Abstract
Alzheimer’s disease (AD) is marked by an increase in the production of extracellular beta amyloid plaques and intracellular neurofibrillary tangles associated with a decline in brain function. Increases in oxidative stress are regarded as an early sign of AD pathophysiology, although the source of reactive oxygen species (ROS) and the mechanism(s) whereby beta amyloid peptides (Aβ) impact oxidative stress have not been adequately investigated. Recent studies provide strong evidence for the involvement of NADPH oxidase and its downstream oxidative signaling pathways in the toxic effects elicited by Aβ. ROS produced by NADPH oxidase activate multiple signaling pathways leading to neuronal excitotoxicity and glial cell-mediated inflammation. This review describes recent studies demonstrating the neurotoxic effects of Aβ in conjunction with ROS produced by NADPH oxidase and the downstream pathways leading to activation of cytosolic phospholipase A2 (PLA2) and secretory PLA2. In addition, this review also describes recent studies using botanical antioxidants to protect against oxidative damage associated with AD. Investigating the metabolic and signaling pathways involving Aβ NADPH oxidase and PLA2 can help understand the mechanisms underlying the neurodegenerative effects of oxidative stress in AD. This information should provide new therapeutic approaches for prevention of this debilitating disease.
Keywords: NADPH oxidase, Cytosolic phospholipase A2, Secretory phospholipase A2, ERK1/2, Botanical phenols, Neurons, Astrocytes, Microglial cells
Increase in Oxidative Stress Is an Early Sign of AD Pathogenesis
Alzheimer’s disease (AD) is the most prominent age-related neurodegenerative disease characterized by accumulation of beta amyloid plaques and intracellular neurofibrillary tangles in the brain. Impairment of cognitive function and memory in this disease is attributed to degeneration of synapses in specific brain regions [1]. Although manifestations of AD have been shown to involve multiple factors, including inherent genetic mutations and environmental conditions, increases in oxidative stress associated with amyloid beta peptide (Aβ) accumulation are considered important underlying factors in the development of this disease [2–5]. Aβ peptides are comprised of 39–43 amino acids and are generated by proteolytic cleavage of amyloid precursor protein (APP) by beta and gamma secretases. Considerable evidence indicates that the cytotoxic effects of Aβ are due to its aggregation to the oligomeric form and not the monomeric or fibrillar forms [6–8]. Oligomeric Aβ can diffuse into cells and alter cellular pathways leading to disruption of synaptic plasticity and inhibition of long-term potentiation [9]. These abnormalities occur before the appearance of Aβ plaques [10].
A number of studies have attempted to elucidate the mechanism(s) whereby Aβ enhances reactive oxygen species (ROS) production and stimulates oxidative signaling pathways in the AD brain. Studies using redox proteomics have identified oxidatively modified proteins as early markers of AD pathogenesis [11]. Earlier studies by Butterfield [12] implicated the possible role of methionine-35 residue of Aβ in conferring neurotoxicity. Transgenic PDAPP mice with an M631L mutation in the APP molecule (corresponding to M35 in Aβ) showed markedly decreased oxidative indices and immunoreactive plaques. Interestingly, this mutation did not prevent memory loss in this mouse model of AD [13]. In recent years, studies have provided compelling evidence for the role of oxidative stress in sustained inflammatory responses in the AD brain [14–20]. Novel strategies have been proposed to prevent neuronal damage due to oxidative stress [21].
Phospholipases A2 in AD—a Link to Oxidative and Pro-inflammatory Pathways
Phospholipases A2 (PLA2) are ubiquitous enzymes known for their role in the maintenance of cell membrane integrity and the production of lipid mediators that regulate cell functions [22, 23]. More than 20 species of PLA2 are present in mammalian cells and are categorized as Ca2+-dependent cytosolic (cPLA2), Ca2+-independent (iPLA2), and secretory (sPLA2) subtypes. In the central nervous system (CNS), PLA2 activity has been implicated to play an important role in neurodegenerative diseases [24]. Recent studies also linked PLA2 activity to ROS production and lipid peroxidation. A major objective of this review is to describe recent studies linking cPLA2 and sPLA2 to oxidative pathways in AD.
Cytosolic Phospholipase A2
In earlier studies, an increase in functional cPLA2 protein or mRNA expression in AD brain has been demonstrated by immunohistochemical analysis [25, 26], reverse transcriptase polymerase chain reaction [27] or detection of lipid hydrolysates [28]. cPLA2 activity is regulated by a number of protein kinases, which phosphorylate the protein on specific serine residues. Extracellular signal-regulated kinase (ERK)-dependent cPLA2 phosphorylation on Ser505 is critical for cPLA2 activity [29]. However, studies to determine cPLA2 activity in human AD brain have provided for contrasting results. For example, increases in levels of activated cPLA2 were observed in the hippocampus of human AD brain and in the hAPP mouse model of AD [30]. The increase in cPLA2 activity in AD brain was marked by the increase in 4-hydroxynonenal, an index of lipid peroxidation [31, 32]. On the contrary, other studies indicated a reduction in cPLA2 activity in blood and brain samples from AD patients [33]. During the early stages of AD, cognitive impairment associated with aberrant cholinergic and glutamatergic activities has been linked to a decrease in both cPLA2 and iPLA2 activities [34]. A study utilizing magnetic resonance imaging also demonstrated reduced phospholipid turnover in the pre-frontal cortex of AD patients [35]. Furthermore, decreased cPLA2 activity was found in cerebrospinal fluid from AD, vascular, and mixed AD–vascular demented patients, as compared to healthy controls [33]. Although the reason for the differences in results is not yet clear, it is recognized that this enzyme is regulated by complex factors depending on the cell types and intracellular signaling pathways. Since cPLA2 and its hydrolytic products are critically important in maintenance of phospholipids in cell membrane, more studies are needed to understand cell specific factors regulating cPLA2 under physiological and pathological conditions.
Secretory Phospholipase A2
Mammalian sPLA2s are generally small molecular weight proteins (~14–19 kDa) with six to eight disulfide bridges. There are more than ten isoforms of sPLA2s in mammals including groups IA-B, IIA-F, III, V, IX, X, XIA-B, XII, XIII, and XIV [22, 36]. Recent studies have focused on sPLA2-IIA because this sPLA2 is a pro-inflammatory protein and is upregulated in coronary artery diseases, atherosclerosis, sepsis, arthritis, and infection [37]. In rodents, most studies on sPLA2-IIA are limited to rats because many mouse strains lack functional sPLA2-IIA [38]. Transgenic mice overexpressing human sPLA2-IIA showed increases in hepatic cholesterol and in collagen deposits in macrophages, suggesting a pro-inflammatory role for this protein [39, 40]. Our studies indicated upregulation of sPLA2-IIA in reactive astrocytes in response to injury due to focal cerebral ischemia in rats [41]. In a subsequent study, we also showed an increase in sPLA2-IIA mRNA expression in AD brain as compared to age-matched controls [42]. These results are in agreement with the recent report indicating an increase in sPLA2 activity in cerebrospinal fluid of AD patients as compared to healthy control subjects [43].
