Abstract
Neurons have two types of processes: axons and dendrites. Axons have an active disassembly program activated by severing. It has not been tested whether dendrites have an analogous program. We sever Drosophila dendrites in vivo and find that they are cleared within 24 h. Morphologically, this clearance resembles developmental dendrite pruning and, to some extent, axon degeneration. Like axon degeneration, both injury-induced dendrite degeneration and pruning can be delayed by expression of Wld(s) or UBP2. We therefore hypothesized that they use common machinery. Surprisingly, comparison of dendrite pruning and degeneration in the same cell demonstrated that none of the specific machinery used to prune dendrites is required for injury-induced dendrite degeneration. In addition, we show that the rapid program of dendrite degeneration does not require mitochondria. Thus, dendrites do have a rapid program of degeneration, as do axons, but this program does not require the machinery used during developmental pruning.
Introduction
It is now well-established that axons have an active program of degeneration that disassembles them after they are severed from the cell body (Raff et al., 2002; Ehlers, 2004; Luo and O'Leary, 2005; Saxena and Caroni, 2007). This program, which is known as Wallerian degeneration, facilitates clearance of badly damaged axons after injury, perhaps in preparation for regeneration (Vargas and Barres, 2007). The fact that distal regions of axons can be stabilized after severing by overexpression of proteins, including Wld(s), led to the idea that endogenous machinery actively disassembles the axon, somewhat analogous to the way that caspases disassemble apoptotic cells (Raff et al., 2002; Ehlers, 2004; Luo and O'Leary, 2005; Saxena and Caroni, 2007).
It is known that dendrites can also be damaged, but it has not been clearly established whether they have a program of degeneration in the same way that axons do. Dendrite beading has been described during excitotoxicity and after ischemia (Oliva et al., 2002; Greenwood et al., 2007; Zeng et al., 2007; Li and Murphy, 2008; Murphy et al., 2008). However, this beading may not be related to that seen after axon severing because it is reversible in at least some cases (Oliva et al., 2002; Greenwood et al., 2007; Zeng et al., 2007; Li and Murphy, 2008; Murphy et al., 2008).
Both axons and dendrites have programs of developmental disassembly known as pruning. Many axons generate extra projections that are removed during development (Luo and O'Leary, 2005; Saxena and Caroni, 2007). Large-scale remodeling of axons and dendrites also takes place during metamorphosis in insects, including Drosophila melanogaster (Luo and O'Leary, 2005; Williams and Truman, 2005). Morphologically, axon and dendrite pruning appear similar to injury-induced axon degeneration. Moreover, axon degeneration is characterized by involvement of the ubiquitin-proteasome system (UPS) (Zhai et al., 2003), and axon and dendrite pruning require the UPS and can be inhibited by expression of the ubiquitin protease UBP2 (Watts et al., 2003; Kuo et al., 2005). Thus, most researchers treat pruning of axons and dendrites and injury-induced axon degeneration interchangeably. Indeed, pruning is often used as a model for studying injury-induced axon degeneration (Raff et al., 2002; Zhai et al., 2003; Ehlers, 2004; Kuo et al., 2005; Luo and O'Leary, 2005; Saxena and Caroni, 2007; Schoenmann et al., 2010). Only one study has suggested that injury-induced degeneration and pruning use different pathways. In this study, the Wld(s) protein was shown to block injury-induced degeneration but not developmental pruning in mammals or Drosophila (Hoopfer et al., 2006). Even in this work, the authors surmise that the two “may converge on a common execution pathway” (Hoopfer et al., 2006). Moreover, the generality of the conclusion that Wld(s) distinguishes developmental pruning and injury-induced degeneration has been challenged recently by the finding that Wld(s) does in fact block developmental pruning of dendrites in Drosophila (Schoenmann et al., 2010). Thus, the simplest hypothesis, and prevailing view in the field, is that there exists a common disassembly pathway that can remove axons after injury and axons and dendrites during developmental pruning.
In this study, we test whether dendrites also have an injury-induced degeneration program that could feed into this common disassembly pathway. We show that dendrites do have a rapid program of degeneration but that it uses machinery different from dendrite pruning.
Materials and Methods
Systems to study dendrite degeneration and pruning.
To study the injury-induced degeneration, we expressed fluorescent proteins in dorsal dendritic arborization neuron E (ddaE) and dorsal dendritic arborization neuron C (ddaC) neurons in Drosophila larvae. ddaE neurons are visualized through EB1–green fluorescent protein (GFP) and mCD8–GFP driven by 221–Gal4. mCD8–GFP driven by pickpocket (ppk)–Gal4 was used to monitor ddaC neurons. Responses to severing were studied in ddaE and ddaC, and pruning was studied in the ddaC neuron.
Dendrite severing was performed by aiming a pulsed UV laser (Photonic Instruments) on a region of the dendrite fairly close to the cell body. Images were acquired right after severing to ensure that dendrites were completely cut and 18 h after severing to examine successful removal of the transected part. All experiments were performed on an LSM510 confocal microscope (Carl Zeiss). In time course experiments, the same larva was mounted on slides every 3 h. Between imaging, larvae were returned to normal Drosophila media as described (Stone et al., 2010).
