Abstract
Biotechnological approaches to practical production of biological protein-based adhesives have had limited success over the last several decades. Broader efforts to produce recombinant adhesive proteins may have been limited by early disappointments. More recent synthetic polymer approaches have successfully replicated some aspects of natural underwater adhesives. For example, synthetic polymers, inspired by mussels, containing the catecholic functional group of 3,4-L-dihydroxyphenylalanine adhere strongly to wet metal oxide surfaces. Synthetic complex coacervates inspired by the Sandcastle worm are water-borne adhesives that can be delivered underwater without dispersing. Synthetic approaches offer several advantages, including versatile chemistries and scalable production. In the future, more sophisticated mimetic adhesives may combine synthetic copolymers with recombinant or agriculture-derived proteins to better replicate the structural and functional organization of natural adhesives.
INTRODUCTION
In the nineteenth century old horses went to the glue factory, as did virtually every other agricultural by-product—cow hides, sheep bones, pigs feet, fish scales, on and on—to be repurposed as water-borne adhesives. The natural functions of the solubilized and denatured biopolymers that comprised the early industrial glues were not, in general, adhesive bonding. Rather, they were adhesive by virtue of being ionizable polyelectrolytes with little higher order structure. Nonetheless, nature is largely held together with highly adapted biopolymeric glues whose natural function is precisely adhesive bonding. Interest in characterizing these natural adhesives is motivated by the prospect of advancing industrial adhesive technology. Since biological glues function for the most part in wet environments, the biomimetic allure is to improve adhesive deliverability and performance in the presence of water. The grandest challenge is to develop effective adhesives for repair of wet living tissues. Twentieth century mechanical fixation with stitches and staples remains very much the norm in medicine.
BIOLOGY LESSONS
Aquatic organisms, freshwater and marine, have evolved a multitude of workable solutions for adhesive bonding of dissimilar materials underwater. Examples include the permanent attachments of sessile mussels and barnacles, temporary attachment of starfish podia during locomotion, and the construction of protective shelters by Sandcastle worms and freshwater insect larva. The bioadhesives were adapted by natural selection for specific roles in the organism’s lifestyle. Nevertheless, in the context of biomimetic engineering, the natural glues should not be considered even optimal biological solutions for underwater adhesion, let alone optimal materials engineering solutions. After all, organisms live under numerous constraints and adaptation is a multivariate optimization process. There would be no selective pressure to improve one function, like adhesive tenacity, to well beyond limitations imposed by factors like availability and capacity to utilize resources. Organisms have limited pre-adapted building blocks to work with and face severe material processing restrictions imposed by narrow physiological conditions and the exigencies of regulated secretion. An example of potential appropriation of pre-adapted components into glue is the hypothesis that the curing mechanism of barnacle cement may be evolutionarily related to the biochemistry of blood clotting (Dickinson et al. 2009).
Underwater bioadhesives are not particularly strong. In controlled laboratory tests, byssal thread and plaque assemblies created by mussels (Mytilus edulis) on glass or Al substrates had tensile bond strengths of 0.2–0.3 MPa (Burkett et al. 2009). Bond strengths of barnacle and Sandcastle worm (Phragmatopoma californica) glues were also in the range of 0.2–0.3 MPa (Conlan et al. 2008; Sun et al. 2007). Though these comparative estimates must be interpreted cautiously because of differences in test methods, the natural bond strengths are a fraction of the 20 MPa bond strengths of contemporary dental adhesives (Van Meerbeek et al. 2001). Clearly the underwater bond strengths of bioadhesives are not miraculous. Mimetic adhesives for human technology must achieve higher underwater bond strengths than the natural adhesives to find broad utility.
