Abstract
Repeated administration of psychostimulants to rodents can lead to behavioral sensitization. Previous studies, using nonspecific opioid receptor (OR) antagonists, revealed that ORs were involved in modulation of behavioral sensitization to methamphetamine (METH). However, the contribution of OR subtypes remains unclear. In the present study, using μ-OR knockout mice, we examined the role of μ-OR in the development of METH sensitization. Mice received daily intraperitoneal injection of drug or saline for 7 consecutive days to initiate sensitization. To express sensitization, animals received one injection of drug (the same as for initiation) or saline on day 11. Animal locomotor activity and stereotypy were monitored during the periods of initiation and expression of sensitization. Also, the concentrations of METH and its active metabolite amphetamine in the blood were measured after single and repeated administrations of METH. METH promoted significant locomotor hyperactivity at low doses and stereotyped behaviors at relative high doses (2.5 mg/kg and above). Repeated administration of METH led to the initiation and expression of behavioral sensitization in wild-type mice. METH-induced behavioral responses were attenuated in the μ-OR knockout mice. Haloperidol (a dopamine receptor antagonist) showed a more potent effect in counteracting METH-induced stereotypy in the μ-OR knockout mice. Saline did not induce behavioral sensitization in either genotype. No significant difference was observed in disposition of METH and amphetamine between the two genotypes. Our study indicated that the μ-opioid system is involved in modulating the development of behavioral sensitization to METH.
Keywords: drug abuse, locomotor activity, stereotyped behaviors
Methamphetamine (METH) is an active analog of amphetamine and has psychostimulant effects very similar to those of amphetamine. Repeated use of the psychostimulants can produce addiction in humans and behavioral sensitization (reverse “behavioral tolerance”) in rodents (Bartlett et al., 1997), characterized by a progressively enhanced locomotor activity and stereotypy. Because the neural alterations that underlie behavioral sensitization are thought to contribute to the development of the compulsive patterns of drug craving that characterizes addiction (Robinson and Berridge, 2008), behavioral sensitization in rodents is widely used as a model for the study of drug addiction (Itzhak and Ali, 2002). Although the definite mechanisms responsible for development of sensitization to METH remain unclear, the mesolimbic dopaminergic pathway in the central nervous system (CNS), originating from the midbrain ventral tegmental area (VTA) and terminating primarily in the nucleus accumbens (NAc) and the medial prefrontal cortex, plays a crucial role in the development of behavioral sensitization to the psychostimulants. Topographic overlaps between opioid and dopamine neurons are found in the CNS, suggesting that interactions exist between these two systems (Sesack and Pickel, 1992). Previous studies have demonstrated that opioid systems are the targets for psychostimulants as well. The opioid receptor (OR) antagonist naloxone can attenuate amphetamine-induced dopamine and behavioral hyperactivity in several species (Feigenbaum and Howard, 1997; Schad et al., 2002). We also found that naltrexone, a long-lasting OR antagonist, can attenuate METH-induced behavioral sensitization in NIH Swiss mice (Chiu et al., 2005). The available evidence strongly indicates that opioidergic systems are involved in modulation of the development of behavioral sensitization to amphetamine and METH.
The opioidergic system consists of a variety of endogenous opioid peptides and their receptors. At least three OR subtypes (μ, δ, and κ) are currently recognized (Knapp et al., 1995). It is well known that selective activation of different subtypes of OR produces different or even opposite behavioral and physiological effects (Devine et al., 1993). Therefore, it is important to clarify the contribution of specific OR subtypes to the development of sensitization to psychostimulants. Although opioid antagonists have been widely used in the studies conducted, they generally lack sufficient selectivity for the opioid subtypes. Their mode of action depends on the manner and dosage of administration. Thus, it has been difficult to clearly pin-point the contribution of each opioid subtype to METH-induced behavioral and neurochemical responses if only a pharmacological agent is used.
The activity of known genes can be modified in vivo by using gene-targeting technology. Currently, opioid receptor-deficient mice have been generated by homologous recombination. The use of transgenic mice has provided an unprecedented opportunity to elucidate the contribution of specific receptor subtypes to the behavioral and neurochemical responses to psychostimulants. The present study was designed to determine whether repeated administration of METH leads to the development of behavioral sensitization to the drug in the μ-OR knockout mice. The disposition characteristics of METH and its metabolite amphetamine in the μ-OR knockout and wild-type mice were examined in this study as well.
MATERIALS AND METHODS
Drugs and Animals
Methamphetamine hydrochloride (Sigma, St. Louis, MO) was dissolved in a saline vehicle and administrated intraperitoneally (i.p.). Haloperidol (Gensia Sicor Pharmaceuticals, Irvine, CA) was diluted in saline and administrated subcutaneously (s.c.). The μ-OR knockout mice used in this study were developed by Loh et al. (1998) and maintained on a 1:1 hybrid genetic background (C57/BL6 and 129/Ola). The μ-OR knockout (−/−), heterozygous (+/−), and wild-type (+/+) mice were bred in our laboratory and maintained on a 12-hr light/dark cycle at a constant temperature (22°C ± 2°C). The mouse genotypes were identified by Southern blots of tail DNA or autoradiography of μ-OR-specific ligand binding in brain tissues. All procedures for animal care and breeding were conducted in accordance with the NIH Guide for the care and use of laboratory animals and were approved by the University of Mississippi Medical Center Animal Care and Use Committee.
