Abstract
Immune activation of fibrosis likely plays a crucial role in the pathogenesis of systemic sclerosis (SSc). The goal of this study was to better understand innate immune regulation and associated IFN- and TGFβ-responsive gene expression in SSc skin and dermal fibroblasts, in particular the effect of different Toll-like receptor (TLR) ligands. To better understand the relationship between inflammation and fibrosis in vivo we developed a murine model for chronic innate immune stimulation. We found that expression of both IFN- and TGFβ-responsive genes are increased in SSc skin and in SSc fibroblasts when stimulated by TLR ligands. In contrast, cutaneous lupus skin showed much more highly upregulated IFN-responsive and much less highly upregulated TGFβ-responsive gene expression. The TLR3 ligand, Poly(I:C), mostly highly increased fibroblast expression of both IFN- and TGFβ-responsive genes as well as TLR3. Chronic subcutaneous immune stimulation by Poly(I:C) stimulated inflammation, and IFN- and TGFβ-responsive gene expression. However, in this model type I IFNs played no apparent role regulating TGFβ activity in the skin. These results suggest that TLR agonists may be important stimuli of dermal fibrosis, potentially mediated by TLR3 or other innate immune receptors.
INTRODUCTION
Systemic sclerosis (SSc) has interrelated pathogenic features involving immune activation and fibrosis. Transforming growth factor-β (TGFβ) has been strongly implicated in SSc-associated fibrosis: it potently induces collagen and collagen processing, it transforms fibroblasts into profibrotic myofibroblasts and it regulates genes key to pathologic fibrosis (Jelaska and Korn, 2000; Kissin et al., 2006). Perivascular and deep dermal inflammatory cell infiltrates are also features of SSc skin. Autoantibodies and elevated circulating immune mediators further indicate immune activation.
IL-13 and TGFβ have been most strongly implicated in linking inflammation and fibrosis. IL-13 contributes to fibrosis in bleomycin-induced ILD and induces fibrosis itself in IL-13 transgenic mice (Belperio et al., 2002). Mice overexpressing IL-13 develop ILD, dependent on enhanced MMP9 activation of TGFβ (Lee et al., 2001). TGFβ, when inducibly expressed in the lung in its active form, induces inflammation and fibrosis (Lee et al., 2004). Less is known about the relationship between inflammation and fibrosis of skin. Dermal fibrosis induced by subcutaneous injection of bleomycin, and in the chronic graft versus host disease murine models of SSc is dependant on TGFβ (Yamamoto et al., 1999; Zhang et al., 2002; Zhang et al., 2003), and bleomycin-induced fibrosis at least partially dependent on IL-13 (Aliprantis et al., 2007; Seki et al., 2007). Although these models have shown that inflammatory changes may underlie fibrosis, and have many useful features that overlap pathological, immune and fibrotic feature of SSc skin, they have not proven readily tractable for examining the role of innate immunity in mediating skin fibrosis.
Toll-like receptors (TLRs) have emerged as commonly used sensors for innate immune activation through recognition of common molecular patterns found on microbes (Akira et al., 2006). Non-immune cells can also respond to inflammatory stimuli via TLRs, including epithelial cells, fibroblasts, endothelial cells and adipocytes (Faure et al., 2000; Guillot et al., 2004; Kollisch et al., 2005; Morris et al., 2006). TLR activation triggers production and secretion of several inflammatory cytokines, notably type I interferons (IFNs) implicated in the pathogenesis of autoimmune diseases, including systemic lupus erythematosus (SLE), a disease with overlapping autoantibody specificities and sometimes overlapping clinical manifestations with SSc (Crow et al., 2003; Hua et al., 2006; van der Pouw Kraan et al., 2007). In SLE, activation of TLR7/8 and TLR9 on dendritic cells through nucleic acid-containing immune-complexes stimulates type I IFNs. Increased IFN “signature” gene expression by peripheral blood mononuclear cells (PBMCs) and in the skin by SSc patients (York et al., 2007), along with evidence suggesting TLR activity in SSc sera supports the notion that TLR activation may initiate the immune response in SSc.
The pivotal role of fibroblasts in fibrosis makes this cell type of particular importance in SSc, yet little is know about the potential importance of TLR activation on SSc dermal fibroblasts. We show here that chronic innate immune stimulation by the TLR3 agonist, Poly(I:C), activates dermal fibrosis in vitro and in vivo, increasing both type I and type II IFNs, as well as TGFβ.
RESULTS
IFN-responsive genes show increased expression in skin from SSc patients
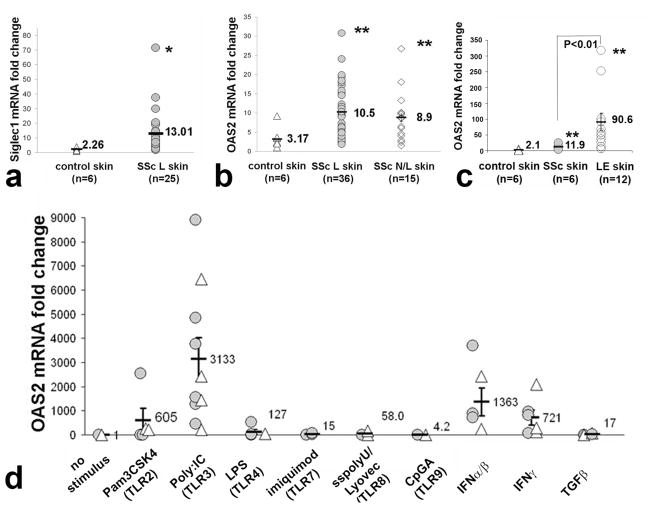
In order to explore the IFN response in SSc skin, we analyzed skin mRNA levels of SIG1, OAS2 and MX2, three genes typically upregulated by type I IFN (York et al., 2007). Expression of SIG1 in lesional skin from patients with SSc was higher than expression from healthy control skin (Fig. 1a; 5.75-fold increase, p<0.05). As with SIG1, OAS2 and MX2 also showed increased expression in lesional skin from SSc patients (Fig. 1b; OAS2 3.31-fold increase, p<0.001; MX2 data not shown). Consistent with previous studies by our group, OAS2 also showed increased expression in non-lesional skin from SSc patients compared to healthy control skin (Farina et al., 2010; Kissin et al., 2006; Milano et al., 2008); Fig. 1b; 2.80-fold increase, p<0.001). Strikingly, skin from patients with lupus erythematosus (LE) show even higher OAS2 expression than SSc (Fig. 1c).