In the mitochondrial involvement assay, mitochondria were labeled with mito-GFP (Pilling et al., 2006) driven by elav (embryonic lethal, abnormal vision, Drosophila)–Gal4, whereas membranes were labeled by mCD8–red fluorescent protein (RFP) (Ye et al., 2007). To image the ddaC neuron during pupal stages, the pupal cases need to be removed as described (Williams et al., 2006) before mounting on slides. NIH ImageJ software was used to analyze and assemble images (http://rsb.info.nih.gov/ij/). Overviews of neurons are maximum projection Z stacks from confocal images.
Drosophila stocks and interference RNA.
The tester lines for RNA interference (RNAi) experiments were as follows: (1) upstream activator sequence (UAS)–dicer2; 221–Gal4, UAS–EB1–GFP, UAS–dicer2; (2) UAS–dicer2; ppk–Gal4, mCD8–GFP/TM6, and (3) UAS–mCD8–mRFP, UAS–dicer2/CyO; elav–Gal4, UAS–mito-GFP/TM6. dicer2 was included in all RNAi experiments to increase the effectiveness of neuronal RNAi (Dietzl et al., 2007). Tester lines for overexpression studies were similar but did not have dicer2.
RNAi or overexpression experiments were performed by crossing the tester lines to the following transgenic fly strains: UAS–dmiro–RNAi [Vienna Drosophila RNAi Centre (VDRC) identification number 106683], UAS–Dronc–RNAi (VDRC identification number 23035), UAS–Katanin–p60L1–RNAi (VDRC identification number 31598), UAS–IK2–RNAi (VDRC identification number 103748), pUAST–Wld(s) (MacDonald et al., 2006), UAS–UBP2 (DiAntonio et al., 2001), UAS–p35 (Bloomington Drosophila Stock Center, Bloomington, IN), and UAS–DIAP1 (Bloomington Drosophila Stock Center). Crosses to UAS–rtnl2–RNAi (VDRC identification number 33318) and yw flies were used as controls.
Results
Dendrites undergo beading and clearance within 24 h after severing
We used Drosophila larval multidendritic neurons as a model system. These neurons lie under the cuticle and are responsible for mechanosensation and nociception (Hughes and Thomas, 2007; Song et al., 2007; Zhong et al., 2010). They are multipolar neurons with several dendrites and a single axon that emerge from the cell body, and they have been used very successfully to study dendrite pruning, development, and branching (Gao et al., 1999; Grueber et al., 2002; Gao and Bogert, 2003; Sugimura et al., 2003; Grueber and Jan, 2004; Kuo et al., 2005, 2006; Williams et al., 2006; Kirilly et al., 2009; Schoenmann et al., 2010).
To test whether dendrites initiate a rapid degeneration program after severing from the cell body, we used a pulsed UV laser to sever dendrites of two different types of multidendritic cells in Drosophila larvae. We chose to use the class I cell ddaE because we have previously studied axon regeneration in this cell (Stone et al., 2010), and we used the class IV ddaC cell because it has been used as a model system in which to study dendrite pruning (Kuo et al., 2005, 2006; Williams et al., 2006; Kirilly et al., 2009; Schoenmann et al., 2010). The class refers to the complexity of dendrite branching pattern: class I multidendritic neurons have the simplest dendritic arbors, and class IV have the most complex (Grueber et al., 2002). A pulsed UV laser can very effectively sever axons or dendrites of these cells (Sugimura et al., 2003; Stone et al., 2010). We labeled class I and class IV neurons by expressing a GFP-tagged membrane marker, mCD8–GFP, or the microtubule and soluble marker EB1–GFP selectively in these cells with class I- and class IV-specific Gal4 drivers.
In both cell types, all dendrite fragments distal to the cut site were cleared within 24 h (Fig. 1). To determine the time course of clearance, we analyzed morphology of ddaC (Fig. 1) and ddaE (supplemental Fig. S1, available at www.jneurosci.org as supplemental material) at multiple time points after dendrite severing. The degeneration process of both cells appeared similar.
Figure 1.