Animal Treatment Protocol
Male wild-type and μ-OR knockout mice ranging from 8 to 12 weeks old (body weight 20–25 g) were used in this study. To test acute administration of METH on locomotor activity, mice from both genotypes were divided into six groups (eight per group). Each group received a single i.p. injection of METH at doses of 0 (saline control), 0.31, 0.62, 1.25, 2.5, or 10 mg/kg. Animal locomotor activity was recorded for 30 min (baseline level) before and 120 min after METH injection. METH-induced locomotor hyperactivity and stereotyped behaviors were used as markers for the development of behavioral sensitization. In the preliminary study, we noticed that, although the single injections of METH at doses of 0.31 and 0.62 mg/kg did not induce an increase in locomotor activity in either genotype of mice, repeated administration of METH at 0.62 mg/kg, but not 0.31 mg/kg, induced an increase in wild-type mice. METH at the dose of 2.5 mg/kg and above produced significant stereotyped behaviors in wild-type mice. Therefore, a dose of 0.62 mg/kg was chosen for investigation of locomotor sensitization, and the doses of 2.5 and 10 mg/kg were chosen for investigation of stereotyped behavioral sensitization in this study.
To initiate locomotor sensitization to METH, eight mice from both genotypes were given a single daily i.p. injection of METH (0.62 mg/kg) or saline (control) for 7 consecutive days. To initiate stereotyped behavioral sensitization to METH, 12 mice from both genotypes were given a single daily i.p. injection of METH (2.5 and 10 mg/kg) for 7 consecutive days. An individual mouse was given the same dose of drug on each day. Animal locomotor activity and stereotyped behaviors were monitored at experimental days 1, 4, and 7. To evaluate the initiation of behavioral sensitization, METH-induced behavioral response at days 4 and 7 was compared with that after first injection at day 1. All behavioral tests were conducted it the same room and at the same time on each day. After the habituation period, mice were injected with saline or METH in their home cages and then moved back into the locomotor arena. Locomotor activity was monitored for 2 hr and stereotyped behaviors were monitored for 5 hr after METH injection.
To investigate the effect of the dopamine receptor antagonist haloperidol on expression of behavioral stereotypy sensitization to METH, four groups of mice (10–12 per group) from each genotype were administered a single daily i.p. injection of METH (10 mg/kg) for 7 consecutive days. On day 11, following 4 abstinent days, each group of mice was s.c. injected with saline or one dose of haloperidol (0.007, 0.02, or 0.06 mg/kg for μ-OR knockout mice and 0.02, 0.06, or 0.18 mg/kg for wild-type mice) and then, 30 min later, injected with METH (10 mg/kg, i.p.). Animal stereotyped behaviors were monitored and then scored at 15-min intervals for 5 hr. Area under the curve (AUC) was calculated from stereotyped behavior scores observed in 5 hr by the trapezoidal role in Graph Pad Prism software (San Diego, CA). The data were fit to a sigmoidal function, which allowed calculation of the median effective dose (ED50) ±95% in Graph Pad Prism.
To examine the disposition characteristics of METH and its active metabolite amphetamine in the body, mice from both genotypes were assigned into two groups with six per group, single treatment group (METH 2.5 mg/kg, i.p., once) and repeated treatment group (METH 2.5 mg/kg, i.p., for 7 consecutive days). Forty microliters of blood was taken by puncture of the retroorbital sinus with a micropipette (Drummond Scientific, Broomall, PA) at various times after METH injection (last injection for repeated treatment group). Blood samples were collected in heparinized test tubes and kept at 4°C for measuring METH and amphetamine.
Locomotor Activity Test
Animal locomotor activity was tested according to our previously described method (Chiu et al., 2005). In brief, locomotor activities were monitored in a plastic cage with top open and without bedding that was the same size as their home cage (28.5 cm × 17.5 cm × 12 cm) and was used as the locomotor arena. On the day of experiment, mice were transferred to the behavioral testing room in their home cages and allowed to acclimatize to the testing room for 60 min undisturbed, with food and water. After the acclimatization period, the mice were individually moved to the locomotor arena to habituate for 60 min. Locomotor activity in the locomotor arena was recorded during the last 30 min of habituation as baseline activity. After the habituation period, mice were injected with saline or METH in their home cages and then moved back into the locomotor arena for recording locomotor activity for 120 min. Twelve arenas can be tracked simultaneously with a CCD camera. Data were quantified by a computer-operated video tracking system (SMART; San Diego Instruments, San Diego, CA).