Substantial cross-talk seems to exist between the path-ways that regulate the expression/activation of cPLA2- and sPLA2-dependent inflammatory responses [44]. It appears that this cross-talk is linked to oxidative stress mediated by NADPH oxidase [45].
NADPH Oxidase is an Important Source of Oxidative Stress in the CNS and in AD
Recent studies have demonstrated the role of NADPH oxidase as an important nonmitochondrial source of ROS in many cell and tissue types, including the CNS [46–48]. Although NADPH oxidase activity is necessary for cell signaling under physiological conditions, its hyperactivation has been shown to contribute to the pathogenesis of a variety of diseases, including neurodegenerative diseases including vascular and neurodegenerative diseases and stroke [49, 50].
There are at least seven known isoforms of NADPH oxidases, NOX1-5, and Duox1 and 2, each with a unique combination of subunits [51]. NOX2 is well studied in phagocytic cells, macrophages, and endothelial cells and is comprised of the subunits p47phox, p67phox, p40phox, and Rac 1 in the cytosol and gp91phox and p22phox in the membrane fraction (plasma membranes or other subcellular membranes). Activation of NOX2 is dependent on phosphorylation of the cytosolic subunits, e.g., phosphorylation of p47phox by protein kinase C in human monocytes [52] and by Src-mediated tyrosine kinase in lung endothelial cells [53]. Subsequently, the cytosolic subunits form a complex and translocated to the membrane-associated gp91phox subunit. The gp91phox subunit has six trans-membrane domains and four heme-binding histidines in transmembrane domains III and V (see Bokoch et al. [54] for a recent review). FAD-binding and NADPH-binding domains are present in the carboxy-terminus of gp91phox [55]. NOX1, 2, and 4 are expressed in neurons, astrocytes, and microglia in the CNS [48]. Although the subcellular sites of NOX isoform expression in cells in the central nervous system have not been clearly determined, recent studies suggest that multiple NOX isoforms can be expressed within the same cell [56].
Recent studies have demonstrated the ability for Aβ to upregulate different NADPH oxidase subunits in microglial cells and increase enzyme activity in AD patients as compared to age-matched controls [57–59]. These studies suggest a relationship between ROS produced from NADPH oxidase and oxidative stress in AD.
Involvement of NADPH Oxidase and cPLA2 in N-Methyl-D-Aspartic Acid-Induced Neuronal Excitotoxicity
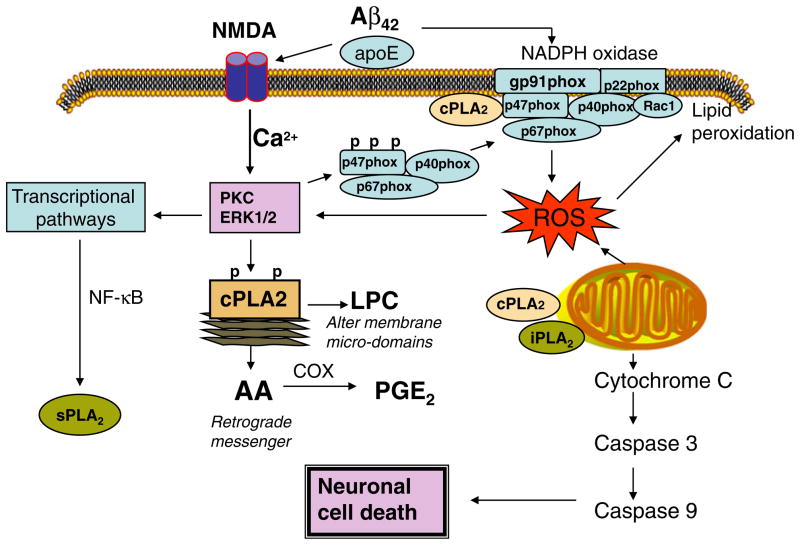
Recent studies with cultured neurons have demonstrated the involvement of NADPH oxidase in excitotoxicity induced by ionotropic glutamate receptors, including the N-methyl-D-aspartic acid (NMDA) subtype [50, 60–62]. NMDA-induced activation of NADPH oxidase can trigger signaling pathways leading to activation of ERK1/2 [63], the protein kinase required for activation of cPLA2 (Fig. 1). Studies in our laboratory with primary cortical neurons demonstrated that oligomeric Aβ can induce ROS production through NADPH oxidase. In turn, ROS from NADPH oxidase can stimulate ERK1/2 phosphorylation and activation of cPLA2 and result in a release of arachidonic acid (AA) [61]. This study further demonstrated that the excitotoxic effect of Aβ is inhibited by NMDA receptor antagonists, including memantine, a drug used to treat AD patients. Stimulation of this signaling pathway should have important physiological consequences since (1) cPLA2 activation modulates membrane phospholipid homeostasis that is known to be altered in a number of neurodegenerative diseases [64, 65], (2) cPLA2-dependent production of AA leads to an increase in the synthesis of bioactive eicosanoids [66, 67], (3) AA itself can act as a retrograde messenger in neurons thereby modulating learning and memory processes [68], and (4) lysophospholipids, e.g., lysophosphatidylcholine, produced by cPLA2 may alter the membrane microenvironment and thereby further alter membrane protein functions [36, 69].
Fig. 1.
The role of Aβ in the coupling of NADPH oxidase and NMDA receptor signaling pathways in neurons
The involvement of cPLA2 in neuronal excitotoxicity, dysfunction, and death in vivo and in vitro has been suggested by several studies. However, despite some indirect evidence, the mechanism whereby cPLA2 alters mitochondrial membrane and triggers mitochondrial apoptotic pathways remains to be investigated. A study by Kriem et al. demonstrated the role of cPLA2 in mediating neuronal apoptosis induced by oligomeric Aβ [70]. Using selective inhibitors, cPLA2 and iPLA2 were shown to play a role in Aβ-mediated loss of mitochondrial membrane potential and increase in ROS in astrocytes [71]. Neurons from cPLA2 knockout (KO) mice show less NMDA-induced injury as compared with wild-type controls [72]. Transgenic mice overexpressing human APP in neurons exhibited a reduced deficit in learning, memory, and behavioral dysfunctions and diminished Aβ-induced neurotoxicity when crossed with cPLA2 KO mice [30]. Similar reduction of Aβ-induced neurotoxicity was observed using cPLA2 inhibitors [70]. Furthermore, squalestatin was shown to be neuroprotective against Aβ-induced neurotoxicity by inhibiting cPLA2 activation [73].