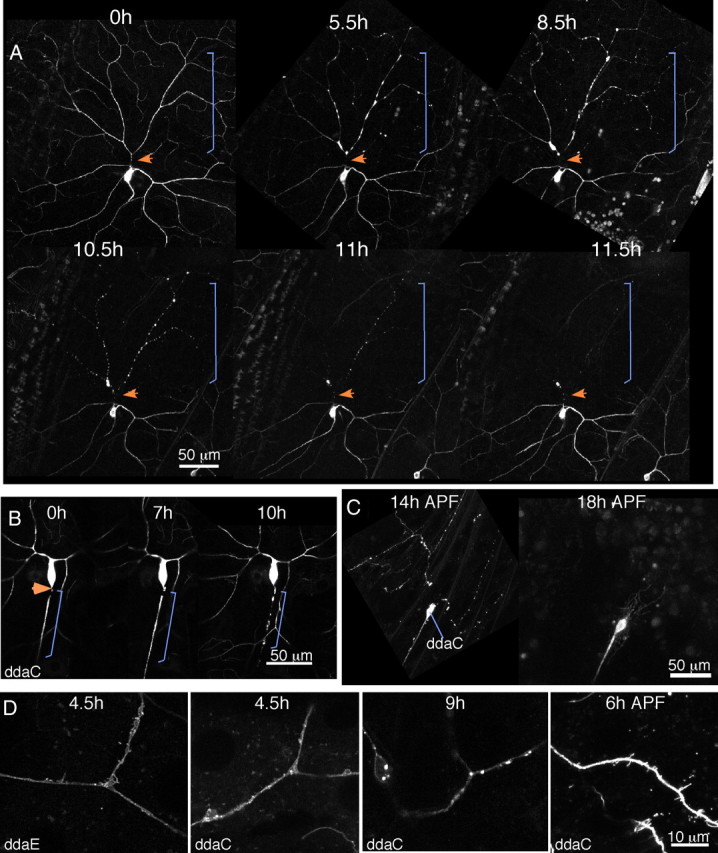
Dendrites degenerate rapidly after severing. A, The multidendritic neuron ddaC was labeled with mCD8–GFP. The binary Gal4–UAS system was used to express the fluorescent protein in a subset of neurons. Whole larvae were mounted on a microscope slide, and a dendrite was severed with a pulsed UV laser. The cell was imaged after severing and at additional later time points. Between imaging sessions, the larva was recovered to food. The orange arrow indicates the site of severing, and the bracket indicates the region of the dendrite separated from the cell body by the cut. B, The ddaC cell was labeled with mCD8–GFP, but the axon was severed rather than the dendrite. As in A, the larva was remounted at different times after severing to track degeneration. Arrow and brackets are as in A. C, White prepupae expressing mCD8–GFP in ddaC under control of ppk–Gal4 were collected and aged until the indicated times APF. At 14 h, dendrites were disconnected from the cell body and fragmented. In the same animal, dendrites were completely cleared at 18 h APF. D, Close-up images of ddaE or ddaC neurons expressing mCD8–GFP at different times after dendrite severing or after pupariation. Supplemental Movies 1–5 (available at www.jneurosci.org as supplemental material) illustrate different aspects of dendrite degeneration. Supplemental Movie 6 (available at www.jneurosci.org as supplemental material) shows a late stage in axon degeneration.
Until 3–4 h after severing, the dendrite remained continuous, and microtubules labeled with EB1–GFP continued to grow in the severed part of the dendrite (supplemental Movie 1, available at www.jneurosci.org as supplemental material). Between 3 and 6 h, dendrite morphology began to change, and at 6 h after injury, dendrite branches often had swollen regions and a beaded appearance. At 9 or 12 h, the region previously occupied by the degenerating dendrite was completely clear (Fig. 1) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). The same general events were observed with both EB1–GFP and mCD8–GFP.
In addition to the swelling and beading, we also observed extensive membrane protrusion before the dendrite fragmented. At approximately 4–5 h after severing, blebs were seen to emerge from the dendrite shaft with both the mCD8–GFP marker (Fig. 1D) (supplemental Movie 2, available at www.jneurosci.org as supplemental material) and EB1–GFP (supplemental Movies 3, 4, available at www.jneurosci.org as supplemental material). At later time points, bright internalized membranes were observed (Fig. 1D) (supplemental Movie 5, available at www.jneurosci.org as supplemental material). Because the membrane protrusion seen at 4–5 h has not been described during axon degeneration (Kerschensteiner et al., 2005) but looks similar to events during dendrite pruning (Kirilly et al., 2009), we wanted to directly compare axon degeneration, dendrite degeneration, and dendrite pruning in the same cell. Membrane protrusion, swelling, and beading were all observed during dendrite pruning of ddaC (Fig. 1C,D). In ddaC axons, we observed swelling, beading (Fig. 1B), and internalized membranes similar to those seen at late stages of dendrite degeneration (supplemental Movie 6, available at www.jneurosci.org as supplemental material), but we did not observe membrane protrusion. The time course of degeneration in the ddaC axon was slightly slower than in the dendrites. Axons could remain intact until 6–7 h (Fig. 1B), but were almost always fragmented by 12 h after severing. Clearance of the severed pieces was more variable than that of dendrites. Most often, all fragments were cleared by 24 h after severing, but in some cases, they could persist until 48–72 h (data not shown).
We conclude that some morphological features are shared between axon and dendrite degeneration and that dendrite degeneration and dendrite pruning are morphologically indistinguishable at this level of resolution. Both axon degeneration and dendrite pruning are known to be active processes; we therefore hypothesized that dendrite degeneration after injury is an active process similar to Wallerian degeneration. Because dendrite degeneration appears extremely similar to dendrite pruning, we also hypothesized that they may use the same machinery.