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Blood METH and Amphetamine
Extraction
METH and amphetamine extraction from mouse blood was accomplished by protein precipitation with acetonitrile containing an internal standard of D8-amphetamine and D8-METH (Cerilliant Corporation, Round Rock, TX). After centrifugation, 1% HCl in methanol was added to the supernatant and the mixture was taken to dryness under a stream of nitrogen gas at 45°C. One milliliter of 0.1 M phosphate buffer, pH 6.0, was added to the sample. METH and amphetamine were extracted from this solution using a modified Bond Elute Certify (SPE column) procedure (Varian, Inc). The eluant was evaporated to one-half the original volume under a stream of nitrogen gas at 45°C, and 1% HCl in methanol was added to the remaining eluant, which was then taken to dryness as described above.
Derivatization
To enhance the chromatography and mass spectrometry response of METH and amphetamine, a trifluoroacetyl derivative was made of each compound. This was accomplished by adding 5% 1-(trifluoroacetyl) imidazole in hexane, and the sample was held at 75°C for 15 min and cooled, and phosphate buffer was added. The samples were held at −80°C for 10 min to freeze the aqueous layer. The hexane layer was transferred to an autosampler vial for GC-MS analysis. The phosphate buffer was added to remove any access 1-(trifluoroacetyl) imidazole from the sample. The samples were analyzed with a Thermo Finnigan Trace GC system (quadrupole MS).
GC conditions
An aliquot of 1-μl of the derivatized sample was injected in splitless mode onto the GC column. The injection port temperature was 220°C; the carrier gas was helium at a flow rate of 1.5 ml/min; and the oven temperature program was 60°C for 1 min raised to 140°C at 30°C/min, held for 1 min, raised to 200°C at 10°C/min, and held for 2 min.
MS conditions
The transfer line and source temperatures were 220°C and 200°C, respectively. Ionization was by electron impact (EI+) using electron energy of 70 eV in the selected ion monitoring (SIM) mode; parameters for acquisition of METH were 7.7–7.9 min m/z 110, 118, 154 and 126, 160 for D8-METH and for 7.9–8.2 min m/z 91, 118, 140 and 126, and143 for D8-amphetamine. METH and amphetamine were quantified by comparison of their peak area with that of their corresponding internal standard to calculate a peak area ratio for amphetamine and peak area ratio for METH.
Stereotyped Behavioral Test
The stereotyped behavior of mice was monitored in a plexiglas box with a CCD camera, recorded on a VCR tape, and analyzed by visual observation. The intensity of stereotyped activity was scored according to an arbitrary four-point scale (0 = normal behavior, 1 = periodic sniffing, 2 = continuous sniffing, 3 = continuous sniffing, periodic licking or gnawing, 4 = continuous licking or gnawing), as described by Costall and colleagues (1972). Each animal was assigned a rating score of 0–4, according to the scale, 30 min before METH injection and every 15 min for 5 hr after METH injection.
Statistical Analysis
All statistical analyses were performed in SigmaStat 3.0 (SPSS, Rochester, MN). Data are expressed as mean ± SEM. All behavioral data were analyzed by two-way ANOVA (genotype vs. dose or genotype vs. day), followed by a post hoc Student-Newman-Keuls multiple-comparisons test. A difference was considered significant at P < 0.05.
RESULTS
Dose Response of Single METH Injection-Induced Locomotor Activity in μ-OR Knockout and Wild-Type Mice
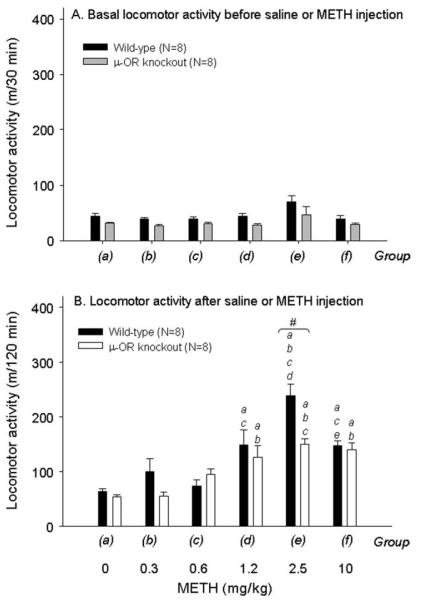
To establish optimal doses for the induction of behavioral sensitization to METH, we first determined the dose–response of single injection of METH-produced locomotor activity. METH doses of 0.31–10 mg/kg were used in this study. Two-way ANOVA (genotype × dose) revealed significant effect for METH-mediated locomotor response [genotype: F(1, 93) = 9.01, P < 0.005; dose: F(5, 93) = 25.2, P < 0.001; interaction: F(5, 93) = 3.43, P < 0.01; Fig. 1]. Subsequent post hoc comparisons indicated that in wild-type mice METH increased locomotor activity at doses of 1.25, 2.5, and 10 mg/kg but not at doses of 0.31 and 0.62 mg/kg. A maximal locomotor response was detected at a dose of 2.5 mg/kg. The highest dose of METH (10 mg/kg) in the study evoked significant stereotyped behaviors (e.g. sniffing, licking, gnawing, and grooming). Therefore, METH-induced locomotor hyperactivity was less in the 10 mg/kg treatment group than in the 2.5 mg/kg treatment group. Similar responses were also found in the μ-OR knockout mice. However, METH-induced locomotor activity was significantly less in the μ-OR knockout mice than in their wild-type controls.
