3 ligands may play a more important role in SSc vascular pathogenesis. Despite a common pathogenesis, elevated levels of ET-1 have not been widely reported in SLE, rheumatoid arthritis or Sjogren’s syndrome and these conditions are not characterized by the same vasculopathy as SSc [55, 56]. We speculate that several factors such as concomitant elevation in TGF-β, interferons, or TNF-α may determine the phenotype and pattern of tissue injury in these diseases.
Supplementary Material
Acknowledgments
This study was supported by: “Norma Nadeau/Mary Van Neste Research Grant” New England chapter of the Scleroderma Foundation, to Dr. Giuseppina Alessandra Farina, Scleroderma Foundation and American College of Rheumatology/Research and Educational Fund grants to Dr. Michael York, and NIH grants U0IAR055063 and R01AR051089 to Dr. Robert Lafyatis. The authors would also like to acknowledge support from the American Society for Scleroderma Research.
Footnotes
The authors have no competing interests.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence).
(http://group.bmj.com/products/journals/instructions-for-authors/licence-forms)
References
- 1.Tabata H, Yamakage A, Yamazaki S. Cutaneous localization of endothelin-1 in patients with systemic sclerosis: immunoelectron microscopic study. Int J Dermatol. 1997;36:272–275. doi: 10.1046/j.1365-4362.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 2.Abraham DJ, Vancheeswaran R, Dashwood MR, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151:831–841. [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaguchi Y, Suzuki K, Hara M, et al. Increased endothelin-1 production in fibroblasts derived from patients with systemic sclerosis. Ann Rheum Dis. 1994;53:506–510. doi: 10.1136/ard.53.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vancheeswaran R, Azam A, Black C, et al. Localization of endothelin-1 and its binding sites in scleroderma skin. J Rheumatol. 1994;21:1268–1276. [PubMed] [Google Scholar]
- 5.Vancheeswaran R, Magoulas T, Efrat G, et al. Circulating endothelin-1 levels in systemic sclerosis subsets--a marker of fibrosis or vascular dysfunction? J Rheumatol. 1994;21:1838–1844. [PubMed] [Google Scholar]
- 6.Shi-wen X, Chen Y, Denton CP, et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soldano S, Montagna P, Villaggio B, et al. Endothelin and sex hormones modulate the fibronectin synthesis by cultured human skin scleroderma fibroblasts. Ann Rheum Dis. 2009;68:599–602. doi: 10.1136/ard.2008.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi-wen X, Renzoni EA, Kennedy L, et al. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol. 2007;26:625–632. doi: 10.1016/j.matbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of Disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:679–685. doi: 10.1038/ncprheum0346. [DOI] [PubMed] [Google Scholar]
- 10.Amiri F, Paradis P, Reudelhuber TL, et al. Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens. 2008;26:1102–1109. doi: 10.1097/HJH.0b013e3282fc2184. [DOI] [PubMed] [Google Scholar]
- 11.Amiri F, Virdis A, Neves MF, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 12.Lamas S, Michel T, Collins T, et al. Effects of interferon-gamma on nitric oxide synthase activity and endothelin-1 production by vascular endothelial cells. J Clin Invest. 1992;90:879–887. doi: 10.1172/JCI115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 14.Saijonmaa O, Nyman T, Fyhrquist F. Endothelin-1 stimulates its own synthesis in human endothelial cells. Biochem Biophys Res Commun. 1992;188:286–291. doi: 10.1016/0006-291x(92)92382-8. [DOI] [PubMed] [Google Scholar]
- 15.Saijonmaa O, Nyman T, Pacek P, et al. Oncostatin M regulates endothelin-1 production in human endothelial cells. Am J Physiol. 1998;275:H662–H667. doi: 10.1152/ajpheart.1998.275.2.H662. [DOI] [PubMed] [Google Scholar]
- 16.Tan FK, Zhou X, Mayes MD, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology(Oxford) 2006;45:694–702. doi: 10.1093/rheumatology/kei244. [DOI] [PubMed] [Google Scholar]
- 17.York MR, Nagai T, Mangini AJ, et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 18.Farina G, Lemaire R, Pancari P, et al. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis. 2009;68:435–441. doi: 10.1136/ard.2007.086850. [DOI] [PubMed] [Google Scholar]
- 19.Fleming JN, Nash RA, McLeod DO, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoSONE. 2008;3:e1452. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farina G, Lafyatis D, Lemaire R, et al. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 62:580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, Peck A, Santer D, et al. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- 22.Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 23.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 24.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 25.Farina G, Lemaire R, Korn JH, et al. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006;25:213–222. doi: 10.1016/j.matbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Kolumam GA, Thomas S, Thompson LJ, et al. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeuke S, Ulmer AJ, Kusumoto S, et al. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002;56:126–134. doi: 10.1016/s0008-6363(02)00512-6. [DOI] [PubMed] [Google Scholar]