Dendrite degeneration is blocked by overexpression of Wld(s) or UBP2
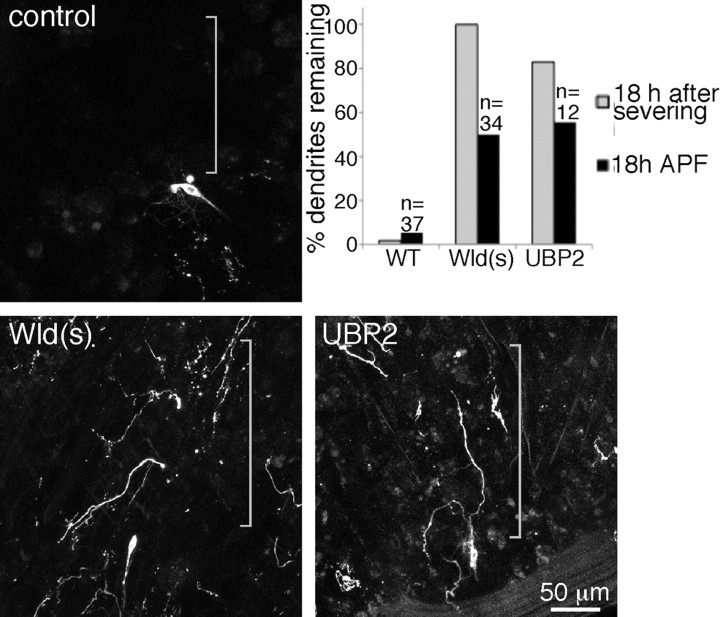
As a first test of whether injury-induced dendrite degeneration might use a mechanism similar to Wallerian degeneration, we tested whether it could be blocked by expression of the Wld(s) protein. Expression of Wld(s) in mammalian or Drosophila neurons delays axon degeneration by days or even weeks after axon severing (Luo and O'Leary, 2005; Hoopfer et al., 2006; MacDonald et al., 2006). In control ddaC cells, ∼98% of animals completely cleared all debris from severed dendrites by 18 h after injury (Fig. 2A,D). Two different genotypes were used as controls: one was a cross of our tester line (UAS–dicer2; ppk–Gal4, UAS–mCD8–GFP) to yw flies, and one was a cross of the same tester line to UAS–rtnl2 hairpin RNA flies. yw was used as a control strain for crosses because it does not contain any transgenes but is a background in which many transgenics are made. RNAi to rtnl2 was used because we have not observed any phenotypes in cells that express this hairpin RNA. Results from both control genotypes were very similar. The cross to yw resulted in 2 of 111 animals with some dendrite remnants present at 18 h after severing, and the cross to rtnl2 RNAi resulted in 0 of 25 animals with any traces of dendrites left 18 h after severing. In all figures, rtnl2 RNAi images are shown, and the numbers in the tables as controls are crosses to yw.
Figure 2.
Expression of Wld(s) or UBP2 blocks dendrite degeneration. Whole larvae expressing mCD8–GFP under control of the class IV da neuron driver ppk–Gal4 were mounted and subjected to dendrite severing with a pulsed UV laser. Animals were then recovered to food and remounted for imaging at later time points. In A, larvae expressed a hairpin RNA directed against rtnl2 (a control); in B, larvae also expressed UAS-controlled Wld(s) (MacDonald et al., 2006), and in C, they expressed UAS-controlled UBP2. For quantitation (D), wild-type (WT) larvae expressing only mCD8–GFP were assayed for the presence of any dendrite traces at 18 h after severing. Similarly, larvae expressing mCD8–GFP and Wld(s) or UBP2 in the ddaC cell were assayed for dendrite traces 18 h after severing. Both transgenes were extremely effective at blocking dendrite degeneration.
In ddaC neurons that expressed the Wld(s) protein, severed dendrites were present for days after injury (Fig. 2B). For quantitation of the effect, we assayed animals 18 h after severing, as in control animals, and found that all animals still had dendrites present 18 h after severing (Fig. 2D). We conclude that, as for axon degeneration, the Wld(s) protein can dramatically delay dendrite degeneration.
To further test whether dendrites have a degeneration program similar to that in axons, we overexpressed the ubiquitin protease UBP2. This manipulation has been shown previously to block both axon and dendrite pruning (Watts et al., 2003; Kuo et al., 2005). Expression of UBP2 also resulted in dendrites that remained for many days after severing (Fig. 2C). In this case, 83% of animals still had distal dendrites 18 h after severing. Thus, dendrites likely have an active program of degeneration that involves the UPS.
Dendrite degeneration is independent of mitochondria
Because the UPS is required for both dendrite pruning and axon degeneration (Watts et al., 2003; Zhai et al., 2003; Kuo et al., 2005) and Wld(s) can also block both, we hypothesized that all three processes may use the same disassembly machinery. Dendrite pruning has been shown to involve caspases (Kuo et al., 2006; Williams et al., 2006), and trophic factor withdrawal also induces a form of axon degeneration that is caspase dependent (Schoenmann et al., 2010), although it is not clear that injury-induced axon degeneration uses caspases (Finn et al., 2000).