Fig. 1.
Dose–response relationship of METH and locomotor activity in wild-type and μ-OR knockout mice. A: Basal locomotor activity before saline or METH injection. B: Locomotor activity after saline or METH injection. a,b,c,d,eP < 0.05 compared with the corresponding dose group within the genotype. #P < 0.05 between the genotypes on the same dose.
Repeated Administration of METH (0.62 mg/kg) Induced Behavioral Locomotor Sensitization in Wild-Type Mice but Not in the μ-OR Knockout Mice
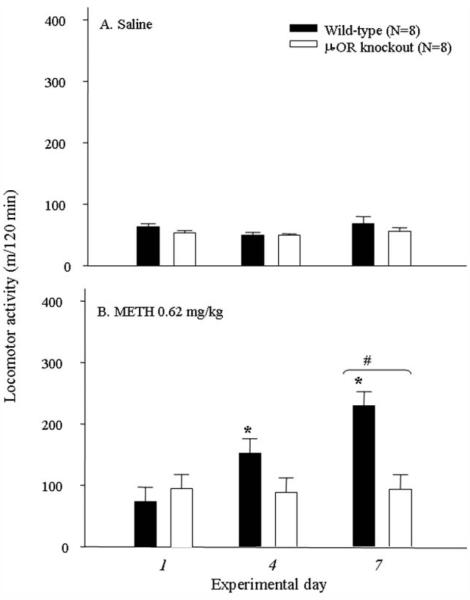
Two-way ANOVA revealed significant effects on locomotor response by METH at dose of 0.62 mg/kg [genotype: F(1, 69) = 36.27, P < 0.001; day: F(4, 69) = 4.72, P < 0.005) = 4.35, P < 0.005; Fig. 2B]. Interaction of genotype with experimental day was significant in METH treatment [F(4, 69) = 4.35, P < 0.005] but was insignificant in saline treatment [F(4, 70) = 0.91, P > 0.05]. The post hoc analyses indicated that METH led to an initiation and expression of behavioral (locomotor) sensitization in wild-type mice but not in μ-OR knockout mice. Repeated administration of saline showed no effect on locomotor response [genotype: F(1, 70) = 0.74, P > 0.05; day: F(4, 70) = 1.57, P > 0.05; interaction: F(4, 70) = 0.91, P > 0.05; Fig. 2A]. These results indicated that repeated administration of METH led to behavioral (locomotor) sensitization in wild-type mice but not in the μ-OR knockout mice.
Fig. 2.
Effect of repeated administration of saline (A) or METH (0.62 mg/kg; B) on locomotor activity in wild-type and μ-OR knockout mice. ⋆P < 0.05 compared with the response detected on day 1 within the genotype. #P < 0.05 between the genotypes on the same experimental day.
Repeated Administration of METH (2.5 and 10 mg/kg)-Induced Behavioral Stereotypy Sensitization Was Attenuated in the μ-OR Knockout Mice
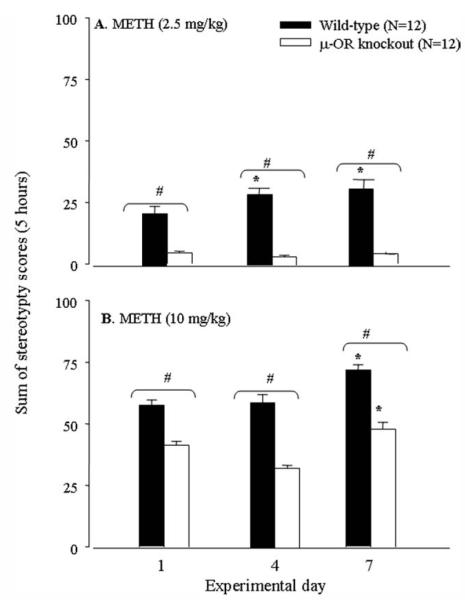
During the experiments, we noticed that METH at the dose of 2.5 mg/kg and above produced significant stereotyped behaviors in wild-type mice. The behaviors lasted for 1–5 hr, depending on METH doses. Furthermore, we determined the effect of repeated administration of METH on stereotyped behaviors in the two genotypes of mice. Two-way ANOVA pointed out significant effects for stereotypy by METH at doses of 2.5 mg/kg [genotype: F(1, 32) = 214.4, P < 0.001; day: F(3, 32) = 5.26, P < 0.01; Fig. 3A] and 10 mg/kg [genotype: F(1, 88) = 173.9, P < 0.001; day: F(3, 88) = 12.6, P < 0.001; Fig. 3B]. Interaction of genotype with experimental day was significant at 2.5 mg/kg METH [F(3, 32) = 4.77, P < 0.01] but was insignificant at 10 mg/kg METH [F(3, 88) = 1.73, P > 0.05]. Subsequent post hoc tests revealed a significant increase in stereotyped behaviors on day 7 in both genotypes of mice following repeated administration of 10 mg/kg METH. At 2.5 mg/kg, METH increased stereotyped behaviors in wild-type mice but not in μ-OR knockout mice on all test days. METH at both doses induced significantly more stereotyped behaviors in the wild-type mice than that in the μ-OR knockout mice on all test days. The results, consistently with locomotor behavior, suggested that the μ-OR knockout mice were less sensitive to METH-induced behavioral stereotypy sensitization.