Bond strength may not be an alluring attribute by itself but what more subtle features of wet bonding might be borrowed from nature? For one, aquatic organisms use multiple interfacial adhesion mechanisms to broaden the types of wet surfaces to which they can adhere. To do so, most aquatic organisms are not satisfied with the 20 genetic amino acids and instead make extensive use of post-translational modifications (PTMs). As a case in point, the mussel proteins at the interface of the plaque and substrate (Zhao et al. 2006), mfp-3 and mfp-5, contain more that 20 mol% hydroxylated tyrosines (3,4-L-dihydroxyphenylalanine (dopa)), as well as 4-hydroxylated arginines (Papov et al. 1995) and phosphorylated serines (Waite and Qin 2001). Dopa and phosphates are likely adhesion promoters. Likewise, the Sandcastle worm glue contains 2–3 mol% dopa and ~30 mol% phosphoserine (Waite et al. 1992; Zhao et al. 2005; Stewart et al. 2004). The underwater adhesive silks of caddisfly (Stewart and Wang 2010) and midge larva (Kao and Case 1985), as well as the sea cucumber Cuvierian tubule adhesive (Flammang et al. 2009), are also extensively phosphorylated on serines. Barnacles are an exception and make due with unmodified amino acids (Kamino 2010) although the compositions of the interfacial proteins, cp-19k and cp-20k, are heavily skewed toward polar residues. Notably, cp-20k contains 17 mol% cysteine, 20 mol% acidic (aspartate and glutamate), and 10 mol% basic (histidine) residues; cp-19k contains 30 mol% hydroxyl (serine and threonine), and 8.5 mol% basic (lysine) residues.
By diversifying the functional groups presented at the interface of glue and substrate, aquatic organisms exploit multiple adhesive interactions which may help them deal with the native surfaces they encounter without elaborate preparation. Submerged surfaces from pristine mountain streams to the depths of the ocean are universally coated with bacterial biofilms (Geesey et al. 1977; Costerton 2007; Thomas and Hermans 1985). The surfaces must be scrubbed clean before bonding or the adhesive adapted to work with the biofilms. When initiating a new byssal thread attachment “the tip of the mussel foot squirm[s] this way and that over the surface prior to thread formation as if it were sensing or clearing away debri” (Waite 1992). Likewise, Sandcastle worms (Stevens et al. 2007) and caddisfly larva thoroughly fondle particles before gluing them onto their tubular composite shells. These pre-bonding exercises may partially or substantially remove surface biofilms. Alternatively, since bacterial biofilms existed long before metazoans came along, it makes biological sense that aquatic organisms might exploit the universal surface films (Zardus et al. 2008). Starfish seem to have taken this latter approach; they walk right over the top of microbial biofilms leaving behind sticky footprints (Thomas and Hermans 1985). In short, aquatic animals may rarely experience the molecularly clean surfaces used in adhesive research in the lab and adhesive diagrams in the literature. And no animal would rely on a single adhesion promoter.
By necessity aquatic organisms formulate their underwater adhesives as water solutions or water suspensions with important consequences. First, the water displaced at the interface of the substrate by polar sidechains readily exchanges into the water carrier. Consider the alternative: non-polar adhesives or adhesives carried in non-polar solvents can physically push aside the bulk water but the two or three layers of ordered water at the interface (Florsheimer et al. 2008), with no where to go, are trapped between adhesive and substrate, which substantially decreases initial bond strength and leads to eventual joint failure as additional water infiltrates the interface. Second, the volume of the water carrier has to be minimized because the water must be either expelled or stowed on board as the glue cures into a solid. Highly diffusible water content is inconsistent with strong glue. Third, the water-borne adhesive has to be delivered underwater without dispersing into the drink before it sets. Mussels solve this problem by creating a space protected from the surrounding seawater using the tip of their foot like a “rubber plunger” (Waite 1987). The tip forms a seal then retracts slightly to create a low-pressure space on the surface into which adhesive precursors are injected. The adhesive sets before exposure to seawater. Barnacles also inject cement into a confined space between the calcareous base plate and substrate (Khandeparker and Anil 2006).
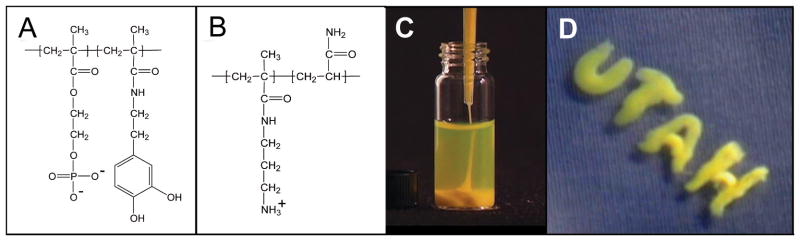
Studies of the Sandcastle Worm suggested a fundamentally different strategy to deliver a water-borne glue underwater—complex coacervation of the adhesive proteins (Stewart et al. 2004; Zhao et al. 2005). Under the right conditions, oppositely charged polyelectrolytes associate electrostatically and separate into a dense water-immiscible fluid called a complex coacervate (Bungenberg de Jong 1949). The water-immiscibility of the complex coacervate ensures that the adhesive stays where it is placed on the surface without dissolving into the ocean (fig. 2D). The interfacial tension between the separated phases is extremely small, on the order of 0.1 mN m−1 (Spruijt et al. 2010), which allows the phase-separated coacervate to readily spread on wet hydrophilic surfaces. The charged sidechains displace surface bound water which readily mixes into the watery bulk of the complex coacervate.