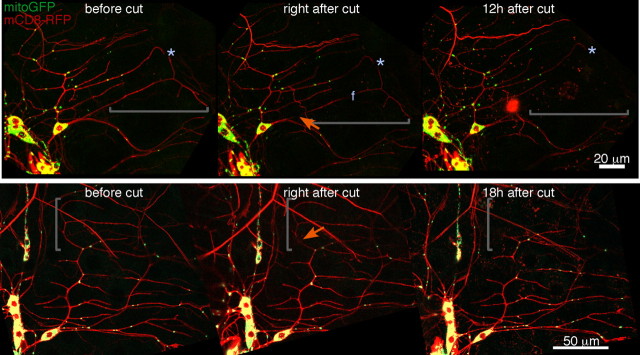
To test whether dendrite degeneration might use a mitochondria-dependent pathway similar to apoptosis, we wanted to determine whether dendrites completely devoid of mitochondria would degenerate with a similar timing to normal dendrites. Mitochondria are typically distributed throughout axons and dendrites, so we needed to reduce overall levels of mitochondria in dendrites to be able to find a dendrite without any mitochondria. The miro protein is required for both anterograde and retrograde mitochondrial transport (Russo et al., 2009), so we reasoned that it might also be required for transport of mitochondria into dendrites. Indeed, when we targeted the miro transcript with a hairpin RNA in the ddaE cell, levels of mitochondria were reduced by ∼50% in the dorsal comb-like dendrite (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
In neurons expressing mito-GFP, which brightly labels mitochondria, and mCD8–RFP to label membranes as well as the hairpin RNA to target miro, we identified dendrites that lacked mitochondria in distal regions. These were then severed and followed over time. Dendrites lacking mitochondria were completely gone by 12–18 h as usual (Fig. 3). We performed this experiment in 20 ddaE neurons. In each case, we identified a region of the neuron completely devoid of mitochondria and severed it from the cell body as shown in Figure 3. In all instances, the distal dendrite was completely cleared by 18 h after severing.
Figure 3.
Mitochondria are not required locally for dendrite degeneration. The number of mitochondria in dendrites was reduced by expression of a hairpin RNA targeting the miro transcript. A pan-neuronal Gal4 driver (elav–Gal4) was used to express this hairpin, mito-GFP, and mCD8–RFP in neurons. Because ddaE cells are relatively separate from other da neurons and also have shorter dendrites, we could identify dendrites that completely lacked mitochondria in this cell. Two examples (of 20) are shown. Severed regions of dendrites that lack mitochondria are indicated by brackets; cut sites are indicated by orange arrows. In both cases, the severed regions of the dendrite were cleared within the normal time frame. The “f” in the top middle panel indicates a region of the dendrite that is in a different focal plane.
We conclude that dendrite degeneration does not require local mitochondria. This suggests that the program of dendrite degeneration may not use apoptotic components, although these are known to play a role in other forms of pruning and degeneration.
Dendrite degeneration is independent of apoptosis machinery
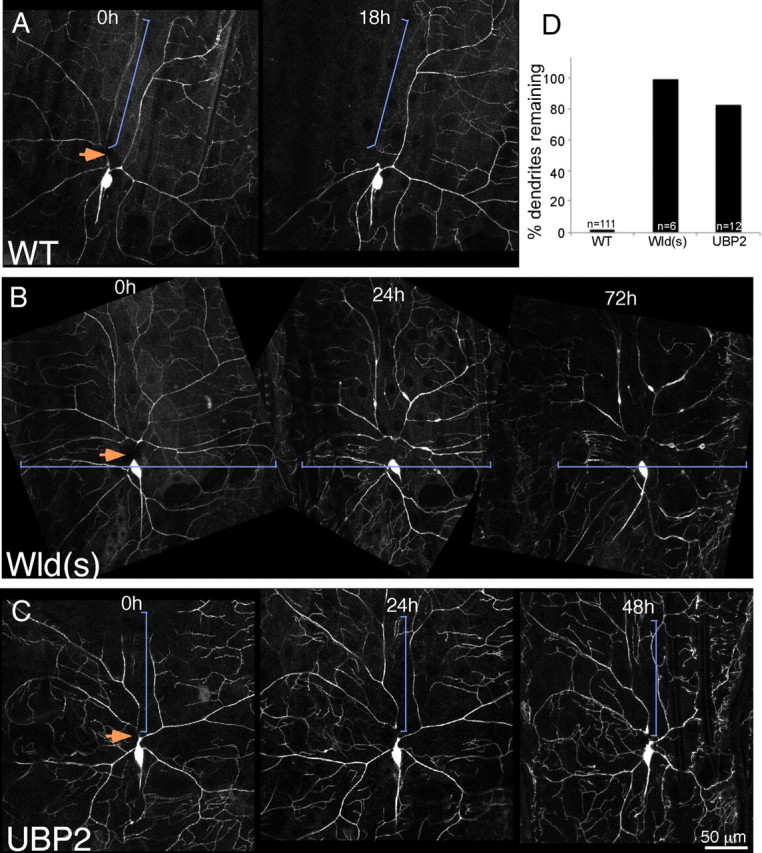
It has not been directly tested whether dendrite pruning is dependent on mitochondria, but dendrite pruning is known to depend on caspases (Kuo et al., 2006; Williams et al., 2006; Schoenmann et al., 2010). We therefore also wanted to determine whether injury-induced dendrite degeneration was caspase dependent. To ensure that the manipulations we performed to alter the apoptotic pathway were effective, we established that we could assay dendrite pruning in the ddaC cell as shown in other studies. We were able to observe complete clearance of ddaC dendrites by 18 h after puparium formation (APF) (Figs. 1C, 4). We could also see that clearance of dendrites was delayed by expression of either Wld(s) or UBP2 in ∼50% of animals (Fig. 4). Expression of either Wld(s) or UBP2 blocked degeneration of ddaC dendrites more effectively than either blocked pruning in the same cell (Fig. 4), so we were reassured that we would be able to determine whether other machinery was shared between degeneration and pruning using this type of comparison.