Fig. 3.
Effect of repeated administration of METH at doses of 2.5 mg/kg (A) or 10 mg/kg (B) on stereotyped behaviors in wild-type and μ-OR knockout mice. ⋆P < 0.05 compared with the response detected on day 1 within the genotype. #P < 0.05 between the genotypes on the same experimental day.
Haloperidol Showed a More Potent Effect in Counteracting METH-Induced Stereotypy in the μ-OR Knockout Mice
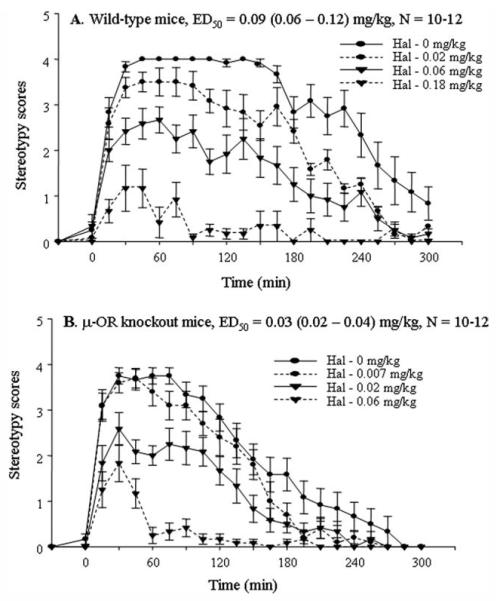
Preadministration of dopamine receptor antagonist haloperidol dose dependently counteracted METH-induced stereotyped behaviors in both genotypes of mice. The subcutaneous ED50s were 0.09 (0.06–0.12) and 0.03 (0.02–0.04) mg/kg in wild-type and μ-OR knockout mice (Fig. 4), respectively. The result indicated that haloperidol was more potent in counteracting METH-mediated stereotypy in the μ-OR knockout mice.
Fig. 4.
Dose–response relationship of haloperidol in counteracting METH-evoked stereotypy in sensitized wild-type mice (A) and μ-OR knockout mice (B). Both genotypes of mice were i.p. injected with METH (10 mg/kg) for 7 consecutive days to initiate sensitization. On day 11, after 4 abstinent days, each group of mice was injected s.c. with saline or one dose of haloperidol and then, 30 min later, injected with METH (10 mg/kg, i.p. injection). Animal stereotyped behaviors were monitored and scored at 15-min intervals for 5 hr.
Disposition of METH in the Wild-Type and μ-OR Knockout Mice Following Single and Repeated Administration of METH
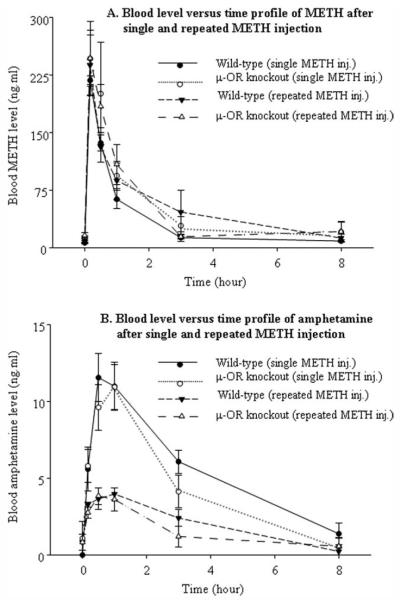
The blood METH and amphetamine concentration–time profiles after single and repeated administration of METH (2.5 mg/kg, i.p.) are shown in Figure 5. Amphetamine is an active metabolite of METH and is mainly metabolized by hepatic cytochrome P-450 isozymes. The pharmacokinetic parameters Cmax (maximum blood concentration), Tmax (time of Cmax), and AUC (area under the blood concentration–time curve, are given in Table I. Based on two-way ANOVA, no significant differences were observed in any pharmacokinetic parameter of blood METH, including Cmax [genotype: F(1, 20) = 2.93, P > 0.05; treatment: F(1, 20) = 0.025, P > 0.05], Tmax [genotype: F(1, 20) = 1.81, P > 0.05; treatment: F(1, 20) = 0.01, P > 0.05], and AUC [genotype: F(1, 20) = 0.53, P > 0.05; treatment: F(1, 20) = 0.55, P > 0.05]. Two-way ANOVA of blood amphetamine parameters showed significant differences in the Cmax [F(1, 20) = 45.9, P < 0.05] and AUC [F(1, 20) = 35.8, P < 0.05] but no significant difference in Tmax [F(1, 20) = 0.04, P > 0.05] between treatment groups. No statistical difference was observed in all blood amphetamine parameters between genotypes, including Cmax [F(1, 20) = 0.049, P > 0.05], Tmax [F(1, 20) = 0.16, P > 0.05], and AUC [F(1, 20) = 2.07, P > 0.05]. The results indicate that there is no genotype difference in the disposition of METH and amphetamine. Repeated administration of METH accelerates the elimination of amphetamine but not of METH itself in both wild-type and μ-OR knockout mice.