Although there is convincing evidence suggesting a role for glial cell NADPH oxidase in Aβ-induced neurotoxicity [74], relatively few studies have investigated the role of NADPH oxidase in neurons. Antisense oligonucleotide knockdown of p22phox expression inhibited Aβ-induced neuronal apoptosis [75]. The NADPH oxidase inhibitor apocynin was also effective in diminishing the cytotoxic effects of Aβ [76]. Furthermore, diminished oxidative stress, neurovascular dysfunction, and behavioral deficits were observed in the Tg2576 mouse model of AD when these mice were crossed with gp91phox KO mice [77].
NADPH Oxidase and cPLA2 and sPLA2 in Glial Cells
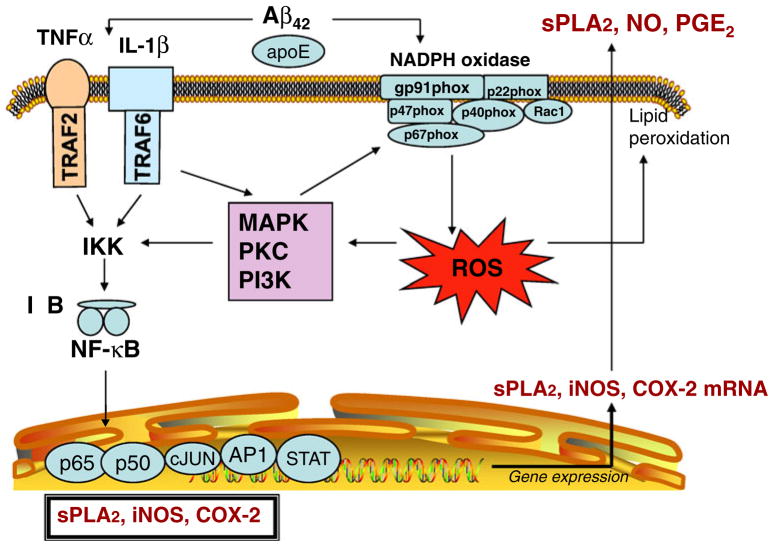
Relatively high levels of NADPH oxidase are found in astrocytes and microglial cells, as compared with neurons, and ROS produced from glial cells has been shown to cause neuronal damage [74, 78]. ROS produced by NADPH oxidase modulates the cytokine-induced activation of NF-κB, a transcription factor that regulates the expression of pro-inflammatory genes, including cyclooxygenase-2 (COX-2), sPLA2-IIA, and inducible nitric oxide synthase (iNOS; Fig. 2). Our studies showed that IL-1β-induced sPLA2-IIA mRNA and protein expression in rat astrocytes could be inhibited by polyphenol antioxidants and apocynin, a known NADPH oxidase inhibitor [79]. These results are consistent with other studies indicating that Aβ and cytokines potentiate ROS production via NADPH oxidase activation in glial cells [80, 81]. Arachidonic acid is another activator of NADPH oxidase that causes superoxide release from microglia and induces their proliferation [82]. Using neuron–glial cell coculture, per-oxynitrite was shown to be produced by NO release from iNOS and ROS from NADPH oxidase in glial cells, and peroxynitrite is the potent cytotoxic factor that kills neurons.
Fig. 2.
Effects of Aβ on cytokine-induced inflammatory responses in glial cells: the role of NADPH oxidase
Although studies with phagocytes indicate that cPLA2 targets NADPH oxidase [83, 84], the role of cPLA2 in the activation of NADPH oxidase in neurons and glial cells has not been well characterized. Studies with neutrophils demonstrate the formation of a complex between the C2 domain of cPLA2 and the p47 phox-PX domain of NADPH oxidase [84]. In BV-2 microglial cells, which lacks sPLA2-IIA, Aβ could induce activation of NADPH oxidase and this was linked to a rapid increase in cPLA2 phosphorylation and a delayed increase in expression which was attributed to the NF-κB pathway. Aβ also enhanced production of PGE2 through conversion of AA by COX-2. In this study, Aβ also led to induction of iNOS and release of NO, but this was thought to be mediated through activation of the PGE2 receptor and the down-stream PKA-CREB pathway. Interestingly, inhibition of cPLA2 by antisense led to a decrease in NADPH oxidase activity and release of superoxides, PGE2 formation, iNOS expression, and NO production [85]. Taken together, this study provided results demonstrating a close relationship between NADPH oxidase and cPLA2 in Aβ-mediated inflammatory responses in microglial cells.
In wild-type mice, Aβ-induced neurotoxicity is enhanced by microglia that produce superoxide from NADPH oxidase, a response that is inhibited in gp91phox KO mice [78]. Aβ also has been shown to increase ROS production in astrocytes through the calcium-dependent activation of NADPH oxidase, and oxidative stress results in glutathione depletion and neuronal death [86]. Peroxynitrite appears to be an important factor mediating neurotoxicity in vitro [87] since generation of pathophysiological concentrations of superoxide and NO are necessary but not sufficient to induce neurodegeneration [88]. It is noted that these studies do not exclude the possibility that neurotoxicity is mediated by other factors, such as pro-inflammatory cytokines and protein modification through cysteine S-nitrosylation [89–92].
Targeting NADPH Oxidase Activity for Neurodegenerative Diseases
Recognition of the important role of NADPH oxidase in generating ROS that regulate receptor signaling pathways has highlighted an urgent need to develop novel approaches and pharmacological agents to regulate this enzyme system [93, 94]. In this regard, it is important to identify compounds that directly modulate the activities of NADPH oxidase, either through binding to specific protein subunits or through scavenging the ROS produced. The recent study by Jaquet et al. provided a list of small molecules useful as NOX inhibitors [95]. Although compounds such as diphenyleneiodonium, apocynin, atorvastatin, and 4-(2-amino-ethyl)benzenesulfonyl fluoride have been used to inhibit NADPH oxidase, these compounds are rather nonspecific. On the other hand, gp91ds-tat, a peptide inhibitor, can specifically block NOX2 [48]. Since different cell types can produce different amount of ROS, it is also important to develop cell-special inhibitors for NADPH oxidase, in particular, for targeting microglial cells [96, 97]. Of particular interest is dextromethorphan, a noncompetitive NMDA receptor antagonist and antitussive agent that has been shown to inhibit several NADPH oxidase-mediated responses, including degeneration of dopaminergic neurons in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinson’s model and endotoxic shock in mice [98, 99]. Another approach is to inhibit NADPH oxidase-mediated responses including inflammation and oxidative stress by nutritional means using natural products from plants [100]. Indeed, the use of plant antioxidants has gained considerable popularity in recent years [95].