Figure 2.
Adhesive complex coacervates inspired by the Sandcastle worm. A.) Synthetic poly(phosphodopa) analog of the highly phosphorylated Pc3 protein. B.) Synthetic polyamine analog of the polybasic Pc1 protein. C.) Pipettable complex coacervate of the mixed synthetic copolymer analogs. D.) Fully submerged application of the adhesive complex coacervate. The water immiscibility and surface wetting properties make complex coacervates ideal vehicles for deliver of underwater adhesives.
PRODUCTION PROSPECTS
The focus turns to production of commercially viable analogs of natural adhesives. The first efforts to exploit natural underwater glues in dentistry and medicine were undertaken with gathered native adhesives. Rumors that native barnacle cement was used in dental procedures in the 1960s are difficult to verify. A report from the early 1980s of tests using native barnacle cement for rabbit bone repair (Papatheofanis and Ray 1982)included the following description of the process, “The barnacles were scraped from the bottom of a marina pier…the cement was collected with a microcapillary tube. Over a seven-day period, only microdrops of the cement were produced.” This is sufficient explanation for why native barnacle cement is not used in dentistry to this day. In a similar vein, native mussel foot proteins (mfps) were tested in rabbit cornea transplants (Robin et al. 1988) and for soft tissue repair (Papatheofanis and Ray 1982; Ninan et al. 2007). Like barnacle cement, native mfps are not used in eye surgery or any other medical procedure, though native mfps isolated from cultured mussels are still commercially available in mg quantities and are priced like life-saving medicine (Cell-Tak, BDBiosciences).
When segments of the mfp-1 gene was cloned into expression vectors in the late 1980s expectations were that an inexpensive supply of mussel adhesive could be produced by heterologous expression in bacteria or yeast (Waite 1987). Several patents were issued for the microbial production of recombinant mfps (Maugh et al. 1990; Maugh et al. 1993; Silverman and Roberto 2006a, b). One of the several complications of this approach was that the extensive PTMs of native mfps are not executed in heterologous hosts. As a workaround, tyrosines were hydroxylated after purification with mushroom tyrosinase (mfp-1). Up to 40% of the tyrosines were converted to dopa although additional purification steps were then required to remove the mushroom tyrosinase (Maugh et al. 1993). The interfacial primers, mfp-3 and mfp-5, were also cloned and expressed in E. coli (Hwang et al. 2005; Hwang et al. 2004). In addition to lack of PTMs, other problems encountered included toxicity to the expression host, low expression levels, and low solubility of the purified proteins. Expression and solubility were improved by fusing six decapeptide repeats from mfp-1, the cuticle protein, to both ends of mfp-5 (Hwang et al. 2007a). Tyrosines were hydroxylated with mushroom tyrosinase post-purification to achieve a final dopa concentration of ~8 mol%, compared to ~27 mol% for native mfp-5. The fusion protein, referred to as fp-151, adhered to gold and supported attachment of cultured cells. Cell adhesion molecule binding RGD sequences were added to the fp-151 fusion protein to enhance attachment of cultured cells (Hwang et al. 2007b). Expression of fp-151 was sufficient to allow bond strength measurements on polished aluminum substrates (Cha et al. 2008). Shear strengths of air cured bonds were ~330 and ~450 kPa before and after treatment with mushroom tyrosinase and ~1 MPa when oxidative crosslinking through dopa was initiated with NaIO4. The recombinant fusion proteins, marketed in mg quantities (Kollodis, Inc), are less expensive than native mfps (Cell-Tak).
Barnacles evolved versatile, general purpose underwater adhesives with bond strengths as high or higher than other natural adhesives using only genetically encoded amino acids, which has important implications for the practical production of recombinant barnacle cement proteins. Both cp-19k and cp-20k were soluble when expressed in E. coli (Urushida et al. 2007; Mori et al. 2007). Recombinant cp-19k irreversibly bound to negative, positive, and hydrophobic surfaces as expected for a versatile general purpose surface primer. Recombinant cp-20k had high affinity for calcite as expected for coupling cement to the barnacles calcareous base plate. The similar behavior of recombinant cp-20k to native cp-20k in a number of analytical methods demonstrated the recombinant and native proteins have the same tertiary structure and confirmed the absence of PTMs. In all, the recombinant barnacle cement proteins are a promising source of material for investigating adhesive proteins with native structures and sequences (Kamino 2010).