Figure 1. Type I IFN-responsive genes: expression in skin from patients with dcSSc and dermal fibroblast induction by specific TLR ligands.
(a and b) mRNA expression of Siglec1 (n=25) and OAS2 in lesional (n=36, SSc L) and non-lesional (n=15, SSc N/L) dcSSc and control skin (n=6). The average fold change in SSc L skin was increased for Siglec1 (5.75-fold increase) and OAS2 (3.17-fold increase). The fold change of OAS2 in SSc N/L skin (n=15) was also increased (2.8-fold increase), * p<0.05; ** p<0.01. (c) mRNA expression of OAS2 (n=6) in SSc (n=6), LE (n=12) and control skin (n=6). OAS2 in LE skin was higher than control skin (43.1-fold increase) and also higher than SSc lesional skin (7.6-fold increase, ** p<0.01). (d) mRNA induction of OAS2 in normal (△) and in SSc (
 ) dermal fibroblasts by TLR ligands: Pam3CSK4 (1μg/ml), Poly(I:C) (2.5μg/ml), LPS (10μg/ml), imiquimod (5μg/ml); sspolyU/Lyovec (100μg/ml) or CpG (5mM); IFN α and β, IFNγ (each at 250 U/ml); or TGFβ (5 ng/ml). TLR2 (p<0.01) and TLR3 ligands (p<0.0001), and IFN α/β (p<0.001) and IFNγ (p<0.01) significantly increased expression of OAS2 in SSc and normal fibroblasts.
) dermal fibroblasts by TLR ligands: Pam3CSK4 (1μg/ml), Poly(I:C) (2.5μg/ml), LPS (10μg/ml), imiquimod (5μg/ml); sspolyU/Lyovec (100μg/ml) or CpG (5mM); IFN α and β, IFNγ (each at 250 U/ml); or TGFβ (5 ng/ml). TLR2 (p<0.01) and TLR3 ligands (p<0.0001), and IFN α/β (p<0.001) and IFNγ (p<0.01) significantly increased expression of OAS2 in SSc and normal fibroblasts.
TLR agonists stimulated IFN-regulated genes in fibroblasts from SSc patient and healthy control skin
To characterize dermal fibroblast innate immune responses in SSc, we tested the effects of TLR ligands on OAS2 expression by dermal fibroblasts from six SSc and four healthy donors. Several TLR agonists stimulated IFN-regulated gene expression by dermal fibroblasts (Fig. 1d). The TLR3 ligand, PolyI(I:C), strongly induced OAS2 mRNA expression by both SSc and control fibroblasts (average 3,133-fold increase compared to control, untreated, p<0.00001). Although we noted significant differences between responses by individual fibroblast cell cultures, there were no consistent differences in Poly(I:C) responses between fibroblasts from SSc compared to control fibroblasts (Fig.1d). TLR2 and TLR4 ligands also induced expression of OAS2 in both SSc and normal cell lines (Fig. 1d, 605-fold and 127-fold increase, respectively, p<0.001).
Increased IFNγ-regulated chemokine genes in SSc skin
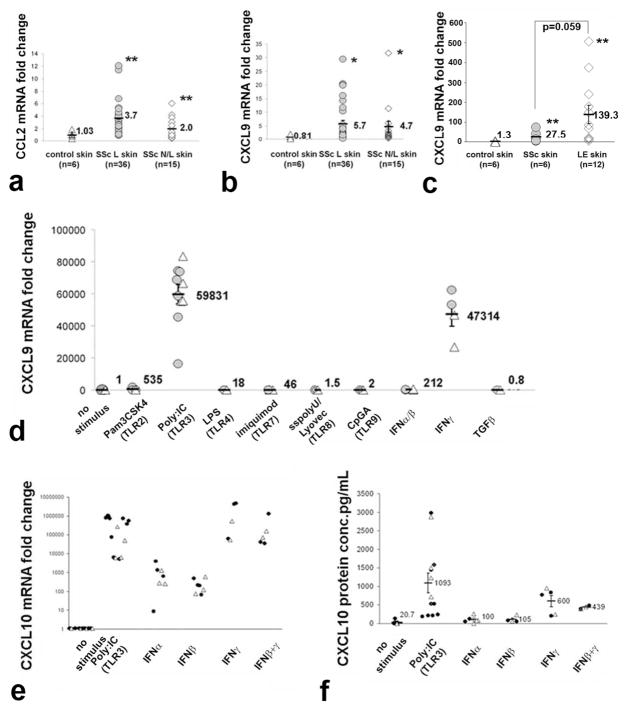
Genes regulated by type I and type II IFNs largely overlap. Secretion of MCP1/CCL2, a potent chemokine for inflammatory cell migration, is synergistically upregulated by TLR and IFNγ stimulation (Yamana et al., 2009; Yoshimura and Takahashi, 2007), and upregulated in SSc skin in both mononuclear cells and dermal fibroblasts (Distler et al., 2001; Galindo et al., 2001). MCP1/CCL2 expression was significantly higher in lesional and non-lesional SSc skin compared to healthy control skin (Fig. 2a, 3.6-fold increase, p<0.01, and 1.94-fold increase p<0.01, respectively). CXCL9, also known as monokine induced by IFNγ, is selectively upregulated by type II IFN relative to type I IFNs (Sanda et al., 2006). CXCL9 expression was also higher in lesional and non-lesional SSc skin compared to healthy controls (Fig. 2b; 7.03-fold increase, p<0.05 and 5.8-fold increase, p<0.05, respectively). CXCL9 was even more strikingly increased in LE skin compared to SSc skin (Fig. 2c; 106.9-fold compared to 21.1, p=0.05).
Figure 2. Type II IFN-responsive genes: expression in skin from patients with dcSSc and dermal fibroblast induction by specific TLR ligands.