Prevention of Neurodegenerative Diseases by Natural Antioxidants
Fruits and vegetables are known to contain phenolic compounds that exhibit antioxidant properties and have been shown to exert health benefits and reduce risk of major diseases, including cardiovascular and neurodegenerative diseases (see review in [101]). The underlying mechanisms for neuroprotection, however, remain incompletely understood but likely involve the ability of polyphenols and plant extracts to scavenge ROS and counteract Aβ formation/aggregation directly or indirectly [102, 103]. Some phytochemicals may exert neurohormetic effects and protect neurons from injury by upregulating cell survival pathways [104]. There is also evidence that some polyphenols, such as curcumin, may inhibit Aβ fibril formation and destabilize preformed fibril Aβ [102, 105–107]. Other studies have demonstrated that some natural products provide neuroprotection by targeting multiple cellular signaling pathways [108, 109]. Recent in vitro and in vivo studies have indicated that purified botanical compounds or plant extracts effectively prevent Aβ-induced neurotoxicity (Table 1) as well as provide neuroprotection in animal models of AD (Table 2). Since our recent review has described the use of phenolic compounds such as resveratrol, curcumin, and apocynin as neuroprotective agents [101], these compounds are not included in Tables 1 and 2.
Table 1.
Polyphenols and botanical extracts tested against amyloid-beta toxicity
| Polyphenol/plant name | Cell type | Effects | References |
|---|---|---|---|
| Apigenin | Cortical neurons | + | [122] |
| Baicalein | PC12 cells | + | [123] |
| [124] | |||
| Biflavonoids | SH-SY5Y cells | +/− | [125] |
| Catechin | PC12 cells | + | [126] |
| [127] | |||
| Cortical neurons | + | [128] | |
| Cocoa | PC12 cells | + | [127] |
| Cyanidin 3-O-glucoside | SH-SY5Y cells | + | [129] |
| Daidzein | Hippocampal neurons | +/− | [130] |
| EGCG | PC12 cells | + | [131] |
| Hippocampal neurons | + | [132] | |
| [133] | |||
| Epicatechin | PC12 cells | + | [127] |
| [124] | |||
| Cortical neurons | + | [128] | |
| Hippocampal neurons | − | [133] | |
| Gallic acid | Hippocampal neurons | + | [133] |
| Genistein | SH-SY5Y cells | + | [134] |
| Hippocampal neurons | +/− | [130] | |
| Hippocampal neurons | +/− | [135] | |
| Cortical neurons | + | [136] | |
| − | [122] | ||
| Ginkgo biloba (EGb 761) | Hippocampal neurons | + | [137] |
| N2a neuroblastoma | + | [138] | |
| SH-SY5Y cells | + | [139] | |
| PC12 cells | +/− | [140] | |
| Grape seed | PC12 cells | + | [141] |
| [142] | |||
| Hibifolin | Cortical neurons | + | [143] |
| PC12 cells | + | [124] | |
| Hypericum perforatum | Hippocampal neurons | + | [144] |
| Icaritin | Cortical neurons | + | [145] |
| Kaempferol | PC12 cells | + | [146] |
| [124] | |||
| Cortical neurons | + | [122] | |
| Morin | HT22 neuroblastoma | + | [106] |
| Mulberry (leaf extract) | Hippocampal neurons | + | [147] |
| Myricetin | Cortical neurons | + | [148] |
| Naringenin | PC12 cells | + | [149] |
| − | [124] | ||
| Cortical neurons | − | [122] | |
| Puerarin | PC12 cells | + | [150] |
| Pycnogenol | PC12 cells | + | [151] |
| Quercetin | Cortical neurons | +/− | [152] |
| − | [122] | ||
| HT22 neuroblastoma | + | [106] | |
| Rhizoma acori graminei | PC12 cells | + | [153] |
| Scutellarin | PC12 cells | + | [124] |
| Smilacis chinae | Cortical neurons | + | [128] |
| Tea extracts | Hippocampal neurons | + | [133] |
Table 2.
Polyphenols and botanical extracts tested in AD animal models
| Polyphenol/plant name | Animal model | Effects | References |
|---|---|---|---|
| Bacopa monniera | PSAPP mice | + | [154] |
| Baicalein | Aβ infusion (i.c.v.) mice | + | [155] |
| Blueberry | Tg2576 mice | + | [156] |
| Cabernet Savignon | Tg2576 mice | + | [157] |
| EGCG | Tg2576 mice | + | [158] |
| [159] | |||
| Aβ infusion (i.c.v.) mice | + | [160] | |
| PS2 mice | + | [160] | |
| LPS (i.c.v.) mice | + | [160] | |
| Epicatechin | Aβ infusion (CA1) rats | + | [161] |
| Ferulic acid | Aβ infusion (i.c.v.) mice | + | [162] |
| [163] | |||
| [164] | |||
| Tg2576 mice | − | [165] | |
| Fustin | Aβ infusion (i.c.v.) mice | + | [166] |
| Garlic | Tg2576 mice | + | [167] |
| [168] | |||
| TgCRND8 mice | + | [169] | |
| Ginkgo biloba | Tg2576 mice | + | [170] |
| [171] | |||
| TgAPP/PS1 mice | + | [172] | |
| [173] | |||
| Ginseng | Tg2576 mice | + | [174] |
| Ginsenoside | Rb1or M1Aβ infusion (i.c.v.) mice | + | [175] |
| Glycyrrhiza uralensis | Aβ infusion (i.c.v.) mice | + | [176] |
| Grape seed | Tg2576 mice | + | [177] |
| Green tea catechins | Aβ infusion (i.c.v.) rats | + | [178] |
| Luteolin | Aβ infusion (i.c.v.) mice | + | [179] |
| Nobiletin | APP–SL 7–5 mice | + | [180] |
| Aβ infusion (i.c.v.) rats | + | [181] | |
| Oroxylin A | Aβ infusion (i.c.v.) mice | + | [118] |
| Pomegranate | Tg2576 mice | + | [182] |
| Rosmarinic acid | Tg2576 mice | + | [165] |
| Silibinin | Aβ infusion (i.c.v.) mice | + | [183] |
| Soy isoflavone | Aβ infusion (i.c.v.) rats | + | [184] |
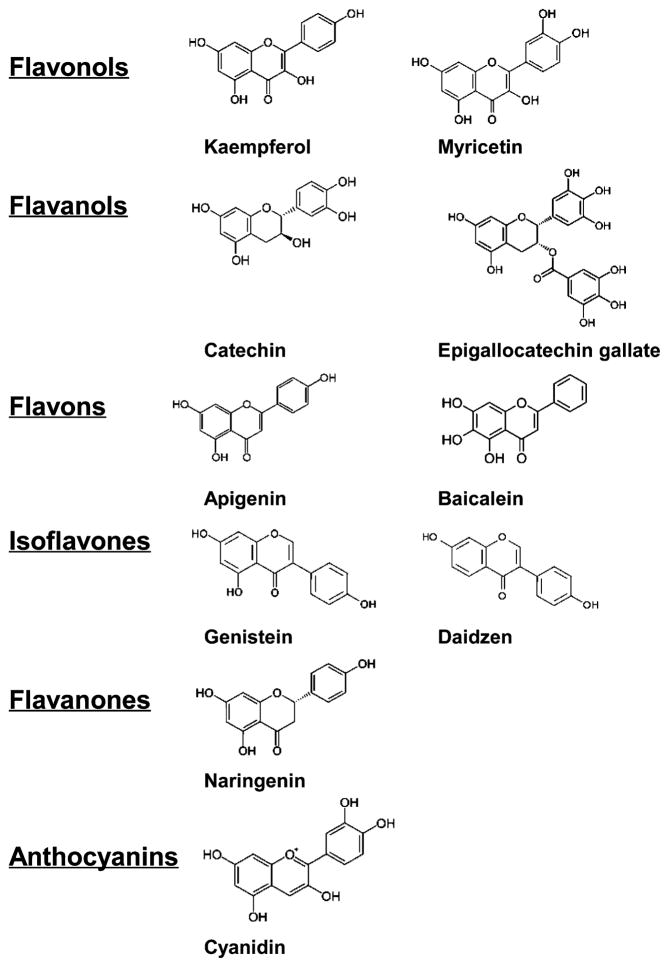
Dietary flavonoids are potent inhibitors of NADPH oxidase. Figure 3 shows the chemical structures of some flavonoids as described in Table 1. A study of 45 compounds indicated that flavanols inhibit NADPH oxidase through an apocynin-like mechanism [110]. Other studies demonstrate the ability of dietary polyphenols to inhibit NADPH oxidase, suggesting that these polyphenols may serve as novel therapeutic agents in neuroinflammation [111]. Some polyphenols, like those from grape seed extract, also have been shown to regulate NADPH oxidase subunit expression [112].
Fig. 3.
Chemical structures of some flavonoids described in Table 1
Dreiseitel et al. used a kinetic photometric model to compare the potency of a number of anthocyanidins for inhibition of sPLA2 [113]. Anthocyanidins have been shown to ameliorate cognitive deficits in AD patients and improve immunocompetency of these patients [114]. The polyphenol genistein was shown to be a potent inhibitor of sPLA2 in inflammatory exudates and in snake venom-induced mouse paw edema [115]. It appears that anti-inflammatory activities of many plant flavonoids are associated with inhibition of PLA2 [116]. These studies provide strong rationale to search for novel compounds as specific inhibitors of PLA2s [117–121].
Summary