Figure 4.
Pruning of ddaC is delayed by Wld(s) and UBP2. Larvae expressing mCD8–GFP in ddaC were aged until 18 h APF and then mounted for imaging. One ddaC cell per animal was scored for the presence of dendrite remnants; any dendrite fragments were scored as a positive. Dendrite fragments were frequently observed in larvae expressing UBP2 or Wld(s) but not control larvae. Quantitation is shown as black bars in the graph. Gray bars are the data from Figure 2 for comparison of the effectiveness of Wld(s) and UBP2 in pruning and degeneration.
We used three previously tested manipulations to block caspase activity in ddaC cells and then compared their effects on dendrite degeneration and pruning. Overexpression of the effector caspase inhibitor p35, the Drosophila inhibitor of apoptosis DIAP1, or RNAi targeting the initiator caspase dronc, all very effectively delayed clearance of dendrites during pruning (Fig. 5). However, in the same cell, none of these manipulations had any effect on degeneration of dendrites after severing (Fig. 5). We conclude that apoptotic machinery is not involved in the program of dendrite degeneration.
Figure 5.
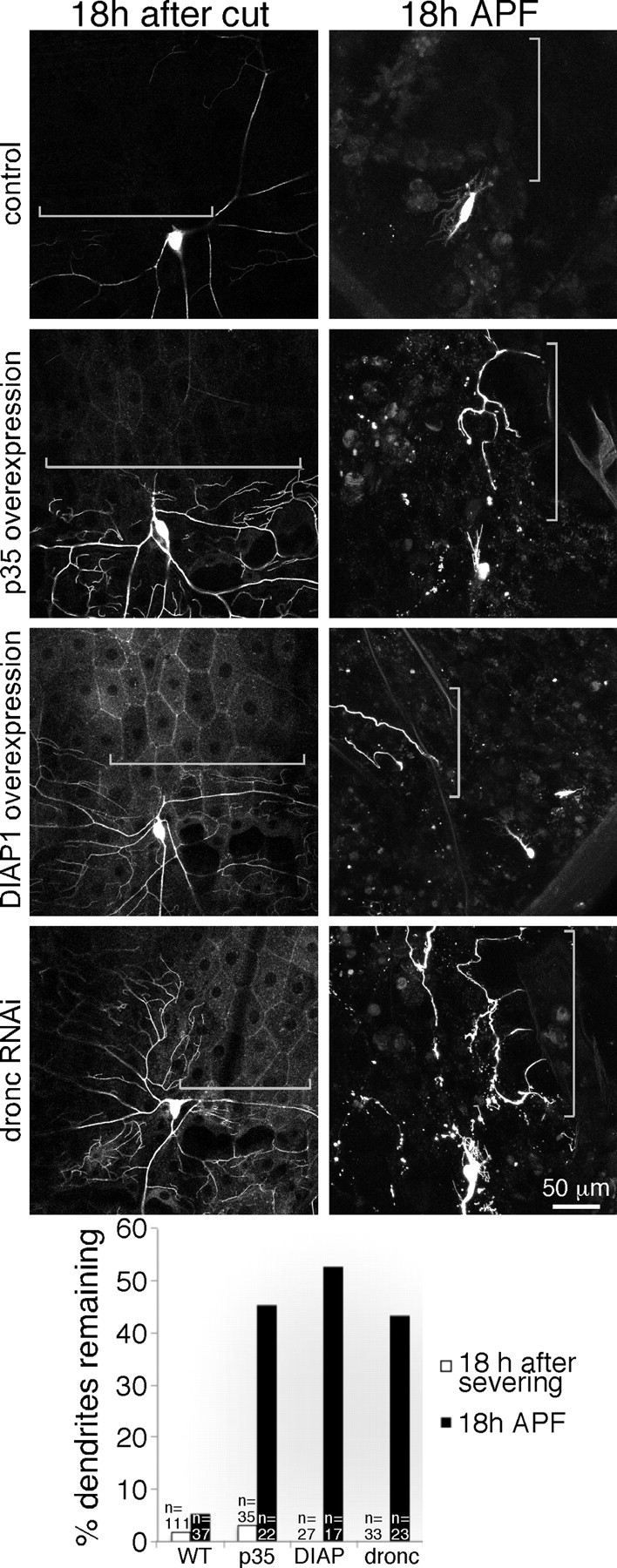
Apoptosis machinery is required for dendrite pruning but not dendrite degeneration. Animals of the same genotypes were assayed for both dendrite degeneration after severing and dendrite pruning during metamorphosis. Control genotypes are as in Figure 2. In addition to mCD8–GFP, experimental animals expressed Gal4-controlled p35, DIAP1, or a hairpin RNA to target the dronc transcript. For severing experiments, larvae were mounted, and a dendrite from ddaC was severed with a pulsed UV laser. After 18 h recovery on food, the presence of dendrite remnants was scored. For pruning experiments, animals were mounted for imaging 18 h APF, and presence of ddaC dendrite remnants was scored. WT, Wild type.