(a and b) Increased mRNA expression of type II IFN-regulated genes, CCL2 (3.5-fold increase, ** p<0.01) and CXCL9 (7.03-fold increase,*, p<0.05) in SSc L, and SSc N/L (1.95 and 5.8-fold increase, * p<0.05; ** p<0.01, respectively) compared to control skin. (c) mRNA induction of CXCL9 in normal (△) and in SSc (
 ) dermal fibroblasts by TLR ligands (as in Fig. 1), IFN α/β, IFNγ and TGFβ. TLR2 (p<0.01) and TLR3 ligand (p<0.001), and IFN α/β (p<0.01) and IFNγ (p<0.001) induced CXCL9 in SSc and in NL fibroblasts compare to unstimulated cell lines. (d and e) mRNA (panel d) and protein (panel e) induction of CXCL10 in normal (△) and in SSc (●) dermal fibroblasts by Poly(I:C) (p<0.001), IFNα (p<0.01), IFNβ (p<0.01), IFNγ (p<0.001) or the combination of IFNβ and IFNγ (p<0.001)..
) dermal fibroblasts by TLR ligands (as in Fig. 1), IFN α/β, IFNγ and TGFβ. TLR2 (p<0.01) and TLR3 ligand (p<0.001), and IFN α/β (p<0.01) and IFNγ (p<0.001) induced CXCL9 in SSc and in NL fibroblasts compare to unstimulated cell lines. (d and e) mRNA (panel d) and protein (panel e) induction of CXCL10 in normal (△) and in SSc (●) dermal fibroblasts by Poly(I:C) (p<0.001), IFNα (p<0.01), IFNβ (p<0.01), IFNγ (p<0.001) or the combination of IFNβ and IFNγ (p<0.001)..
TLRs regulate chemokine genes in dermal fibroblasts from SSc skin
To further investigate the potential role of innate immune activation on SSc fibroblasts, we tested the effects of TLR ligands on fibroblast expression of CXCL9. The TLR3 ligand, Poly(I:C), strikingly upregulated CXCL9 expression by dermal fibroblasts derived from SSc and healthy control skin (Fig. 2d, average 59,831-fold increase, p<0.00001). TLR2 activation also modestly induced CXCL9 (average 535-fold increase<0.001). Other TLR ligands had little effect on CXCL9 expression. In contrast to OAS2 expression, which showed higher expression upon treatment with type I IFNs than IFNγ (see Fig. 1c), CXCL9 showed markedly higher expression upon treatment with IFNγ than type I IFNs (Fig. 2d, 47,314-fold compared to 212-fold increase). Thus, Poly(I:C) stimulates fibroblast expression of genes regulated by both type I and type II IFNs.
Poly(I:C) also markedly induced CXCL10 (also know as IFNγ-inducible protein 10) mRNA expression (Fig. 2e, average 35,2339 ± 10,0459-fold increase). IFNγ, or IFNγ in combination with IFNβ strongly induced CXCL10. Type I IFNs, IFNα or IFNβ, induced significant but far less (10–100,000-fold less) CXCL10 expression than IFNγ, confirming that this chemokine is selectively upregulated by IFNγ. CXCL10 secretion paralleled mRNA expression, with average CXCL10 secretion strongly induced in Poly(I:C)-treated fibroblasts (Fig. 2f, average 1,093 pg/ml compared to control 20.7 pg/ml). Type I IFNs had relatively little effect on CXCL10 secretion, whereas IFNγ or IFNβ+IFNγ treatment strongly stimulated CXCL10 secretion.
IFN type I and type II secretion in TLR3 ligand activation of IFN-responsive genes in mouse dermal fibroblasts
The TLR3 ligand, Poly(I:C), induces IFNβ secretion by many cell types, such as fibroblasts, epithelial cells and dendritic cells. Recently TLR3 activation has also been shown to stimulate IFNγ secretion (Kleinman et al., 2008; Negishi et al., 2008). To clarify the relative roles of type I and II IFN secretion on Poly(I:C)-induced upregulation of IFN-responsive genes, we utilized mouse fibroblasts obtained from skin of WT and type I IFN receptor (IFNAR)-deleted mice. Poly(I:C), IFNβ and IFNγ significantly upregulated MX2 expression in WT fibroblasts (Fig. S1); MX2 expression was induced more by IFNβ than IFNγ. Poly(I:C) and IFNβ failed to induce MX2 expression in dermal fibroblasts deleted of IFNAR, although IFNγ slightly increased MX2 expression in IFNAR−/− fibroblasts.
In contrast to MX2, IFNγ stimulated CXCL9 expression as strongly as IFNβ in WT murine fibroblasts (Fig. S1). IFNAR-deletion blocked IFNβ, but not IFNγ, and partially blocked Poly(I:C) stimulation of CXCL9. Thus Poly(I:C) induction in CXCL9 is only partially mediated by type I IFN, IFNγ possibly mediating part of the effect of Poly(I:C) on CXCL9.
Upregulated TGFβ-responsive genes in SSc and LE skin
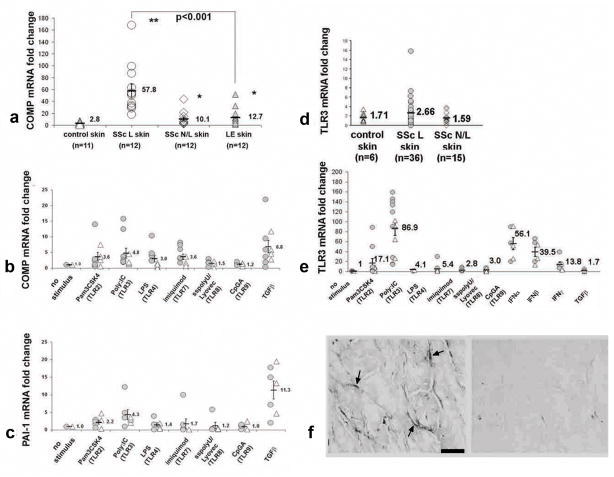
We have shown that COMP expression is upregulated in SSc skin (Farina et al., 2009). To further compare TLR targets in SSc and LE, we assessed COMP mRNA in skin. SSc skin showed markedly upregulated COMP (Fig. 3a, 20.6-fold), while LE skin showed only modest upregulation and significantly less expression of COMP compared to SSc skin (3.8-fold, p<0.001).