Fig. 5.
The blood METH (A) and amphetamine (B) concentration–time profiles after single and repeated i.p. injection of METH (2.5 mg/kg).
TABLE I.
Pharmacokinetic Parameters of METH and Amphetamine in Blood After Single or Repeated Injections of METH in Wild-Type and μ-OR Knockout Mice Mean ± SEM; (N = 6)
| After single METH injection |
After repeated METH injections |
|||
|---|---|---|---|---|
| Wild-type | μ-OR knockout | Wild-type | μ-OR knockout | |
| Blood METH | ||||
| Cmax (ng · ml−1) | 218 ± 6 | 305 ± 53 | 251 ± 33 | 283 ± 29 |
| Tmax (hr) | 0.17 ± 0 | 0.22 ± 0.06 | 0.19 ± 0.02 | 0.22 ± 0.06 |
| AUC (ng · hr · ml−1) | 262 ± 20 | 419 ± 89 | 420 ± 129 | 381 ± 36 |
| Blood amphetamine | ||||
| Cmax (ng · ml−1) | 13 ± 1.7 | 12 ± 1.4 | 4.6 ± 0.4* | 4.8 ± 0.4* |
| Tmax (hr) | 0.75 ± 0.11 | 0.78 ± 0.15 | 0.69 ± 0.15 | 0.78 ± 0.15 |
| AUC (ng · hr · ml−1) | 45 ± 4.4 | 35 ± 5.3 | 16 ± 2.3* | 14 ± 3.8* |
P < 0.05 compared with the single METH injection group within the same genotype of mice.
DISCUSSION
The present study revealed that the μ-OR knockout mice were less sensitive to METH-induced behavioral sensitization. The results clearly demonstrate that the μ-subtype of OR is implicated in the development of behavioral sensitization to the psychostimulant. METH is metabolized by cytochrome P-450 N-demethylation to amphetamine. Both METH and amphetamine undergo further metabolism by hydroxylation, deamination, and/or conjugation to nonactive metabolites (NTP-CERHR, 2005). Pharmacokinetic parameters (such as Cmax and AUC) of a drug in blood represent the total body burden of the drug. Therefore, we measured Cmax and AUC of METH and its major active metabolite amphetamine in the blood. The pharmacokinetic parameters of blood METH showed no difference between the genotypes in either single or repeated METH treatment group. Also, there were no differences in the Cmax or AUC of blood METH between single and repeated METH treatment groups in either genotype (Table I). The Cmax and AUC values of blood amphetamine were significantly lower in the repeated METH treatment group than in the single treatment group in both genotypes, which reflects a lower body burden of amphetamine following repeated administration of METH. There were no significant differences in the Cmax and AUC of blood amphetamine between wild-type and μ-OR knockout mice. Obviously, the absence of genotype difference in the pharmacokinetic parameters of METH could not account for a less sensitive behavioral response to the drug in μ-OR knockout mice than in wild-type mice. Also, the result of a lower body burden of amphetamine could not account for a higher behavioral response after repeated administration of METH in both genotypes. It must be determined whether repeated administrations of METH induce enzymes responsible for amphetamine metabolism and thus lead to the lower Cmax and AUC values of mouse blood amphetamine.
The dopamine system has traditionally been considered crucial to the ability of amphetamines to stimulate locomotor activity and stereotyped behaviors. Stimulation of dopamine activity in the NAc is responsible for amphetamine-induced locomotor activity (Iversen et al., 1975; Pijnenburg et al., 1976), whereas the nigrostriatal dopaminergic system, projecting from the substantia nigra (SN) to the neostriatum (i.e., putamen and caudate nucleus), is linked to the focused stereotyped behaviors produced by high doses of the drugs (Kelley et al., 1988). METH induced hyperlocomotor activity at low dose (0.62 mg/kg) and stereotyped behaviors at high doses (2.5 and 10 mg/kg) in wild-type mice, which may indicate the NAc is more sensitive than the striatum to the psychostimulant. The most characteristic change produced by amphetamines is an increase in extracellular dopamine level. Amphetamines not only inhibit the uptake of released dopamine in the synaptic cleft but also trigger dopamine release from the cytosol to the extracellular space by means of reverse transport through the plasma membrane dopamine transporter (Hyman et al., 2006). The exact mechanism of how the μ-opioid system modulates dopaminergic neurotransmission and thus influences METH-produced behavioral responses is unclear. Based on data in the literature, however, it can be proposed that METH-induced increase in CNS opioid peptides plays an important role in the development of behavioral sensitization to the drug. Previous studies in our laboratory and other laboratories have demonstrated that amphetamines induce an increase in expression of the opioid peptide precursor preproenkephalin mRNA in rodent brains. For example, acute and repeated administration of amphetamine induced an increase in expression of preproenkephalin mRNA in the rat striatum (Wang and McGinty, 1995). Acute administration of fenfluramine, a halogenated derivative of amphetamine, led to an increase in expression of preproenkephalin mRNA in the rat striatum (Liste et al., 2000). No behavioral sensitization to amphetamine was detected in the enkephalin knockout mice (Hodler et al., 2005). Recently, we found that there was an increase in expression of preproenkephalin mRNA in the NAc and striatum in METH-sensitized wild-type mice but not in μ-OR knockout mice (Tien et al., 2007). These results indicated that endogenous enkephalin is involved in the development of behavioral sensitization to amphetamine and METH.