Natural underwater adhesives seem to have bumped up against a bond strength ceiling somewhere short of one MPa. Gene technology approaches to produce mimetic adhesives cannot overcome the biological and material limitations. How to make stronger underwater glues? One approach is to combine functional elements or design principles from biological glues with the versatility of synthetic polymer chemistry and the gathered know-how of adhesive engineers. The allure of synthetic approaches are the broad range of polymer backbone chemistries, the diverse sidechain functionalities that can be built in during polymerization, and cost-effective commercial scale-up. The most unique chemical elements are the protein sidechains, including post-translational modifications; other features like exact amino acid sequences and polyamide backbones are common to all proteins. By narrowing the problem down to the sidechains it becomes a relatively simple matter to create synthetic polymer analogs that replicate some of the features of biological glues.
Deming and coworkers demonstrated it was sufficient to copy only “functionality, and not amino acid sequence” to replicate bulk adhesion with synthetic random copolypeptides (Deming 1999; Yu and Deming 1998). Air cured bond strengths on aluminum were nearly 10X higher with random dopa and L-lysine copolypeptides (~4 MPa) than L-lysine polypeptide controls (~0.5 MPa). Bonds were stronger without oxidant (5.7 MPa) than with oxidant (4.9 MPa) implicating the catechol form of dopa rather than the oxidized o-quinone form as being directly responsible for robust interfacial adhesion (Yu et al. 1999). The focus has been on the o-dihydroxyphenyl sidechain of dopa as the critical functionality of mussel adhesive proteins ever since. The Messersmith group went further to reduce mussel surface adhesion to the single functionality of dopa. In a clever twist, non-fouling, protein-resistant surfaces were created by exploiting the fouling chemistry of mussels (Dalsin et al. 2003; Dalsin et al. 2005). In particular, dopa and a dopa-containing decapeptide corresponding to the repeating peptide motif of mefp-1 were conjugated to linear poly(ethylene glycol) (PEG), a well-known biocompatible surface passivating polymer. Both constructs worked equally well at preventing fibroblast attachment to Au and Ti surfaces. The conclusion—a single dopa residue in a non-polypeptide context was sufficient to anchor PEG to TiO2 surfaces. The effect was later quantified in single-molecule experiments with PEG-tethered dopa on an AFM tip (Lee et al. 2006). The catechol bound ~4X stronger than the quinone and ~8X stronger than tyrosine, which confirmed the importance of the ortho-hydroxyl groups of dopa for interfacial adhesion. Another example of reducing mussel adhesion to the single functionality of catechol sidechains was reported by Wilker’s group who synthesized copolymers of styrene and 3,4-dihydroxystyrene, a dopa analog. Copolymers containing pendant catechol groups had higher dry bond strengths on Al than polystyrene (Westwood et al. 2007).
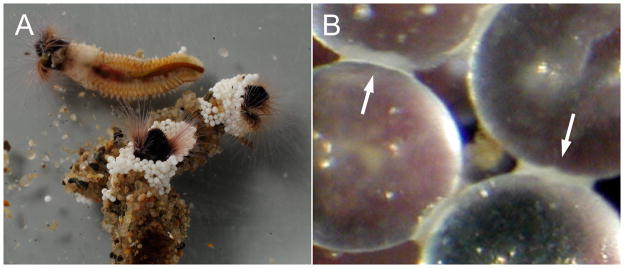
The adhesive of the Sandcastle worm has also served as a model for entirely synthetic biomimetic underwater adhesives (Shao and Stewart 2010; Shao et al. 2009; Rovner 2009). Sandcastle worms glue hard wet parts to similar hard wet parts to assemble a tubular composite shell (fig. 1); a much simpler underwater bonding problem than that of the mussels. Though the glue genes are cloned (Zhao et al. 2005; Endrizzi and Stewart 2009) the extensive phosphorylation of serines (Stewart et al. 2004) and hydroxylation of tyrosines (Waite et al. 1992) precluded a practical heterologous expression approach. Instead, the sidechains of the glue proteins were copied in the same molar ratio as the natural glue proteins into a set of copolymers with polymethacrylate backbones, the phosphate and catechol functionalities in the first, the amine functionality in the second (fig. 2A,B). Dense, cohesive, and pipettable complex coacervates formed when aqueous solutions of the oppositely charged biomimetic copolymers were mixed in similar proportions as the natural proteins (fig. 2C). The coacervates did not disperse, adhered to fully submerged glass, and slowly spread on the wet surface (fig. 2D). The phosphate, amine, and catechol sidechains can all promote wet adhesion depending on the substrate. In sum, the analog sandcastle glue qualitatively replicated the full range of properties predicted for the natural sandcastle glue—underwater deliverability, wet adhesion, a pH triggered initial setting reaction, and hardening by dopa-mediated covalent crosslinking (Shao and Stewart 2010). Underwater bond strengths of the adhesive complex coacervates were more than 2X the estimated bond strength of the natural adhesive (Sun et al. 2007). The inexpensive adhesive can be synthesized on any scale. Degradable adhesive complex coacervates formulated with gelatin as the polyamine were used to repair rat calvarial bone defects (Winslow et al.). The adhesive complex coacervate maintained alignment of bone segments during healing without effect on adjacent uninjured bone tissue and was gradually resorbed and replaced by new bone. The inflammatory response was commensurate with normal wound healing.