Figure 3. Induction of TGFβ-responsive genes by TLR ligands in SSc and normal dermal fibroblasts.
(a) Increased COMP mRNA expression in dcSSc and LE skin compared to control skin. (b and c) COMP and PAI-1 mRNA expression by dermal fibroblasts from dcSSc (
 ) and control (△) subjects treated with TLR agonists (as in Fig. 1), IFN α/β, IFNγ or TGFβ. (b and c) COMP and PAI-1 expression show significant induction with agonists of TLR2 (p<0.05), TLR3/Poly(I:C) (p<0.05), and TGFβ, (p<0.001). (d) mRNA expression of TLR3 mRNA expression in lesional (SSc L; 1.5-fold increase, p=ns), in non-lesional skin (SSc N/L; 0.92-fold increase, p=ns) from dcSSc patients compared to control skin (n=6). (e) Increased TLR3 mRNA expression by dermal fibroblasts from control (△) and dcSSc (
) and control (△) subjects treated with TLR agonists (as in Fig. 1), IFN α/β, IFNγ or TGFβ. (b and c) COMP and PAI-1 expression show significant induction with agonists of TLR2 (p<0.05), TLR3/Poly(I:C) (p<0.05), and TGFβ, (p<0.001). (d) mRNA expression of TLR3 mRNA expression in lesional (SSc L; 1.5-fold increase, p=ns), in non-lesional skin (SSc N/L; 0.92-fold increase, p=ns) from dcSSc patients compared to control skin (n=6). (e) Increased TLR3 mRNA expression by dermal fibroblasts from control (△) and dcSSc (
 ) subjects cultured as in Fig. 2: TLR2 agonist (17.1 fold-increase, p<0.05); TLR3 agonist (86.9 fold-increase, p<0.00001); IFNα (56.1 fold-increase, p<0.00001); IFNβ (39.5 fold-increase, p<0.00001); and IFNγ (13.8 fold-increase, p<0.01). (f) Immunohistochemical staining with anti-TLR3 (left panel) and control Ig (right panel) in dcSSc skin.
) subjects cultured as in Fig. 2: TLR2 agonist (17.1 fold-increase, p<0.05); TLR3 agonist (86.9 fold-increase, p<0.00001); IFNα (56.1 fold-increase, p<0.00001); IFNβ (39.5 fold-increase, p<0.00001); and IFNγ (13.8 fold-increase, p<0.01). (f) Immunohistochemical staining with anti-TLR3 (left panel) and control Ig (right panel) in dcSSc skin.
TLR ligands regulate TGF-β responsive genes in dermal fibroblasts
To test whether TLR activation might control TGFβ-responsive genes expression in SSc dermal fibroblasts, COMP and PAI-1, two TGF-β inducible genes, were assessed after TLR stimulation. Several TLR ligands, most strongly Poly(I:C), induced COMP and PAI-1 mRNA expression in SSc and control skin fibroblasts (Fig. 3b and c, average increase of 4.8-fold, p<0.01; and 4.3-fold, p<0.05, respectively). To show that the effects of poly(I:C) are specific to this double-stranded RNA ligand, polyI and polyC were also tested, showing no effect on TGFβ- or IFN-responsive gene expression (Fig. S2). To clarify that TGFβ mediates the effect of Poly(I:C) on COMP and PAI-1 expression, we blocked TGFβ in poly(I:C)-treated fibroblasts. Neutralizing anti-TGFβ antibody inhibited Poly(I:C) induced COMP and PAI-1 expression (65% and 56%, respectively), but not IFN-responsive genes, MX2 and CXCL9 (Fig. S4), similar to its effect on poly(I:C)-induced smooth muscle actin on lung fibroblasts (Sugiura et al., 2009).
TLR3 in skin and regulation by TLR ligands in dermal fibroblasts
Since Poly(I:C) highly upregulated several IFN-responsive genes in fibroblasts, we assessed expression of the Poly(I:C) ligand, TLR3 in SSc skin. TLR3 mRNA was detectable by RT-PCR in both lesional and non-lesional SSc skin. Average TLR3 expression in SSc lesional skin trended slightly higher than in control skin, but this difference was not statistically significant (Fig. 3d, average fold-change of 1.55 and 0.93, for lesional and non-lesional skin, respectively, compared to normal skin. p=ns). TLR3 was also detectable by immunohistochemistry, in skin from SSc patients (Fig. 3f) and control skin (not shown) at similar levels. TLR3 staining was seen in spindle shaped cells in the dermis consistent with dermal fibroblasts.
TLR3 ligand induces TLR3 expression in macrophages through autocrine upregulation of IFNβ (Doyle et al., 2003; Miettinen et al., 2001). Poly(I:C) induced TLR3 expression in both SSc and normal fibroblasts (Fig. 3e, average 86.9-fold induction). Other TLR ligands also stimulated TLR3 expression, including TLR2 and TLR7 ligands (average 17.1-fold increase, p<0.05, and 5.4-fold increase, p<0.001, respectively). IFNα, IFNβ and, more modestly, IFNγ, also induced TLR3 mRNA expression (p<0.01).