The μ-ORs are widely distributed in the CNS, including the thalamus, hippocampus, striatum, NAc, amygdala, midbrain, brainstem, and spinal cord (Mansour et al., 1995). The μ-ORs are localized mainly on inhibitory γ-aminobutyric acid (GABA) interneurons that synapse on dopamine neurons. For example, the VTA and SN in the midbrain are composed of both dopamine and GABA neurons. Activation of μ-OR decreases GABA release, which consequently disinhibits dopamine neurons and increases extracellular dopamine release in the terminals (Di Chiara and Imperato, 1988). Systemic or intra-VTA administration of exogenous μ-OR agonist morphine inhibited the firing of GABAergic interneurons and increased the firing of dopamine neurons (Matthews and German, 1984; Johnson and North, 1992; Leite-Morris et al., 2004). Intra-SN administration of the enkephalinase inhibitor thiorphan, which slows the degradation rate of endogenous enkephalin, potentiated the amphetamine-induced dopamine release in the striatum (Schad et al., 2002). Enkephalin acts as an endogenous agonist and has a high affinity for μ- and δ-ORs. Thus, it is possible that endogenous opioid peptides enhance dopamine release in the terminals through activation of μ-OR on GABA neurons and then disinhibition of dopamine neurons of the VTA and SN. The METH-produced enhancement of dopamine release and behavioral response should be attenuated in μ-OR knockout mice, as observed in the present study.
In addition to dopamine release and reuptake, its metabolic enzymes are also affected by METH (Pereira et al., 2006). Dopamine is oxidized to 3,4-dihydroxyphenylacetic acid (DOPAC) by sequential action of monoamine oxidase (MAO) within cytosol after reuptake from the synaptic cleft. Catechol-O-methyltransferase (COMT) then oxidizes DOPAC to HVA. Alternatively, dopamine is methylated to 3-methoxytyramine by COMT, and then oxidized to HVA by MAO (Standaert and Galanter, 2008). METH inhibits the intraneuronal oxidative metabolism of dopamine through MAO inhibition (Fenton, 2002) and leads to a decrease in tissue concentration of DOPAC (Pereira et al., 2006). As a result, the cytosolic dopamine homeostasis would be disrupted. In a recent in vivo microdialysis study, we found that the basal values of DOPAC and HVA in striatal dialysates in wild-type mice was lower (P < 0.05) following seven consecutive daily administrations of METH (0.62 and 2.5 mg/kg). This phenomenon was not observed in μ-OR knockout mice (Lan et al., 2008). This result may suggest that dopamine metabolic enzymes in wild-type mice are more sensitive to METH than those that in the μ-OR knockout mice.
However, amphetamine-induced stereotyped behaviors are not completely the result of increased extracellular dopamine levels. Depletion of endogenous dopamine stores by pretreatment with reserpine fails to block and even enhances the amphetamine-induced behavioral responses (Callaway et al., 1989). Dopamine receptor supersensitivity following reserpine treatment is considered to be responsible for this phenomenon (Feldman et al., 1997). Multiple doses of METH have been reported to cause dopamine 2 (D2) receptor supersensitivity, which is related to the drug-induced locomotor hyperactivity and stereotyped behaviors (Ujike et al., 1990). In a previous study, we treated mice with different doses of METH for 7 consecutive days and sacrificed them on day 11 (4 days after the last injection) for neurochemical measurements. Quantitative autoradiographic analysis of striatum and NAc showed that METH treatment leads to a decrease in D1 receptor ligand binding in μ-OR knockout mice but not in wild-type mice. METH at 10 mg/kg enhances D2 receptor ligand binding in both genotypes. However, METH at doses of 0.62 and/or 2.5 mg/kg produce a decrease in D2 receptor ligand binding in μ-OR knockout mice but not in wild-type mice (Tien et al., 2007). These results indicate that a decrease in striatal and NAc D1 receptor in METH-sensitized μ-OR knockout mice is related to a decrease in the behavioral response in these animals.
Haloperidol is a D2/D1 receptor antagonist with relatively higher affinity for D2 sites. In the present study, haloperidol showed a more potent effect in counteracting METH-induced stereotyped behaviors in μ-OR knockout mice than that in wild-type controls. Because there are lower extracellular dopamine and D1 receptor levels in μ-OR knockout mice after repeated administrations of METH, a higher dopamine receptor occupancy by haloperidol in the brains of μ-OR knockout mice would be expected, which may explain a more potent efficacy of the antagonist in counteracting METH-induced stereotyped behaviors in these mice.