Figure 1.
Sandcastle worms. A.) The top worm was removed from its tubular shell. The bottom worms have reconstructed portions of their natural sand and seashell tubes with white zirconium oxide beads (0.5 mm) in the laboratory. B.) Close-up of laboratory rebuilt tube. Arrows indicate spots of glue holding beads together.
The preceding examples demonstrated that bioadhesive sidechain functionalities can be taken out of the context of specific amino acid sequences and polypeptide backbones and incorporated into a range of synthetic polymers to replicate aspects of natural underwater bonding. To take the opposite view for a moment, sequence does matter, if they did not contribute to important structure the repeating peptide motifs that comprise many of the mussel, barnacle, and Sandcastle worm proteins would have diverged much more. Structure matters, as evident in nanoscale studies of adhesion and cohesion of native mfps. Two opposed mica surfaces were cohered by mfp-3, whereas mfp-1 adhered to one surface did not adhere to the other, i.e., no cohesion (Lin et al. 2007). Mica surfaces coated with mfp-2 did not cohere to either another mfp-2 coated surface nor to bare mica until Ca2+ or Fe3+ were added, in which case strong cohesion occurred. Furthermore, mfp-2 adhered strongly to interfacial mfp-5 but not mfp-3 (Zeng et al. 2010). Structure matters in the amyloid-like nanofibrillar network of barnacle cement (Barlow et al. 2010).
To conclude, there are more tricks to be learned from natural underwater adhesives. The interactions between individual mussel foot proteins in vitro are displaying how the proteins fit together like puzzle pieces into an elaborate hierarchical structure that connects collagenous threads to wet rocks. Physiological studies of the Sandcastle worm are revealing how its multipart adhesive is packaged, delivered, mixed, and cured underwater (Wang et al. in press). Rational mutagenesis of the expressible barnacle proteins may perhaps uncover the role of sequence and structure in interfacial adhesion. The first examples of synthetic biomimetic adhesives demonstrated the potential to combine lessons from bioadhesives with the versatility and practicality of polymer chemistry. There will soon be synthetic underwater adhesives that combine the deliverability and wet adhesion of natural adhesives with the superior bond strengths of synthetic adhesives. In the longer run, synthetic analogs may incorporate hierarchical structure and cooperative assembly mechanisms similar to the natural adhesives and may incorporate a combination of synthetic polymers and modified proteins.
Finally, to come full circle, the author of a 1905 book (Dawidowsky 1905) on the subtle art of manufacturing hide glues for carpentry mentioned in passing “…when cabinet makers cut themselves, they apply glue to the wound with the best success.” Plainly, the logic of repairing wounds with glue has been long appreciated and state-of-the-art glue of every period has been tested on tissue (Leonard 1970; Donkerwolcke et al. 1998; Heiss 2006). Perhaps biomimetic adhesive development will lead to long-sought technology to replace the stitches, staples, and screws used to this day to repair damaged tissues.
Acknowledgments
Support from the National Institutes of Health (R01EB006463), the National Science Foundation (DMR0906014), and the Office of Naval Research (N00014-10-1-0108) are gratefully acknowledged.