TLR3 induces inflammation and fibrosis in skin
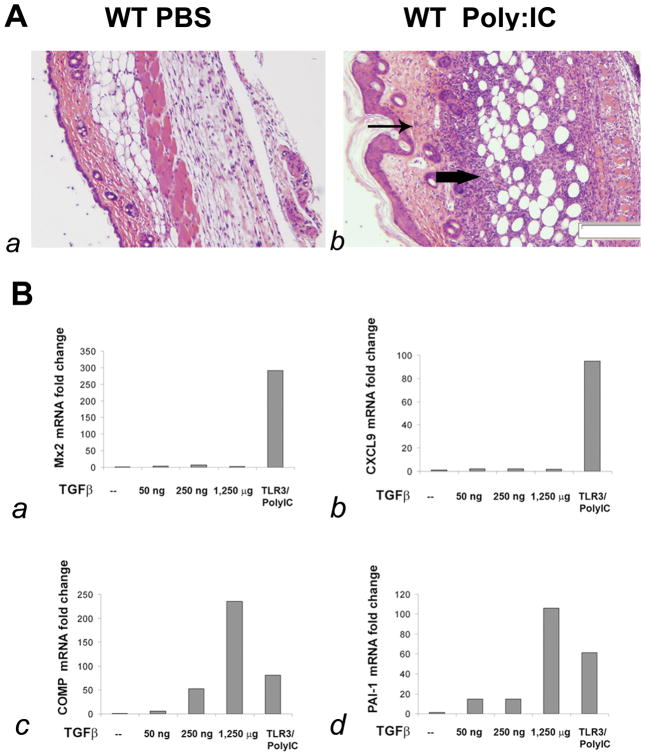
To more clearly define the effect of TLR3 ligand on dermal fibrosis in vivo, we tested the effect of continuous stimulation with Poly(I:C) for one week by subcutaneous osmotic pump. Skin histology showed striking inflammation in the deep dermis and fat (Fig. 4A, b), accompanied by increased dermal matrix and epidermal hyperplasia. Intradermal muscle myofibers were markedly fewer in number and some fibers had central nuclei suggesting regenerating fibers. Skin from mice treated with Poly(I:C) showed dramatically increased expression of genes typically regulated by type I and type II IFNs: MX2 and CXCL9, respectively (Fig. 4B, a and b; MX2 average fold-change 48.19 ± 15.11; CXCL9 average fold-change 85.12 ± 24.13). To show that the effects of poly(I:C) are specific to this double-stranded RNA ligand, polyI and polyC were also tested in mice, showing no effect on IFN-responsive gene expression (Fig. S3). To extend these observations, mice were treated with subcutaneous Poly(I:C) for 28-days. Skin from these mice showed less inflammation, increased fibrosis and the development of myofibroblasts, not seen in 7-day treated skin (Fig. S5).
Figure 4. TLR3 ligand, Poly(I:C), causes inflammation and fibrosis in mouse skin.
(A) H&E stained cross section of skin from a C57BI6 mouse one week after subcutaneous pump injection of poly(I:C) (A, b) or PBS (A, a). Arrows indicates inflammatory infiltrates (heavy arrow) and dermal fibrosis (light arow). Magnification 40X. (B) Expression of the IFN-responsive genes: MX2 (B, a) and CXCL9 (B, b) or TGFβ-responsive genes COMP (B, c) and PAI-1 (B, d) by RT-PCR of skin RNA from control or Poly(I:C) treated mice compared to mice injected by subcutaneous pump with increasing doses of TGFβ. Fold-change shown on the graph is normalized to mRNA expression by control PBS treated mouse.
Dermal remodeling induced by Poly(I:C)
To assess the link between TLR agonists and matrix remodeling in SSc, we tested expression of TGFβ-responsive genes in 7-day Poly(I:C) treated mice and compared this to mice treated with three concentrations of TGFβ. We found COMP and PAI1 mRNAs strikingly increased in the skin of TGFβ-treated and also Poly(I:C) treated mice (Fig. 4B, c and d, Poly(I:C) treated mice COMP average 81.01-fold increase, PAI-1 average 61.18-fold increase).
MX2 regulation by type I IFN secretion in Poly(I:C) pump treated mice
To clarify the role of type I IFNs in Poly(I:C)-induced gene expression, Poly(I:C) containing pumps were placed in wild type (WT, C57Bl/6) and interferon receptor I-deleted (B6IFNAR −/−) mice. Poly(I:C) induced expression of MX2 was completely blocked in IFNAR−/− mice (Fig. 5a). In contrast, IFNAR deletion only partially inhibited Poly(I:C) induced CXCL9, a type II selective IFN-responsive gene (Fig. 5b), and had no effect on expression of the TGFβ-responsive genes, PAI-1 and TSP-1 (Fig. 5c and d), suggesting that type I IFNs do not regulate TGFβ activation in Poly(I:C) treated skin.
Figure 5. Effect of IFN or TRIF/TICAM deletion, or TGFβ inhibition on Poly(I:C) induction of IFN- and TGFβ-responsive genes.
Expression of IFN-responsive genes: MX2 (a) and CXCL9 (b), and TGFβ responsive genes PAI-1 (c) and TSP1 (d) by RT-PCR analysis of skin mRNA from C57BI/6 WT (n=10), C57BI/6/IFNAR−/− (n=8) and C57BI/6 TICAM−/− (n=10) mice one week after Poly(I:C) pump insertion; * p<0.05; ** p<0.01. Expression of IFN-responsive genes: MX2 (e) and CXCL9 (f) and TGFβ-responsive genes: PAI-1 (g) and TSP1 (h) mRNA from untreated C57BI/6 WT (n=3) mice one week after PBS pump insertion, or mice treated with TGFβ neutralizing antibody (n=3), one week after Poly(I:C) pump insertion. Fold-changes shown on the graph are normalized to mRNA expression by control mice. Results presented are means ± SE and are representative of three independent experiments. *p<0.05; ** p<0.01.
Deletion of TRIF/TICAM1 partially blocks Poly(I:C) induction of IFN- and TGFβ-responsive genes
Poly(I:C) stimulates innate immune activation through both TLR3 and RIG1/MDA5 double-stranded RNA sensors (Takeuchi and Akira, 2009). To clarify the role of TLR3 in altered gene expression in Poly(I:C) treated mice, WT and TICAM1−/− mice were treated with Poly(I:C) by osmotic pumps as above. TICAM deletion reduced expression of both IFN-and TGFβ-responsive genes in Poly(I:C) treated mice (Fig. 5a–d), confirming that TLR3 is partially, though not completely responsible for mediating the effect of Poly:(I:C) on dermal inflammation and fibrosis, as TICAM −/− mice continued to show significantly increased expression of these genes, suggesting that RIG-I/MDA5 dsRNA sensors might also contribute to increase IFN and TGFβ-responsive gene expression.