In summary, AD progression is marked by an increase in oxidative stress and chronic inflammation that is attributed in part to the toxic effects of Aβ. Studies in recent years have identified NADPH oxidase as an important source of ROS that contributes to Aβ-induced neuronal damage and glial cell activation. ROS produced by NADPH oxidase activate MAPK and subsequently cPLA2, a key enzyme that releases AA from phospholipids for the synthesis of eicosanoids (Fig. 1). cPLA2 activation and AA release have been associated with neuronal excitotoxicity, impairment of mitochondrial dysfunction, and neuronal apoptosis. In addition, ROS produced by NADPH oxidase can activate NF-κB to promote pro-inflammatory gene transcription, thereby enhancing the synthesis of sPLA2, iNOS, and COX-2, enzymes that play a role in neurodegenerative diseases (Fig. 2). There is compelling evidence for the beneficial effects of antioxidants from botanical sources as inhibitors of NADPH oxidase and PLA2, and these polyphenolic compounds may become useful therapeutic agents to alleviate oxidative stress and chronic inflammation in neurodegenerative diseases, including AD.
Acknowledgments
This work was supported by grants P02 AG018357 and 1R21 AT003859 from the NIH. Thanks are due to Mr. Dennis Reith for his help in editing the manuscript.
Contributor Information
Agnes Simonyi, Biochemistry Department, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA.
Yan He, Biochemistry Department, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA.
Wenwen Sheng, Biochemistry Department, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA. Department of Pathology and Anatomical Sciences, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA.
Albert Y. Sun, Department of Pathology and Anatomical Sciences, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA
W. Gibson Wood, Department of Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Gary A. Weisman, Biochemistry Department, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA. Bond Life Sciences Center, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA
Grace Y. Sun, Email: sung@missouri.edu, Biochemistry Department, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA. Department of Pathology and Anatomical Sciences, University of Missouri, 117 Schweitzer Hall, Columbia, MO, USA
References
- 1.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield DA. Amyloid beta-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer’s disease brain: mechanisms and consequences. Curr Med Chem. 2003;10:2651–2659. doi: 10.2174/0929867033456422. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 4.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. J Neurovirology. 2002;8:539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 9.Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ. Alzheimer’s disease amyloid beta-protein and synaptic function. Neuromolecular Med. 2010 doi: 10.1007/s12017-009-8091-0. in press. [DOI] [PubMed] [Google Scholar]
- 10.Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer’s disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2009;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez A, Llacuna L, Fernandez-Checa JC, Colell A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J Neurosci. 2009;29:6394–6405. doi: 10.1523/JNEUROSCI.4909-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, McGeer EG, Yasojima K. Alzheimer disease and neuroinflammation. J Neural Transm Suppl. 2000;59:53–57. doi: 10.1007/978-3-7091-6781-6_8. [DOI] [PubMed] [Google Scholar]
- 18.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker DG, Link J, Lue LF, Dalsing-Hernandez JE, Boyes BE. Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 2006;79:596–610. doi: 10.1189/jlb.0705377. [DOI] [PubMed] [Google Scholar]
- 21.Zafrilla P, Morillas JM, Rubio-Perez JM, Cantos Villar E. Ingredients for functional drinks in neurodegenerative diseases: a review. Nat Prod Commun. 2009;4:719–740. [PubMed] [Google Scholar]
- 22.Murakami M, Kudo I. Phospholipase A2. J Biochem (Tokyo) 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 23.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 27.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 28.Walter A, Korth U, Hilgert M, Hartmann J, Weichel O, Hilgert M, Fassbender K, Schmitt A, Klein J. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging. 2004;25:1299–1303. doi: 10.1016/j.neurobiolaging.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castegna A, Lauderback CM, Mohmmad-Abdul H, Butterfield DA. Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: implications for Alzheimer’s disease. Brain Res. 2004;1004:193–197. doi: 10.1016/j.brainres.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Neely MD, Sidell KR, Graham DG, Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- 33.Smesny S, Stein S, Willhardt I, Lasch J, Sauer H. Decreased phospholipase A2 activity in cerebrospinal fluid of patients with dementia. J Neural Transm. 2008;115:1173–1179. doi: 10.1007/s00702-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 34.Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl) 2008;198:1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- 35.Forlenza OV, Schaeffer EL, Gattaz WF. The role of phospholipase A2 in neuronal homeostasis and memory formation: implications for the pathogenesis of Alzheimer’s disease. J Neural Transm. 2007;114:231–238. doi: 10.1007/s00702-006-0597-0. [DOI] [PubMed] [Google Scholar]
- 36.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibeas E, Fuentes L, Martin R, Hernandez M, Nieto ML. Secreted phospholipase A2 type IIA as a mediator connecting innate and adaptive immunity: new role in atherosclerosis. Cardiovasc Res. 2009;81:54–63. doi: 10.1093/cvr/cvn234. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 39.Eckey R, Menschikowski M, Lattke P, Jaross W. Increased hepatic cholesterol accumulation in transgenic mice overexpressing human secretory phospholipase A2 group IIA. Inflammation. 2004;28:59–65. doi: 10.1023/b:ifla.0000033021.44105.9c. [DOI] [PubMed] [Google Scholar]
- 40.Ghesquiere SA, Gijbels MJ, Anthonsen M, van Gorp PJ, van der Made I, Johansen B, Hofker MH, de Winther MP. Macrophage-specific overexpression of group IIa sPLA2 increases atherosclerosis and enhances collagen deposition. J Lipid Res. 2005;46:201–210. doi: 10.1194/jlr.M400253-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 42.Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: a new inflammatory factor for Alzheimer’s disease. J Neuroinflammation. 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalbot S, Zetterberg H, Blennow K, Fladby T, Grundke-Iqbal I, Iqbal K. Cerebrospinal fluid secretory Ca2+-dependent phospholipase A2 activity is increased in Alzheimer disease. Clin Chem. 2009;55:2171–2179. doi: 10.1373/clinchem.2009.130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun GY, Shelat PB, Jensen MD, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2009 doi: 10.1007/s12017-009-8092-z. 101007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 46.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 48.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 49.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Brennen AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 53.Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 54.Bokoch GM, Diebold B, Kim JS, Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid Redox Signal. 2009;11:2429–2441. doi: 10.1089/ars.2009.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen K, Craige SE, Keaney JF., Jr Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal. 2009;11:2467–2480. doi: 10.1089/ars.2009.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cave A. Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol. 2009;9:208–213. doi: 10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AE, Ebenezer PJ, Weidner AM, Levine H, Keller J, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 59.Zekry D, Epperson TK, Krause KH. A role for NOX NADPH oxidases in Alzheimer’s disease and other types of dementia? IUBMB Life. 2003;55:307–313. doi: 10.1080/1521654031000153049. [DOI] [PubMed] [Google Scholar]