- 29.Cekic C, Casella CR, Eaves CA, et al. Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J Biol Chem. 2009;284:31982–31991. doi: 10.1074/jbc.M109.046383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imaizumi T, Yoshida H, Nishi N, et al. Double-stranded RNA induces galectin-9 in vascular endothelial cells: involvement of TLR3, PI3K, and IRF3 pathway. Glycobiology. 2007;17:12C–15C. doi: 10.1093/glycob/cwm045. [DOI] [PubMed] [Google Scholar]
- 31.Doyle S, Vaidya S, O'Connell R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 32.Farina GA, York MR, Di Marzio M, et al. Poly(I:C) Drives Type I IFN- and TGFbeta-Mediated Inflammation and Dermal Fibrosis Simulating Altered Gene Expression in Systemic Sclerosis. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalali BN, Kollisch G, Mages J, et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- 34.McCartney S, Vermi W, Gilfillan S, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009 doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake T, Kumagai Y, Kato H, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 36.Wornle M, Sauter M, Kastenmuller K, et al. Novel role of toll-like receptor 3, RIG-I and MDA5 in poly (I:C) RNA-induced mesothelial inflammation. Mol Cell Biochem. 2009;322:193–206. doi: 10.1007/s11010-008-9957-4. [DOI] [PubMed] [Google Scholar]
- 37.Kariko K, Bhuyan P, Capodici J, et al. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 38.Kleinman ME, Yamada K, Takeda A, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar H, Koyama S, Ishii KJ, et al. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–687. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 40.Humbert M, Cabane J. Successful treatment of systemic sclerosis digital ulcers and pulmonary arterial hypertension with endothelin receptor antagonist bosentan. Rheumatology (Oxford) 2003;42:191–193. doi: 10.1093/rheumatology/keg050. [DOI] [PubMed] [Google Scholar]
- 41.Korn JH, Mayes M, Matucci Cerinic M, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–3993. doi: 10.1002/art.20676. [DOI] [PubMed] [Google Scholar]
- 42.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 43.Omagari D, Mikami Y, Suguro H, et al. Poly I:C-induced expression of intercellular adhesion molecule-1 in intestinal epithelial cells. Clin Exp Immunol. 2009;156:294–302. doi: 10.1111/j.1365-2249.2009.03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orita T, Kimura K, Zhou H, et al. Poly(I:C)-Induced Adhesion Molecule Expression Mediated by NF-{kappa}B and Phosphoinositide 3-Kinase-Akt Signaling Pathways in Human Corneal Fibroblasts. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4909. [DOI] [PubMed] [Google Scholar]
- 45.Oshiumi H, Matsumoto M, Funami K, et al. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Sato S, Mori K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 47.Harari OA, Alcaide P, Ahl D, et al. Absence of TRAM restricts Toll-like receptor 4 signaling in vascular endothelial cells to the MyD88 pathway. Circ Res. 2006;98:1134–1140. doi: 10.1161/01.RES.0000220105.85182.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spirig R, Potapova I, Shaw-Boden J, et al. TLR2 and TLR4 agonists induce production of the vasoactive peptide endothelin-1 by human dendritic cells. Mol Immunol. 2009;46:3178–3182. doi: 10.1016/j.molimm.2009.05.179. [DOI] [PubMed] [Google Scholar]
- 49.Groskreutz DJ, Monick MM, Powers LS, et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 50.Tissari J, Siren J, Meri S, et al. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174:4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 51.Jorgenson RL, Young SL, Lesmeister MJ, et al. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum Immunol. 2005;66:469–482. doi: 10.1016/j.humimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim DM, Narasimhan S, Michaylira CZ, et al. TLR3-mediated NF-{kappa}B signaling in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1172–1180. doi: 10.1152/ajpgi.00065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueta M, Hamuro J, Kiyono H, et al. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–294. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- 54.Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Julkunen H, Saijonmaa O, Gronhagen-Riska C, et al. Raised plasma concentrations of endothelin-1 in systemic lupus erythematosus. Ann Rheum Dis. 1991;50:526–527. doi: 10.1136/ard.50.7.526-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pache M, Schwarz HA, Kaiser HJ, et al. Elevated plasma endothelin-1 levels and vascular dysregulation in patients with rheumatoid arthritis. Med Sci Monit. 2002;8:CR616–619. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.