TGF-β activation in Poly(I:C) pump treated mice
To confirm that Poly(I:C) augments TGF-β activity in vivo, Poly(I:C) pump treated mice were additionally treated with or without anti-TGF-β antibody. Poly(I:C)-induced PAI-1 and TSP-1 expression were partially blocked in mice receiving neutralizing anti-TGF-β antibody (Fig. 5g and h), indicating that Poly:(I:C)/TLR3 induction modulates PAI-1 and TSP-1 gene expression at least in part through TGF-β. Although anti-TGF-β treatment did not show any significant effect on the type I IFN-responsive gene MX2, it also modestly reduced Poly(I:C) induced CXCL9 expression (Fig. 5e and f).
DISCUSSION
We show that IFN-responsive genes are increased in SSc skin and that TLR agonists, particularly TLR3 agonist Poly(I:C), highly upregulate IFN- and TGFβ-responsive genes expressed by dermal fibroblasts. Moreover, we show in vivo that mice exposed to chronic stimulation with dsRNA/Poly(I:C) develop dermal inflammation and skin remodeling similar to SSc skin, including TGF-β dependent upregulation of TGF-β responsive genes. IFNAR-deletion in vitro and in vivo blocked Poly(I:C)-induced expression of MX2, a type I IFN-responsive gene, and partially blocked expression of CXCL9, a gene selectively regulated by type II IFN, but did not alter Poly(I:C)-induced TGFβ-responsive gene expression. Poly(I:C)-stimulated IFN- and TGFβ-responsive gene expression in vitro and in vivo was partially dependent on TICAM indicating that TLR3 was at least partially responsible for upregulating these signals.
Emerging evidence points to the importance of TLR activation in structural cells, in particular fibroblasts, because they regulate inflammatory signals, tissue regeneration and fibrosis (Kluwe et al., 2009). Several observations support a role for TLR stimulation of fibroblasts in promoting inflammatory fibrogenic responses (Meneghin and Hogaboam, 2007; Pierer et al., 2004; Proost et al., 2004). TLR4 activation in hepatic stellate cells sensitizes these cells to TGFβ, promoting TGFβ-dependent activation and collagen production (Seki et al., 2007). One recent study has postulated that TLR4 mediates fibroblast inducing chemokine production in response to anti-fibroblast antibodies described in SSc serum (Fineschi et al., 2008).
Our in vitro data show that SSc, as well as healthy, dermal fibroblasts express several functional TLRs, showing the ability to respond to innate immune stimuli mainly through TLR3, and to a lesser extent TLR2 and TLR4. DsRNA/Poly(I:C) provided the most powerful stimulus, strongly upregulating type I and type II selective IFN-responsive genes: OAS2, and CXCL9 and CXCL10, respectively. Experiments utilizing IFNAR-deleted fibroblasts suggested that Poly(I:C)-TLR3 activation induced both type I and II IFN secretion by dermal fibroblasts. Although TLR3 ligands are well known to stimulate type I IFNs, new evidence suggests that TLR3 ligands can also induce IFNγ as mice deleted in TLR3 receptors are not able to produce IFNγ and develop more severe viral infections (Negishi et al., 2008). Poly(I:C)-TLR3 activation also stimulated TGFβ-dependent expression of two well known TGFβ inducible genes, PAI-1 and COMP, by both SSc and normal fibroblasts. These data indicate important roles of dermal fibroblasts in responding directly to immune stimuli and the potential for dysregulation of these pathways to lead to dermal inflammation and fibrosis.
Chronic in vivo administration of Poly(I:C) strongly supported in vitro results, showing highly upregulated IFN- and TGFβ-responsive gene expression. As for fibroblasts in vitro, IFNAR deletion blocked expression of the type I selective IFN-responsive gene, MX2, but only partially blocked increased expression of the type II selective IFN-responsive gene CXCL9, suggesting that Poly(I:C) stimulates IFNγ, as well as type I IFNs in skin. We show both type I (OAS2, Siglec-1) and type II (CXCL9) selective IFN-responsive genes are upregulated in SSc skin, again suggesting parallels between this model and SSc skin. Notably, IFNγ and in some reports, type I IFNs downregulate TGFβ signaling and have been previously tested as possible therapeutics in several open label trials (Varga, 1997). Although not controlled experiences, in some cases these trials suggested that treatment with IFN actually aggravates SSc disease activity (Black et al., 1999). Our data presented here, as well as our previous data showing increased IFN-responsive gene expression by SSc PBMCs, in the context of the more completely defined pathogenic roles of TLR7/8 and TLR9 in the pathogenesis of SLE support this notion, suggesting that IFNs might play pathogenic roles in SSc.
Our data do not exclude important contributions by other inflammatory cells resident or recruited to the skin. Indeed, chronic Poly(I:C) stimulation leads to a marked mononuclear cell accumulation and in SSc, resident macrophages appear to be important targets of IFN, showing increased Siglec-1 expression in SSc skin. Our data also do not examine protein expression or directly address the functional roles of OAS2, Siglec1, MX2, CXCL9, CXCL10, PAI1 or COMP in pathogenesis.
Despite the effects of IFNAR deletion on IFN-regulated gene expression, no effect was seen on TGFβ-regulated gene expression. Since TGFβ-responsive gene expression was partially inhibited by blocking TGFβ, these results suggest that TLR3 activation stimulates TGFβ independent of its effects on IFNs. The intracellular pathway for this effect is not known, but Poly(I:C) might act either by increasing latent TGFβ production or by affecting TGFβ activation. A recent study has shown that Poly(I:C) affects myofibroblast differentiation in human lung fibroblasts through an NF-kB dependent pathway (Sugiura et al., 2009). Although our Poly(I:C) model results cannot be directly extrapolated to SSc, they raise the possibility that IFN inhibition in SSc, currently in early phases of investigation, might not block fibrotic aspects of the disease.