- 60.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 62.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- 64.Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bazan NG, Colangelo V, Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimer’s disease. Prostaglandins Other Lipid Mediat. 2002;68–69:197–210. doi: 10.1016/s0090-6980(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 67.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res. 2009;50(Suppl):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leu BH, Schmidt JT. Arachidonic acid as a retrograde signal controlling growth and dynamics of retinotectal arbors. Dev Neurobiol. 2008;68:18–30. doi: 10.1002/dneu.20561. [DOI] [PubMed] [Google Scholar]
- 69.Li XH, Long DX, Li W, Wu YJ. Different mechanisms of lysophosphatidylcholine-induced Ca(2+) mobilization in N2a mouse and SH-SY5Y human neuroblastoma cells. Neurosci Lett. 2007;424:22–26. doi: 10.1016/j.neulet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Kriem B, Sponne I, Fifre A, Malaplate-Armand C, Lozac’h-Pillot K, Koziel V, Yen-Potin FT, Bihain B, Oster T, Olivier JL, Pillot T. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. Faseb J. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- 71.Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Y, Kishimoto K, Linden DJ, Sapirstein A. Cytosolic phospholipase A(2) alpha mediates electrophysiologic responses of hippocampal pyramidal neurons to neurotoxic NMDA treatment. Proc Natl Acad Sci USA. 2007;104:6078–6083. doi: 10.1073/pnas.0605427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bate C, Williams A. Squalestatin protects neurons and reduces the activation of cytoplasmic phospholipase A2 by Abeta(1–42) Neuropharmacology. 2007;53:222–231. doi: 10.1016/j.neuropharm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos Trans R Soc Lond B Biol Sci. 2005;360:2309–2314. doi: 10.1098/rstb.2005.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Abeta1–40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 79.Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochem Int. 2009;55:362–368. doi: 10.1016/j.neuint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. beta-Amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- 81.Shen S, Yu S, Binek J, Chalimoniuk M, Zhang X, Lo SC, Hannink M, Wu J, Fritsche K, Donato R, Sun GY. Distinct signaling pathways for induction of type II NOS by IFNgamma and LPS in BV-2 microglial cells. Neurochem Int. 2005;47:298–307. doi: 10.1016/j.neuint.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Daniels I, Lindsay MA, Keany CI, Burden RP, Fletcher J, Haynes AP. Role of arachidonic acid and its metabolites in the priming of NADPH oxidase in human polymorphonuclear leukocytes by peritoneal dialysis effluent. Clin Diagn Lab Immunol. 1998;5:683–689. doi: 10.1128/cdli.5.5.683-689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shmelzer Z, Haddad N, Admon E, Pessach I, Leto TL, Eitan-Hazan Z, Hershfinkel M, Levy R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J Cell Biol. 2003;162:683–692. doi: 10.1083/jcb.200211056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shmelzer Z, Karter M, Eisenstein M, Leto TL, Hadad N, Ben-Menahem D, Gitler D, Banani S, Wolach B, Rotem M, Levy R. Cytosolic phospholipase A2alpha is targeted to the p47phox-PX domain of the assembled NADPH oxidase via a novel binding site in its C2 domain. J Biol Chem. 2008;283:31898–31908. doi: 10.1074/jbc.M804674200. [DOI] [PubMed] [Google Scholar]
- 85.Szaingurten-Solodkin I, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1-42 in microglia. Glia. 2009;57:1727–1740. doi: 10.1002/glia.20886. [DOI] [PubMed] [Google Scholar]
- 86.Abramov AY, Canevari L, Duchen MR. beta-Amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 88.Borutaite V, Brown G. What else has to happen for nitric oxide to induce cell death? Biochem Soc Trans. 2005;33:1394–1396. doi: 10.1042/BST0331394. [DOI] [PubMed] [Google Scholar]
- 89.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75:349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 92.Beltran B, Orsi A, Clementi E, Moncada S. Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol. 2000;129:953–960. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 94.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 95.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 96.Block ML. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19:550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- 98.Li G, Liu Y, Tzeng NS, Cui G, Block ML, Wilson B, Qin L, Wang T, Liu B, Liu J, Hong JS. Protective effect of dextromethorphan against endotoxic shock in mice. Biochem Pharmacol. 2005;69:233–240. doi: 10.1016/j.bcp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, Hong JS, Liu B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J. 2004;18:589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 100.Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Subcell Biochem. 2007;42:299–318. [PubMed] [Google Scholar]
- 101.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ono K, Naiki H, Yamada M. The development of preventives and therapeutics for Alzheimer’s disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des. 2006;12:4357–4375. doi: 10.2174/138161206778793010. [DOI] [PubMed] [Google Scholar]
- 103.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 104.Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 106.Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, Lee SE. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 107.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 108.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr. 2008;138:1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 109.Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Vafeiadou K, Vauzour D, Spencer JP. Neuroinflammation and its modulation by flavonoids. Endocr Metab Immune Disord Drug Targets. 2007;7:211–224. doi: 10.2174/187153007781662521. [DOI] [PubMed] [Google Scholar]