References
- Barlow DE, Dickinson GH, Orihuela B, Kulp I, JL, Rittschof D, Wahl KJ. Characterization of the adhesive plaque of the barnacle balanus amphitrite: Amyloid-like nanofibrils are a major component. Langmuir. 2010;26:6549–6556. doi: 10.1021/la9041309. [DOI] [PubMed] [Google Scholar]
- Bungenberg de Jong HG. Morphology of coacervates. In: Kruyt HR, editor. Colloid science. II. Elsevier Publishing Company, Inc; 1949. pp. 431–482. [Google Scholar]
- Burkett JR, Wojtas JL, Cloud JL, Wilker JJ. Method for measuring the adhesion strength of marine mussels. The Journal of Adhesion. 2009;85:601–615. [Google Scholar]
- Cha HJ, Hwang DS, Lim S, White JD, Matos-Perez CR, Wilker JJ. Bulk adhesive strength of recombinant hybrid mussel adhesive protein. Biofouling. 2008:1–9. doi: 10.1080/08927010802563108. [DOI] [PubMed] [Google Scholar]
- Conlan SL, Mutton RJ, Aldred N, Clare AS. Evaluation of a fully automated method to measure the critical removal stress of adult barnacles. Biofouling. 2008;24:471–481. doi: 10.1080/08927010802353716. [DOI] [PubMed] [Google Scholar]
- Costerton JW. Springer series on biofilms. Springer; Berlin: 2007. The biofilm primer. [Google Scholar]
- Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125 (14):4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- Dalsin JL, Lin L, Tosatti S, Voros J, Textor M, Messersmith PB. Protein resistance of titanium oxide surfaces modified by biologically inspired mpeg-dopa. Langmuir. 2005;21 (2):640–646. doi: 10.1021/la048626g. [DOI] [PubMed] [Google Scholar]
- Dawidowsky F. Glue, gelatine, cements, and pastes. 2. Henry Carey Baird and Co; Philadelphia: 1905. [Google Scholar]
- Deming TJ. Mussel byssus and biomolecular materials. Curr Opin Chem Biol. 1999;3 (1):100–105. doi: 10.1016/s1367-5931(99)80018-0. [DOI] [PubMed] [Google Scholar]
- Dickinson GH, Vega IE, Wahl KJ, Orihuela B, Beyley V, Rodriguez EN, Everett RK, Bonaventura J, Rittschof D. Barnacle cement: A polymerization model based on evolutionary concepts. J Exp Biol. 2009;212:3499–3510. doi: 10.1242/jeb.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkerwolcke M, Burny F, Muster D. Tissues and bone adhesives--historical aspects. Biomaterials. 1998;19(16):1461–1466. doi: 10.1016/s0142-9612(98)00059-3. S0142-9612(98)00059-3 [pii] [DOI] [PubMed] [Google Scholar]
- Endrizzi BJ, Stewart RJ. Glueomics: An expression survey of the adhesive gland of the sandcastle worm. The Journal of Adhesion. 2009;85 (8):546–559. [Google Scholar]
- Flammang P, Lambert A, Bailly P, Hennebert E. Polyphosphoprotein-containing marine adhesives. The Journal of Adhesion. 2009;85:447–464. [Google Scholar]
- Florsheimer M, Kruse K, Polly R, Abdelmonem A, Schimmelpfennig B, Klenze R, Fanghanel T. Hydration of mineral surfaces probed at the molecular level. Langmuir. 2008;24:13434–13439. doi: 10.1021/la801677y. [DOI] [PubMed] [Google Scholar]
- Geesey GG, Richardson WT, Yeomans HG, Irvin RT, Costerton JW. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol. 1977;23 (12):1733–1736. doi: 10.1139/m77-249. [DOI] [PubMed] [Google Scholar]
- Heiss C, Kraus R, Schluckebier D, Stiller AC, Wenisch S, Schnettler R. Bone adhesives in trauma and orhopedic surgery. Eur J Trauma. 2006;32:141–148. [Google Scholar]
- Hwang DS, Gim Y, Cha HJ. Expression of functional recombinant mussel adhesive protein type 3a in escherichia coli. Biotechnol Prog. 2005;21 (3):965–970. doi: 10.1021/bp050014e. [DOI] [PubMed] [Google Scholar]
- Hwang DS, Gim Y, Yoo HJ, Cha HJ. Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials. 2007a;28 (24):3560–3568. doi: 10.1016/j.biomaterials.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Hwang DS, Sim SB, Cha HJ. Cell adhesion biomaterial based on mussel adhesive protein fused with rgd peptide. Biomaterials. 2007b;28 (28):4039–4046. doi: 10.1016/j.biomaterials.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Hwang DS, Yoo HJ, Jun JH, Moon WK, Cha HJ. Expression of functional recombinant mussel adhesive protein mgfp-5 in escherichia coli. Appl Environ Microbiol. 2004;70 (6):3352–3359. doi: 10.1128/AEM.70.6.3352-3359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K. Molecular design of barnacle cement in comparision with those of mussel and tubeworm. Journal of Adhesion. 2010;86:96–110. [Google Scholar]