Our in vivo model shows that chronic TLR3 stimulation can induce fibrosis, but do not exclude possible roles for other TLR agonists or innate immunity modulators in dermal fibrosis. Synthetic dsRNA/Poly(I:C), as well as viral dsRNA can be recognized by other innate immune sensors, including RIG-I, MDA5 and PKR. Notably, deletion of TICAM/TRIF, a required signaling molecule for TLR3, only partially blocked upregulated IFN- and TGFβ-responsive genes in Poly(I:C) treated mice. These results suggest that Poly(I:C) in this model is acting in part through such non-TLR3 dependent receptors and that these receptors lead to similar alterations in gene expression. Additional candidates for TLR activation in SSc include autoantibody-nucleic acids complexes discussed above and matrix molecules shown to be TLR ligands, such as hyluronan, a ligand for TLR2 and TLR4 (Jiang et al., 2005). Intriguingly, IFN-responsive genes are upregulated in SSc, but more highly in LE skin, where they are associated with pDC activation (Meller et al., 2005). In contrast, TGFβ-responsive gene COMP, which is also stimulated upon TLR activation, is more highly upregulated in SSc compared to SSc skin. This distinctive ratio between IFN- and TGFb-responsive gene expression possibly reflects key differences either in the type of innate immune receptor activated or in the genetically defined response to innate immune activation.
Epidermal hyperplasia seen early in our model may be relevant to psoriasis, in that psoriatic skin shows evidence of activation by IFN, the TLR7 agonist, imiquimod, induces psoriatic-like disease in mice and humans, and LL-37 released in psoriatic skin complexed to self DNA can trigger TLR9 (Lande et al., 2007; van der Fits et al., 2009; van der Fits et al., 2004). However, the epidermal hyperplasia we see largely resolves in mice treated for 28-days, suggesting that it may be related to the inflammatory phase of the process.
In conclusion, our data show increased expression of genes upregulated by IFNα and TGFβ in SSc skin and in chronic TLR3-stimulated murine skin. These data support important roles for TLRs or other innate immune activators in SSc pathogenesis and provide a new model system for understanding the relationship between dermal inflammation and matrix remodeling.
MATERIALS AND METHODS
Study subjects
All study subjects met the criteria for dcSSc with proximal skin disease (LeRoy et al., 1988), or subacute (n=7) or discoid (n=5) cutaneous lupus erythematosus (LE). The study was conducted under a protocol approved by the Boston University Medical Center, Institutional Review Board and the ethics committee of Heinrich-Heine University. All subjects gave written informed consent. For SSc subjects, biopsies were performed over the dorsal mid-forearm (lesional skin), or shoulder or back (non-lesional skin) and placed immediately into RNAlater (Qiagen) and stored at −20°C until preparation of RNA, or utilized for explant fibroblast cultures. LE biopsies were performed over lesional skin and processed for RNA as described (Meller et al., 2005).
Fibroblast culture
Primary human dermal fibroblast explant cultures from diffuse cutaneous SSc (dcSSc) and control subjects were established as described previously (Jelaska et al., 1996). Mouse fibroblasts were obtained from 2–3 day old C56BI/6 and B6/IFNAR −/− mice. Skin was treated overnight with 0.025% trypsin in HBSS at 4°C and the next day dermis separated from epithelium and digested with collagenase (0.4 mg/ml, Collagenase type II, Worthington Biochemical Corp., Lakewood, NJ). Mouse and human fibroblasts were cultured in DME supplemented with 10% FBS and penicillin/streptomycin and utilized at passage 3–6 (human) or 2–4 (mouse). Fibroblasts were incubated in serum-free DME for 24h prior to the addition of TLR agonists: Pam3CSK4, Poly(I:C), LPS, imiquimod, sspolyU/Lyovec, CpGA-ODNM362 (Invivogen); control polynucleotides: Poly Cytidylic Acid (Poly C) or Poly Inosinic Acid (Midland Certified Reagent Company, Midland, Texas); or cytokines: rHu-IFNα or IFNβ (Biomedical Laboratories, Piscataway, NJ); IFNγ (R&D System) or TGFβ (R&D System) for 24h in 0.1% FBS. In some experiments, fibroblast cultures were pre-treated with monoclonal anti-TGFβ antibody (R&D System) or control human IgG (AY1498-23, Genzyme).
RNA preparation and real-time polymerase chain reaction (RT-PCR)
Human tissue and fibroblasts were transferred into 600 μl of RLT buffer (Qiagen, Valencia, CA) plus β-mercapto-ethanol, minced and disrupted using a Polytron homogenizer. RNAs were purified using the RNeasy total kit protocol (Qiagen). RNA was extracted from murine skin using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. cDNAs were synthesized from 0.2-μg of total RNA using Superscript II RNase H− reverse transcriptase and random primers (Invitrogen Life Technologies, Rockville, MD) and used as templates for quantitative real-time PCR using primers and normalization described in supplementary methods.
Immnohistochemistry and ELISA
TLR3 was detected in AZF (Newcomer Supply, Middletown, WI) fixed, paraffin embedded, rehydrated skin sections from SSc patients and healthy controls using goat control or anti-TLR3 IgG (T-17, Santa Cruz Biotechnology). Following anti-TLR3 incubation, sections were sequentially incubated with biotinylated mouse anti-goat IgG (Jackson ImmunoResearch, West Grove, PA). avidin/biotinylated peroxidase complex (Vectastain Elite kit, Vector Labs, Burlingame, CA) and Diaminobenzidine. IP-10/CXCL10 ELISA was conducted according to the supplied protocol (BD Biosciences)
In vivo administration of PolyI:C, TGFβ and anti-TGFβ antibody
C57BI/6 WT and C57BI/6 TICAM−/− mice were obtained from The Jackson Laboratory; C57BI/6/IFNAR−/− mice were obtained from Dr. John Sprent (Kolumam et al., 2005). Osmotic pumps designed to deliver Poly:(I:C) (0.5 mg/ml, 0.1 mg total dose in 200 micro-liters released over 7 or 28 days, Alzet), PBS or TGFβ (50ng, 250ng 1.25 μg total dose) were implanted in 4–8 week old mice. Anti-TGFβ antibody (5mg/kg) was injected intraperitoneally on day 0, 3 and 5. After 7 days mice were sacrificed and skin (~1 cm2) surrounding the pump outlet was homogenized in Trizol (Invitrogen) for preparation of RNA or in some experiments fixed in formalin for histology and immunohistochemistry.