The pruning machinery is not required for injury-induced dendrite degeneration
Several additional proteins that contribute to removal of dendrites during pruning have been identified. The putative microtubule severing protein Katanin–p60L1 (Kat–60L1) is required for an early step in pruning; the disconnection of dendrites from the cell body (Lee et al., 2009). The kinase IK2 seems to play a role in the same pathway (Lee et al., 2009), and large multidomain protein Mical seems to function at a similar step in dendrite pruning (Kirilly et al., 2009). These pruning studies were performed in the ddaC cell, so we confirmed that in our hands RNAi to target each of these players would delay ddaC pruning. In all cases, we found a strong effect (Fig. 6). When we severed dendrites in the same cells during larval life, dendrite degeneration proceeded as usual (Fig. 6). Thus, although both dendrite pruning and dendrite degeneration can be blocked by expression of Wld(s) or UBP2, the specific machinery used to disassemble dendrites in these two cases seems to be entirely different. We conclude that dendrites have a rapid program of degeneration and uses a different set of machinery from dendrite pruning.
Figure 6.
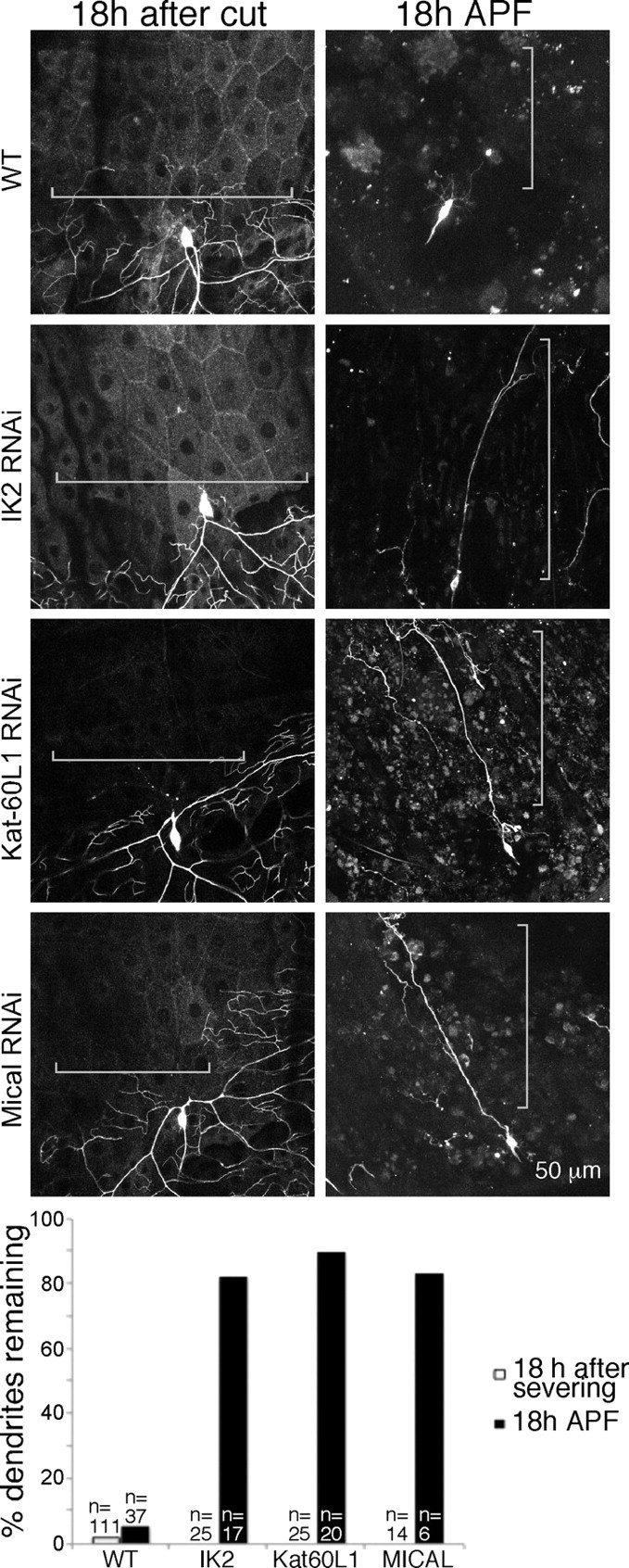
Specific players required for dendrite pruning are not required for dendrite degeneration. The same strategy shown in Figure 5 was used to test the role of IK2, Kat–60L1, and Mical in dendrite degeneration, again compared with pruning in cells of the same genotype. RNAi to reduce each of these proteins was performed by expressing UAS-controlled hairpin RNAs. The presence of dendrite remnants in ddaC was scored 18 h after severing or 18 h APF.
Discussion
Although it is well established that axons have an active program of degeneration, it has not been directly tested whether dendrites have a similar program. We show that, after severing, dendrites are rapidly cleared and that they undergo morphological changes similar to those seen during dendrite pruning. Moreover, like dendrite pruning and axon degeneration, dendrite degeneration can be blocked by overexpression of Wld(s) or UBP2.