- Kao WY, Case ST. A novel giant secretion polypeptide in chironomus salivary glands: Implications for another balbiani ring gene. J Cell Biol. 1985;101 (3):1044–1051. doi: 10.1083/jcb.101.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandeparker L, Anil AC. Underwater adhesion: The barnacle way. Int J Adhesion and Adhesives. 2006;27:165–172. [Google Scholar]
- Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103 (35):12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard F. Hemostatic applications of alpha-cyanoacrylates: Bonding mechanism and physiological degradation of bonds. In: Manly RS, editor. Adhesion in biological systems. Academic Press; New York: 1970. pp. 185–199. [Google Scholar]
- Lin Q, Gourdon D, Sun C, Holten-Andersen N, Anderson TH, Waite JH, Israelachvili JN. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc Natl Acad Sci U S A. 2007;104 (10):3782–3786. doi: 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugh KJ, Anderson DM, Strausberg R, Strausberg SL. Method of producing bioadhesive protein. 5,202,236 USA Patent. 1993
- Maugh KJ, Anderson DM, Strausberg R, Strausberg SL, McCandliss R, Wei T, Filpula D. 5049504 Bioadhesive coding sequences. 1990
- Mori Y, Urushida Y, Nakano M, Uchiyama S, Kamino K. Calcite-specific coupling protein in barnacle underwater cement. FEBS J. 2007;274 (24):6436–6446. doi: 10.1111/j.1742-4658.2007.06161.x. [DOI] [PubMed] [Google Scholar]
- Ninan L, Stroshine RL, Wilker JJ, Shi R. Adhesive strength and curing rate of marine mussel protein extracts on porcine small intestinal submucosa. Acta Biomater. 2007;3 (5):687–694. doi: 10.1016/j.actbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheofanis BA, Ray RD. Experimental use of adhesives in the repair of transverse fractures of the rat and rabbit. Biomat, Med Dev, Art Org. 1982;10:247–265. doi: 10.3109/10731198209118784. [DOI] [PubMed] [Google Scholar]
- Papov VV, Diamond TV, Biemann K, Waite JH. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel mytilus edulis. J Biol Chem. 1995;270 (34):20183–20192. doi: 10.1074/jbc.270.34.20183. [DOI] [PubMed] [Google Scholar]
- Robin JB, Picciano P, Kusleika RS, Salazar J, Benedict C. Preliminary evaluation of the use of mussel adhesive protein in experimental epikeratoplasty. Arch Ophthalmol. 1988;106 (7):973–977. doi: 10.1001/archopht.1988.01060140119037. [DOI] [PubMed] [Google Scholar]
- Rovner SL. Worm inspires medical adhesive. Chemical and Engineering News. 2009;87 (34):32. [Google Scholar]
- Shao H, Bachus KN, Stewart RJ. A water-borne adhesive modeled after the sandcastle glue of p. Californica. Macromol Biosci. 2009;9 (5):464–471. doi: 10.1002/mabi.200800252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Stewart RJ. Biomimetic underwater adhesives with environmentally triggered setting mechanisms. Adv Mater. 2010;22 (6):729–733. doi: 10.1002/adma.200902380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman HG, Roberto FF. Cloning and expression of recombinant protein mefp-1 of the blue mussel, mytilus edulis. 6987170 USA Patent. 2006a
- Silverman HG, Roberto FF. Cloning and expression of recombinant protein mefp-2 of the blue mussel, mytilus edulis. 6995012 USA Patent. 2006b
- Spruijt E, Sprakel J, Cohen Stuart MA, van der Gucht J. Interfacial tension between a complex coacervate phase and its coexisting aqueous phase. Soft Matter. 2010;6:172–178. [Google Scholar]