Supplementary Material
Acknowledgments
This study was supported by: “Norma Nadeau/Mary Van Neste Research Grant” New England chapter of the Scleroderma Foundation, to Dr. Giuseppina Alessandra Farina, and NIH grants U0IAR055063 and R01AR051089 to Dr. Robert Lafyatis. Dr. Stephen Meller and Bernhard Homey are supported by the Deutsche Forschungsgemeinschaft. The authors would also like to acknowledge support from the American Society for Scleroderma Research.
Abbreviations
- SSc
systemic sclerosis
- TLR
toll-like receptor
- ECM
extracellular matrix
- TGFβ
transforming growth factor-β
- IFN
Interferon
- SLE
systemic lupus erythematosus
- PBMC
peripheral blood mononuclear cells
- IFNAR1
Interferon alpha receptor-1
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci U S A. 2007;104:2827–2830. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–427. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- Black CM, Silman AJ, Herrick AI, Denton CP, Wilson H, Newman J, et al. Interferon-alpha does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999;42:299–305. doi: 10.1002/1529-0131(199902)42:2<299::AID-ANR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, et al. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- Distler O, Pap T, Kowal-Bielecka O, Meyringer R, Guiducci S, Landthaler M, et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 2001;44:2665–2678. doi: 10.1002/1529-0131(200111)44:11<2665::aid-art446>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Doyle SE, O’Connell R, Vaidya SA, Chow EK, Yee K, Cheng G. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4. J Immunol. 2003;170:3565–3571. doi: 10.4049/jimmunol.170.7.3565. [DOI] [PubMed] [Google Scholar]
- Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis and rheumatism. 2010;62:580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina G, Lemaire R, Pancari P, Bayle J, Widom RL, Lafyatis R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis. 2009;68:435–441. doi: 10.1136/ard.2007.086850. [DOI] [PubMed] [Google Scholar]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- Fineschi S, Goffin L, Rezzonico R, Cozzi F, Dayer JM, Meroni PL, et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 2008;58:3913–3923. doi: 10.1002/art.24049. [DOI] [PubMed] [Google Scholar]
- Galindo M, Santiago B, Rivero M, Rullas J, Alcami J, Pablos JL. Chemokine expression by systemic sclerosis fibroblasts: abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 2001;44:1382–1386. doi: 10.1002/1529-0131(200106)44:6<1382::AID-ART231>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- Jelaska A, Arakawa M, Broketa G, Korn JH. Heterogeneity of collagen synthesis in normal and systemic sclerosis skin fibroblasts. Increased proportion of high collagen-producing cells in systemic sclerosis fibroblasts. Arthritis and rheumatism. 1996;39:1338–1346. doi: 10.1002/art.1780390811. [DOI] [PubMed] [Google Scholar]
- Jelaska A, Korn JH. Role of apoptosis and transforming growth factor beta1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 2000;43:2230–2239. doi: 10.1002/1529-0131(200010)43:10<2230::AID-ANR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kim D, Peck A, Santer D, Patole P, Schwartz SM, Molitor JA, et al. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis and rheumatism. 2006;54:3655–3660. doi: 10.1002/art.22186. [DOI] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med. 2009;87:125–138. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol. 2006;176:603–615. doi: 10.4049/jimmunol.176.1.603. [DOI] [PubMed] [Google Scholar]
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Meller S, Winterberg F, Gilliet M, Muller A, Lauceviciute I, Rieker J, et al. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis Rheum. 2005;52:1504–1516. doi: 10.1002/art.21034. [DOI] [PubMed] [Google Scholar]
- Meneghin A, Hogaboam CM. Infectious disease, the innate immune response, and fibrosis. J Clin Invest. 2007;117:530–538. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GE, Parker LC, Ward JR, Jones EC, Whyte MK, Brightling CE, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. Faseb J. 2006;20:2153–2155. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- Negishi H, Osawa T, Ogami K, Ouyang X, Sakaguchi S, Koshiba R, et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci U S A. 2008;105:20446–20451. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- Pierer M, Rethage J, Seibl R, Lauener R, Brentano F, Wagner U, et al. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172:1256–1265. doi: 10.4049/jimmunol.172.2.1256. [DOI] [PubMed] [Google Scholar]
- Proost P, Verpoest S, Van de Borne K, Schutyser E, Struyf S, Put W, et al. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol. 2004;75:777–784. doi: 10.1189/jlb.1003524. [DOI] [PubMed] [Google Scholar]
- Sanda C, Weitzel P, Tsukahara T, Schaley J, Edenberg HJ, Stephens MA, et al. Differential gene induction by type I and type II interferons and their combination. J Interferon Cytokine Res. 2006;26:462–472. doi: 10.1089/jir.2006.26.462. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ichikawa T, Koarai A, Yanagisawa S, Minakata Y, Matsunaga K, et al. Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am J Respir Cell Mol Biol. 2009;40:654–662. doi: 10.1165/rcmb.2008-0371OC. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- van der Fits L, van der Wel LI, Laman JD, Prens EP, Verschuren MC. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. The Journal of investigative dermatology. 2004;122:51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Voskuyl AE, Rustenburg F, Baggen JM, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66:1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J. Recombinant cytokine treatment for scleroderma. Can the antifibrotic potential of interferon-gamma be realized clinically? Arch Dermatol. 1997;133:637–642. doi: 10.1001/archderm.133.5.637. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takagawa S, Katayama I, Nishioka K. Anti-sclerotic effect of transforming growth factor-beta antibody in a mouse model of bleomycin-induced scleroderma. Clin Immunol. 1999;92:6–13. doi: 10.1006/clim.1999.4720. [DOI] [PubMed] [Google Scholar]
- Yamana J, Santos L, Morand E. Enhanced induction of LPS-induced fibroblast MCP-1 by interferon-gamma: involvement of JNK and MAPK phosphatase-1. Cell Immunol. 2009;255:26–32. doi: 10.1016/j.cellimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Takahashi M. IFN-gamma-mediated survival enables human neutrophils to produce MCP-1/CCL2 in response to activation by TLR ligands. J Immunol. 2007;179:1942–1949. doi: 10.4049/jimmunol.179.3.1942. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. 2002;168:3088–3098. doi: 10.4049/jimmunol.168.6.3088. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McCormick LL, Gilliam AC. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2003;121:713–719. doi: 10.1046/j.1523-1747.2003.12517.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.