Because axon degeneration, dendrite pruning, and dendrite degeneration involve similar morphological changes and can all be blocked by overexpression of Wld(s) or UBP2, it seems logical that they might all involve the same disassembly machinery. Indeed, it has been suggested previously that pruning might be a good model system to use to understand injury-induced degeneration (Raff et al., 2002; Zhai et al., 2003; Ehlers, 2004; Kuo et al., 2005; Luo and O'Leary, 2005; Saxena and Caroni, 2007; Schoenmann et al., 2010). We have rigorously tested this idea by comparing dendrite pruning and injury-induced dendrite degeneration in the same cell. We confirm that pruning uses caspases, IK2, Kat–60L1, and Mical, but none of these proteins is required for injury-induced dendrite degeneration. Mitochondria are also dispensable for injury-induced dendrite degeneration. Mitochondria have been implicated previously in the protective effect of Wld(s) or nmnat protection of axons from degeneration (Yahata et al., 2009). It is therefore possible that the protective effect of Wld(s) does not arise from blocking the intrinsic degeneration machinery but rather by activating a different pathway.
We propose that at least two pathways exist to disassemble axons and dendrites. One pathway is caspase dependent and is activated during pruning. Recent studies have also shown that caspases are involved in axon degeneration after trophic-factor withdrawal (Schoenmann et al., 2010), so pruning and loss of trophic support could activate the same disassembly machinery. In contrast, none of the specific machinery used for dendrite pruning is used for injury-induced dendrite degeneration. Thus, an entirely different, and as yet unidentified, set of machinery must be used in this process. Injury-induced axon degeneration may share this machinery because it also seems to be a caspase-independent process (Finn et al., 2000), but testing this idea will first require identifying specific proteins required for either axon or dendrite degeneration after injury.
All disassembly pathways can be blocked by Wld(s) or UBP2. It is still unclear how Wld(s) protects axons or dendrites, but this may involve changes in nicotinamide-adenine dinucleotide (Coleman and Freeman, 2010). These changes must have a very general blocking effect because they inhibit both dendrite pruning and dendrite degeneration, which seem to be separate pathways. The UPS also seems to play a role in both pathways because UBP2 can inhibit both pruning and degeneration. The UPS could be a common effector of both pathways, or different specific substrates could be targeted by the UPS in each pathway.
IK2, Kat–60L1, and Mical seem to be involved in a specific early step in pruning in which the dendrite is clipped from the cell body (Kirilly et al., 2009; Lee et al., 2009). Typically, dendrites are first disconnected from the cell body at their base (Fig. 5) and then the distal region fragments. When caspases are inhibited, this early step can still occur (Fig. 5), but when IK2, Kat–60L1, or Mical is targeted, dendrites typically remain connected to the cell body (Fig. 6) (Kirilly et al., 2009; Lee et al., 2009). It is unclear whether machinery is needed to fulfill this early disconnection function after dendrite injury, because the injury itself may replace this step. However, some other machinery must substitute for the later caspase-dependent steps of fragmentation.
In the current study, we show that at least two disassembly pathways exist in the same compartment of a single cell and that one is used to remove dendrites developmentally and one is used to remove them after injury. Additional pathways may exist in the axon of this cell or in dendrites and axons of different cells. Until now, it has been assumed that all active disassembly of axons or dendrites would use ubiquitous machinery. Because this does not seem to be the case, it will be extremely important to identify the specific machinery activated in each type of disassembly.
Footnotes
This work was supported by National Institutes of Health Grant R21 NS066216. M.M.R. is a Pew Scholar in the Biomedical Sciences. We are very grateful to Wes Grueber, Bing Ye, Marc Freeman, the Bloomington Drosophila Stock Center, and the Vienna Drosophila RNAi Center (VDRC) for Drosophila lines.
References
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Deconstructing the axon: Wallerian degeneration and the ubiquitin-proteasome system. Trends Neurosci. 2004;27:3–6. doi: 10.1016/j.tins.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Bogert BA. Genetic control of dendritic morphogenesis in Drosophila. Trends Neurosci. 2003;26:262–268. doi: 10.1016/S0166-2236(03)00078-X. [DOI] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 2007;282:26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan YN. Dendritic development: lessons from Drosophila and related branches. Curr Opin Neurobiol. 2004;14:74–82. doi: 10.1016/j.conb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Gu Y, Huang Y, Wu Z, Bashirullah A, Low BC, Kolodkin AL, Wang H, Yu F. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat Neurosci. 2009;12:1497–1505. doi: 10.1038/nn.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci U S A. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A. 2009;106:6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28:11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jr, Lam TT, Swann JW. Distally directed dendrotoxicity induced by kainic acid in hippocampal interneurons of green fluorescent protein-expressing transgenic mice. J Neurosci. 2002;22:8052–8062. doi: 10.1523/JNEUROSCI.22-18-08052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci U S A. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 2010;21:767–777. doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Yamamoto M, Niwa R, Satoh D, Goto S, Taniguchi M, Hayashi S, Uemura T. Distinct developmental modes and lesion-induced reactions of dendrites of two classes of Drosophila sensory neurons. J Neurosci. 2003;23:3752–3760. doi: 10.1523/JNEUROSCI.23-09-03752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Williams DW, Truman JW. Remodeling dendrites during insect metamorphosis. J Neurobiol. 2005;64:24–33. doi: 10.1002/neu.20151. [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J Neurosci. 2007;27:11604–11613. doi: 10.1523/JNEUROSCI.0983-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]