In summary, the present results clearly define participation of the μ-OR in development of behavioral sensitization to METH. Lacking μ-OR does not alter the overall disposition of METH in the mouse. Further clarification of how endogenous opioid peptides and dopamine enzymes, transporters, and receptors are involved in the development of METH sensitization will be needed.
ACKNOWLEDGMENTS
The authors thank Mrs. Helen J. Mitchell for her technique assistance in mouse behavioral test. We also thank Dr. David B. Couch and Mrs. Marsha Manuel for their careful proofreading of the manuscript.
Contract grant sponsor: Center for Psychiatric Neuroscience at the University of Mississippi Medical Center, supported by the NIH; Contract grant number: P20 RR0l7701 (to T.M., subproject PI).
REFERENCES
- Bartlett E, Hallin A, Chapman B, Angrist B. Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability? Neuropsychopharmacology. 1997;16:77–82. doi: 10.1016/S0893-133X(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Kuczenski R, Segal DS. Reserpine enhances amphetamine stereotypies without increasing amphetamine-induced changes in striatal dialysate dopamine. Brain Res. 1989;505:83–90. doi: 10.1016/0006-8993(89)90118-2. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67:100–109. doi: 10.1016/j.brainresbull.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Olley JE. The substantia nigra and stereotyped behaviour. Eur J Pharmacol. 1972;18:95–106. doi: 10.1016/0014-2999(72)90136-7. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Wise RA. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. Eur J Pharmacol. 1993;243:55–64. doi: 10.1016/0014-2999(93)90167-g. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Feigenbaum JJ, Howard SG. Effects of naloxone on amphetamine induced striatal dopamine release in vivo: a microdialysis study. Life Sci. 1997;60:1659–1668. doi: 10.1016/s0024-3205(97)00108-2. [DOI] [PubMed] [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of neuropsychopharmacology. Sinauer Associates Inc.; Sunderland, MA: 1997. pp. 549–590. [Google Scholar]
- Fenton JJ. Drug of abuse. In: Fenton JJ, editor. Toxicology: a case-oriented approach. CRC Press; Boca Raton, FL: 2002. pp. 359–402. [Google Scholar]
- Hodler C, Paquet B, Gilbert F, Drolet G, Levesque D, Rouillard C. Role of endogenous enkephalin and dynorphin in amphetamine-induced behavioral sensitization. Society of Neuroscience 35th Annual Meeting; Washington, DC. 2005. abstract 1029.7. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Behavioral consequences of methamphetamine-induced neurotoxicity in mice: relevance to the psychopathology of methamphetamine addiction. Ann N Y Acad Sci. 2002;965:127–135. doi: 10.1111/j.1749-6632.2002.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Kelly PH, Miller RJ, Seviour P. Proceedings: amphetamine and apomorphine responses in the rat after lesion of mesolimbic or striatal dopamine neurones. Br J Pharmacol. 1975;54:244. [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology. 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, Roeske WR, Yamamura HI. Molecular biology and pharmacology of cloned opioid receptors. FASEB J. 1995;9:516–525. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- Lan KC, Ma T, Lin-Shiau SY, Liu SH, Ho IK. Methamphetamine-elicited alterations of dopamine- and serotonin-metabolite levels within mu-opioid receptor knockout mice: a microdialysis study. J Biomed Sci. 2008;15:391–403. doi: 10.1007/s11373-007-9218-7. [DOI] [PubMed] [Google Scholar]
- Leite-Morris KA, Fukudome EY, Shoeb MH, Kaplan GB. GABAB receptor activation in the ventral tegmental area inhibits the acquisition and expression of opiate-induced motor sensitization. J Pharmacol Exp Ther. 2004;308:667–678. doi: 10.1124/jpet.103.058412. [DOI] [PubMed] [Google Scholar]
- Liste I, Munoz A, Guerra MJ, Labandeira-Garcia JL. Fenfluramine-induced Increase in preproenkephalin mRNA levels in the striatum: interaction between the serotonergic, glutamatergic, and dopaminergic systems. Synapse. 2000;35:182–191. doi: 10.1002/(SICI)1098-2396(20000301)35:3<182::AID-SYN3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. Mu opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- NTP-CERHR . Monograph on the potential human reproductive and developmental effects of amphetamines. Bethesda, MD: 2005. NIH Publication No. 05–4474. [PubMed] [Google Scholar]
- Pereira FC, Lourenξ̧o ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR, Ali SF. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–193. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad CA, Justice JB, Jr, Holtzman SG. Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther. 2002;300:932–938. doi: 10.1124/jpet.300.3.932. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J Neurosci. 1992;12:1335–1350. doi: 10.1523/JNEUROSCI.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Galanter JM. Pharmacology of dopaminergic neurotransmission. In: Gola DE, Tashjian AH Jr, Armstrong EJ, Amstrong AW, editors. Principles of pharmacology—the pathophysiologic basis of drug therapy. 2nd ed. Lippincott Williams and Wilkins; Philadelphia: 2008. pp. 185–205. [Google Scholar]
- Tien L-T, Ho IK, Loh HH, Ma T. Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res. 2007;85:673–680. doi: 10.1002/jnr.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology. 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat fore-brain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]