- Stevens MJ, Steren RE, Hlady V, Stewart RJ. Multiscale structure of the underwater adhesive of phragmatopoma californica: A nanostructured latex with a steep microporosity gradient. Langmuir. 2007;23 (9):5045–5049. doi: 10.1021/la063765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RJ, Wang CS. Adaptation of caddisfly larval silks to aquatic habitats by phosphorylation of h-fibroin serines. Biomacromolecules. 2010;11 (4):969–974. doi: 10.1021/bm901426d. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Weaver JC, Morse DE, Waite JH. The tube cement of phragmatopoma californica: A solid foam. J Exp Biol. 2004;207 (Pt 26):4727–4734. doi: 10.1242/jeb.01330. [DOI] [PubMed] [Google Scholar]
- Sun C, Fantner GE, Adams J, Hansma PK, Waite JH. The role of calcium and magnesium in the concrete tubes of the sandcastle worm. J Exp Biol. 2007;210 (Pt 8):1481–1488. doi: 10.1242/jeb.02759. [DOI] [PubMed] [Google Scholar]
- Thomas LA, Hermans CO. Adhesive interactions between the tube feet of a starfish, leptasterias hexactis, and substrata. Biol Bull. 1985;169:675–688. [Google Scholar]
- Urushida Y, Nakano M, Matsuda S, Inoue N, Kanai S, Kitamura N, Nishino T, Kamino K. Identification and functional characterization of a novel barnacle cement protein. FEBS J. 2007;274 (16):4336–4346. doi: 10.1111/j.1742-4658.2007.05965.x. [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, Inoue S, Pedigao J. Fundamentals of operative dentistry. 2. Quintessence Publishing; Carol Stream, Ill: 2001. pp. 194–214. [Google Scholar]
- Waite JH. Nature’s underwater adhesive specialist. Chemtech. 1987;17:692–697. [Google Scholar]
- Waite JH. The formation of mussel byssus: Anatomy of a natural manufacturing process. Results Probl Cell Differ. 1992;19:27–54. doi: 10.1007/978-3-540-47207-0_2. [DOI] [PubMed] [Google Scholar]
- Waite JH, Jensen RA, Morse DE. Cement precursor proteins of the reef-building polychaete phragmatopoma californica (fewkes) Biochemistry. 1992;31 (25):5733–5738. doi: 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- Waite JH, Qin X. Polyphosphoprotein from the adhesive pads of mytilus edulis. Biochemistry. 2001;40 (9):2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- Wang CS, Svendsen KK, Stewart RJ. Morphology of the adhesive system in the sandcastle worm, phragmatopoma californica. In: von Byern J, Grunwald I, editors. Biological adhesive systems. Springer; New York: in press. [Google Scholar]
- Westwood G, Horton TN, Wilker JJ. Simplified polymer mimics of cross-linking adhesive proteins. Macromolecules. 2007;40:3960–3964. [Google Scholar]
- Winslow BD, Shao H, Stewart RJ, Tresco PA. Biocompatibility studies of adhesive complex coacervates modeled after the sandcastle glue of p. Californica. Biomaterials. doi: 10.1016/j.biomaterials.2010.07.078. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Deming TJ. Synthetic polypeptide mimics of marine adhesives. Macromolecules. 1998;31 (15):4739–4745. doi: 10.1021/ma980268z. [DOI] [PubMed] [Google Scholar]
- Yu M, Hwang J, Deming TJ. Role of l-3,4-dihydroxyphenylalanine in mussel adhesive protein. J Am Chem Soc. 1999;121:5825–5826. [Google Scholar]
- Zardus JD, Nedved BT, Huang Y, Tran C, Hadfield MG. Microbial biofilms facilitate adhesion in biofouling invertebrates. 2008;214:91–98. doi: 10.2307/25066663. [DOI] [PubMed] [Google Scholar]
- Zeng H, Hwang DS, Israelachvili JN, Waite JH. Strong reversible fe3+-mediated bridging between dopa-containing protein films in water. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1007416107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Robertson NB, Jewhurst SA, Waite JH. Probing the adhesive footprints of mytilus californianus byssus. J Biol Chem. 2006;281 (16):11090–11096. doi: 10.1074/jbc.M510792200. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sun C, Stewart RJ, Waite JH. Cement proteins of the tube-building polychaete phragmatopoma californica. J Biol Chem. 2005;280 (52):42938–42944. doi: 10.1074/jbc.M508457200. [DOI] [PubMed] [Google Scholar]