- 112.Decorde K, Teissedre PL, Sutra T, Ventura E, Cristol JP, Rouanet JM. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res. 2009;53:659–666. doi: 10.1002/mnfr.200800165. [DOI] [PubMed] [Google Scholar]
- 113.Dreiseitel A, Korte G, Schreier P, Oehme A, Locher S, Hajak G, Sand PG. sPhospholipase A(2) is inhibited by anthocyanidins. J Neural Transm. 2009;116:1071–1077. doi: 10.1007/s00702-009-0268-z. [DOI] [PubMed] [Google Scholar]
- 114.Zhao B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem Res. 2009;34:630–638. doi: 10.1007/s11064-008-9900-9. [DOI] [PubMed] [Google Scholar]
- 115.Dharmappa KK, Mohamed R, Shivaprasad HV, Vishwanath BS. Genistein, a potent inhibitor of secretory phospholipase A (2): a new insight in down regulation of inflammation. Inflammopharmacology. 2009;18:25–31. doi: 10.1007/s10787-009-0018-8. [DOI] [PubMed] [Google Scholar]
- 116.Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol Aging. 2004;25:431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 117.da Silva SL, Calgarotto AK, Maso V, Damico DC, Baldasso P, Veber CL, Villar JA, Oliveira AR, Comar M, Jr, Oliveira KM, Marangoni S. Molecular modeling and inhibition of phospholipase A2 by polyhydroxy phenolic compounds. Eur J Med Chem. 2009;44:312–321. doi: 10.1016/j.ejmech.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 118.Kim DH, Kim S, Jeon SJ, Son KH, Lee S, Yoon BH, Cheong JH, Ko KH, Ryu JH. The effects of acute and repeated oroxylin A treatments on Abeta(25-35)-induced memory impairment in mice. Neuropharmacology. 2008;55:639–647. doi: 10.1016/j.neuropharm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 119.Lattig J, Bohl M, Fischer P, Tischer S, Tietbohl C, Menschikowski M, Gutzeit HO, Metz P, Pisabarro MT. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. J Comput Aided Mol Des. 2007;21:473–483. doi: 10.1007/s10822-007-9129-8. [DOI] [PubMed] [Google Scholar]
- 120.Moon TC, Quan Z, Kim J, Kim HP, Kudo I, Murakami M, Park H, Chang HW. Inhibitory effect of synthetic C-C biflavones on various phospholipase A(2)s activity. Bioorg Med Chem. 2007;15:7138–7143. doi: 10.1016/j.bmc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 121.Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants—a new role as anti-inflammatory molecule. Curr Top Med Chem. 2007;7:765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 122.Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–5295. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- 123.Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY. Potent Inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neurotoxicity. J Agric Food Chem. 2004;52:4128–4132. doi: 10.1021/jf049953x. [DOI] [PubMed] [Google Scholar]
- 124.Zhu JT, Choi RC, Chu GK, Cheung AW, Gao QT, Li J, Jiang ZY, Dong TT, Tsim KW. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death. J Agric Food Chem. 2007;55:2438–2445. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
- 125.Kang SS, Lee JY, Choi YK, Song SS, Kim JS, Jeon SJ, Han YN, Son KH, Han BH. Neuroprotective effects of naturally occurring biflavonoids. Bioorg Med Chem Lett. 2005;15:3588–3591. doi: 10.1016/j.bmcl.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 126.Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Res Bull. 2003;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Heo HJ, Lee CY. Epicatechin and catechin in cocoa inhibit amyloid beta protein induced apoptosis. J Agric Food Chem. 2005;53:1445–1448. doi: 10.1021/jf048989m. [DOI] [PubMed] [Google Scholar]
- 128.Ban JY, Jeon SY, Bae K, Song KS, Seong YH. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79:2251–2259. doi: 10.1016/j.lfs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Tarozzi A, Merlicco A, Morroni F, Franco F, Cantelli-Forti G, Teti G, Falconi M, Hrelia P. Cyanidin 3-O-glucopyranoside protects and rescues SH-SY5Y cells against amyloid-beta peptide-induced toxicity. NeuroReport. 2008;19:1483–1486. doi: 10.1097/WNR.0b013e32830fe4b8. [DOI] [PubMed] [Google Scholar]
- 130.Zhao L, Chen Q, Diaz Brinton R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp Biol Med (Maywood) 2002;227:509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- 131.Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (–)-epigallocatechin-3-gallate. FASEB J. 2003;17:952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 132.Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI. The green tea polyphenol (−)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 133.Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur J NeuroSci. 2006;23:55–64. doi: 10.1111/j.1460-9568.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 134.Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, Mook-Jung I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis. 2004;16:21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 135.Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25–35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med. 2004;36:180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 136.Valles SL, Borras C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardo FV, Vina J. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7:112–118. doi: 10.1111/j.1474-9726.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 137.Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J NeuroSci. 2000;12:1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 138.Longpre F, Garneau P, Christen Y, Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic Biol Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 139.Shi C, Zhao L, Zhu B, Li Q, Yew DT, Yao Z, Xu J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem Biol Interact. 2009;181:115–123. doi: 10.1016/j.cbi.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Yao Z, Drieu K, Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001;889:181–190. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- 141.Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004;1030:317–329. doi: 10.1196/annals.1329.040. [DOI] [PubMed] [Google Scholar]
- 142.Ono K, Condron MM, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu JT, Choi RC, Xie HQ, Zheng KY, Guo AJ, Bi CW, Lau DT, Li J, Dong TT, Lau BW, Chen JJ, Tsim KW. Hibifolin, a flavonol glycoside, prevents beta-amyloid-induced neurotoxicity in cultured cortical neurons. Neurosci Lett. 2009;461:172–176. doi: 10.1016/j.neulet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 144.Silva BA, Dias AC, Ferreres F, Malva JO, Oliveira CR. Neuroprotective effect of H. perforatum extracts on beta-amyloid-induced neurotoxicity. Neurotox Res. 2004;6:119–130. doi: 10.1007/BF03033214. [DOI] [PubMed] [Google Scholar]
- 145.Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience. 2007;145:911–922. doi: 10.1016/j.neuroscience.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 146.Roth A, Schaffner W, Hertel C. Phytoestrogen kaempferol (3, 4′, 5, 7-tetrahydroxyflavone) protects PC12 and T47D cells from beta-amyloid-induced toxicity. J Neurosci Res. 1999;57:399–404. [PubMed] [Google Scholar]
- 147.Niidome T, Takahashi K, Goto Y, Goh S, Tanaka N, Kamei K, Ichida M, Hara S, Akaike A, Kihara T, Sugimoto H. Mulberry leaf extract prevents amyloid beta-peptide fibril formation and neurotoxicity. NeuroReport. 2007;18:813–816. doi: 10.1097/WNR.0b013e3280dce5af. [DOI] [PubMed] [Google Scholar]
- 148.Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Multifunction of myricetin on A beta: neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. J Neurosci Res. 2008;86:368–377. doi: 10.1002/jnr.21476. [DOI] [PubMed] [Google Scholar]
- 149.Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH. Effect of antioxidant flavanone, naringenin, from Citrus junoson neuroprotection. J Agric Food Chem. 2004;52:1520–1525. doi: 10.1021/jf035079g. [DOI] [PubMed] [Google Scholar]
- 150.Zhang HY, Liu YH, Wang HQ, Xu JH, Hu HT. Puerarin protects PC12 cells against beta-amyloid-induced cell injury. Cell Biol Int. 2008;32:1230–1237. doi: 10.1016/j.cellbi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 151.Peng QL, Buz’Zard AR, Lau BH. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain Res Mol Brain Res. 2002;104:55–65. doi: 10.1016/s0169-328x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 152.Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Irie Y, Keung WM. Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-beta peptide. Brain Res. 2003;963:282–289. doi: 10.1016/s0006-8993(02)04050-7. [DOI] [PubMed] [Google Scholar]
- 154.Holcomb LA, Dhanasekaran M, Hitt AR, Young KA, Riggs M, Manyam BV. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimer’s Dis. 2006;9:243–251. doi: 10.3233/jad-2006-9303. [DOI] [PubMed] [Google Scholar]
- 155.Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on beta-amyloid peptide-(25-35)-induced amnesia in mice. Eur J Pharmacol. 2004;506:55–61. doi: 10.1016/j.ejphar.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 156.Joseph JA, Denisova NA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 157.Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 158.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 160.Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, Oh KW, Hong JT. Green tea (−)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 161.Cuevas E, Limon D, Perez-Severiano F, Diaz A, Ortega L, Zenteno E, Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25–35 in rats. Eur J Pharmacol. 2009;616:122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 162.Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001;133:89–96. doi: 10.1038/sj.bjp.0704047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim HS, Cho JY, Kim DH, Yan JJ, Lee HK, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1–42) in mice. Biol Pharm Bull. 2004;27:120–121. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- 164.Cho JY, Kim HS, Kim DH, Yan JJ, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of beta-amyloid peptide (1–42) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:901–907. doi: 10.1016/j.pnpbp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 165.Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-{beta} aggregation pathway. Am J Pathol. 2009;175:2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jin CH, Shin EJ, Park JB, Jang CG, Li Z, Kim MS, Koo KH, Yoon HJ, Park SJ, Choi WC, Yamada K, Nabeshima T, Kim HC. Fustin flavonoid attenuates beta-amyloid (1–42)-induced learning impairment. J Neurosci Res. 2009;87:3658–3670. doi: 10.1002/jnr.22159. [DOI] [PubMed] [Google Scholar]
- 167.Chauhan NB. Anti-amyloidogenic effect of Allium sativum in Alzheimer’s transgenic model Tg2576. J Herb Pharmacother. 2003;3:95–107. [PubMed] [Google Scholar]
- 168.Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J Ethnopharmacol. 2006;108:385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 169.Chauhan NB, Sandoval J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother Res. 2007;21:629–640. doi: 10.1002/ptr.2122. [DOI] [PubMed] [Google Scholar]
- 170.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 171.Augustin S, Rimbach G, Augustin K, Schliebs R, Wolffram S, Cermak R. Effect of a short- and long-term treatment with Ginkgo biloba extract on amyloid precursor protein levels in a transgenic mouse model relevant to Alzheimer’s disease. Arch Biochem Biophys. 2009;481:177–182. doi: 10.1016/j.abb.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 172.Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65:1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- 173.Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 174.Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer’s amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20:1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 175.Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Abeta(25–35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004;29:860–868. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- 176.Ahn J, Um M, Choi W, Kim S, Ha T. Protective effects of Glycyrrhiza uralensis Fisch. on the cognitive deficits caused by beta-amyloid peptide 25–35 in young mice. Biogerontology. 2006;7:239–247. doi: 10.1007/s10522-006-9023-0. [DOI] [PubMed] [Google Scholar]
- 177.Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1–40 in rats. J Nutr Biochem. 2008;19:619–626. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 179.Liu R, Gao M, Qiang GF, Zhang TT, Lan X, Ying J, Du GH. The anti-amnesic effects of luteolin against amyloid beta (25–35) peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience. 2009;162:1232–1243. doi: 10.1016/j.neuroscience.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 180.Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, Tetsu N, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:739–744. doi: 10.1124/jpet.108.140293. [DOI] [PubMed] [Google Scholar]
- 181.Matsuzaki K, Yamakuni T, Hashimoto M, Haque AM, Shido O, Mimaki Y, Sashida Y, Ohizumi Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci Lett. 2006;400:230–234. doi: 10.1016/j.neulet.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 182.Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Lu P, Mamiya T, Lu LL, Mouri A, Niwa M, Hiramatsu M, Zou LB, Nagai T, Ikejima T, Nabeshima T. Silibinin attenuates amyloid beta(25–35) peptide-induced memory impairments: implication of inducible nitric-oxide synthase and tumor necrosis factor-alpha in mice. J Pharmacol Exp Ther. 2009;331:319–326. doi: 10.1124/jpet.109.155069. [DOI] [PubMed] [Google Scholar]
- 184.Ma WW, Xiang L, Yu HL, Yuan LH, Guo AM, Xiao YX, Li L, Xiao R. Neuroprotection of soyabean isoflavone co-administration with folic acid against beta-amyloid 1–40-induced neurotoxicity in rats. Br J Nutr. 2009;102:502–505. doi: 10.1017/S0007114509274757. [DOI] [PubMed] [Google Scholar]