Summary
Eight right-handed subjects were asked to silently generate a verb to a visual stimulus while the magnetic flux normal to the scalp surface was recorded with a whole-head neuromagnetometer. The spatiotemporal patterns of activation in lateral occipital, inferior parietal, superior temporal, basal temporal, and inferior frontal cortices were estimated using minimum estimation, a distributed source analysis methodology. Although there was significant variability among subjects, averaged data indicated that latencies of peak activation in these regions of interest progressed from posterior to anterior. Peak latencies were earliest in lateral occipital cortex and latest in pars opercularis and pars triangularis in the inferior frontal gyrus. Lateralization of activation was strongest in pars opercularis, which is part of classical Broca’s area, with activation being stronger in this area within the left hemisphere in every subject. Results provide support for the use of magnetoencephalography in conjunction with MNE analysis for the purpose of lateralizing and localizing language-specific activation in frontal areas as well as the study of the spatiotemporal parameters of brain activation associated with cognitive function.
Keywords: Magnetoencephalography, Cognition, Naming
Broca’s region includes the pars opercularis and pars triangularis of the inferior frontal gyrus (IFG) in the dominant (usually left) hemisphere. Originally thought to be primarily involved in assembly of phonological representations for the purpose of speech production (Broca, 1861), relatively recent functional imaging studies have found these regions to be active during a variety of linguistic tasks, including those requiring phonological (Thierry et al., 1999), syntactical (Heim et al., 2003), and semantic (Amunts et al., 2004) processing. It has therefore been proposed that IFG may contain subdivisions involved in computations for specific linguistic functions (Bookheimer, 2002). The purpose of the current study is to characterize the spatiotemporal pattern of activation associated with silent naming of an action represented in a picture using magnetoencephalography (MEG), focusing on IFG. Functional imaging modalities that rely on hemodynamic response, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), consistently detect activation in the left inferior frontal lobe, including the classical Broca’s area, during the performance of verb generation (Blacker et al., 2006; Costafreda et al., 2006; Gaillard et al., 2000; Harrington et al., 2006) and picture naming (Harrington et al., 2006; Moore and Price, 1999) tasks. Activation in temporoparietal language areas of the left hemisphere (including posterior superior temporal and supramarginal gyri) is also reported during verb generation (Harrington et al., 2006; Holland et al., 2001) and naming (Bright et al., 2004; Harrington et al., 2006; Moore and Price, 1999; Perani et al., 1999) tasks. Activation in left (Bright et al., 2004; Moore and Price, 1999) and right (Saccuman et al., 2006) fusiform gyrus during picture naming is also reported. Such tasks have been used to successfully determine hemispheric dominance for language function (Baciu et al., 1999; Harrington et al., 2006; Kamada et al., 2007). Although studies using fMRI or PET have produced important data regarding the spatial pattern of activation during covert and overt production of verbs and naming of pictures, one of the distinct advantages of MEG in comparison to other in vivo imaging modalities is its ability to provide temporal in addition to spatial information regarding the pattern of brain activation that occurs in conjunction with task performance. In an early MEG study regarding the spatiotemporal pattern of activation associated with picture naming Salmelin et al. (1994) reported a progression of activation beginning in occipital areas bilaterally within the first 200 ms after stimulus presentation, progressing to left temporal areas between 200 and 400 ms after stimulus presentation and then to left frontal areas at 400 to 600 ms post stimulus presentation. Levelt et al. (Levelt et al., 1998) found a similar temporal progression during picture naming, as well as an additional area in right inferior parietal cortex just posterior to the superior temporal gyrus that overlapped temporally with the left temporal activation. Other investigators have identified a similar time course and lateralization of activation for these regions in picture (Kober et al., 2001; Soros et al., 2003) and action (Soros et al., 2003) naming studies using MEG. Although some MEG studies report difficulty detecting activation in IFG during either noun or verb generation tasks (Levelt et al., 1998; Soros et al., 2003), others report consistent detection of activation in this area during, generally greater in the left as compared with the right hemisphere (Bowyer et al., 2004; Kober et al., 2001). In the current study we apply minimum norm estimation (MNE) methodology to determining the current distribution at any point in time that contributes to the pattern of magnetic flux that is observed at the scalp using the MEG scanner (Hamalainen and Ilmoniemi, 1994; Sarvas, 1987). In comparison to approaches that restrict solutions to single sources at any given point in time, MNE allows for the distribution of current among a number of sources distributed throughout the brain. A distributed source methodology may have advantages in modeling brain activation during the performance of cognitive tasks where a number of areas may be actively involved in computations at any given point in time. We hypothesized that, as in previous studies activation would spread from posterior, occipital cortices, to temporal and then anterior, frontal areas. We also expected that lateralization of activity would progress from primary bilateral to predominantly leftward as activation spread from posterior, sensory areas, to inferior frontal areas.
METHODS
Participants
Participants were eight right-handed adults (ages 18 to 75, male = 43, SD = 19), with no history of neurologic dysfunction. Handedness was determined using the Edinburgh Handedness Inventory (Oldfield, 1971). Participants were presented with a simple line drawing taken from the Action Naming Test (Obler and Albert, 1979) and told to silently name the action it depicted. Event-related fields (ERFs) were recorded to each presentation of the visual stimulus. Stimuli consisted of 160 drawings. A short practice period during which the subjects were introduced to the task preceded data collection. Two separate blocks of trials were obtained at different time points. Stimuli were presented via an arrangement of mirrors with a variable interstimulus interval (0.6 to 1.0 seconds) for 1 second. Participants were asked to silently name the action depicted by the picture as quickly as possible. The participant was asked to keep his/her eyes open during stimulus presentation, fixating on a dark dot placed in the center of the mirror, to reduce eye movements or blinks and prevent ERF contamination by rhythmic activity (typically in the alpha band), which can seriously interfere with the accurate detection of task-related brain activity.
MEG Data Acquisition and Analysis
ERFs time-locked to the presentation of pictures were recorded in a magnetically shielded room, using a whole-head neuromagnetometer (4D 3600, 4D NeuroImaging; San Diego, CA) equipped with an array of 248 gradiometer sensors and housed in a magnetically shielded room designed to reduce environmental magnetic noise that might interfere with biologic signals. The signal was recorded with a band pass filter set at 0.1 and 50 Hz. One second of data was recorded with a sample rate of 254 Hz, including a 150-ms prestimulus period.
Single-trial ERF segments identified as contaminated by eye-related or head movement–related magnetic artifacts (defined as magnetic flux deflections in excess of 3 picoTesla (pT) peak-to-peak in the recordings from magnetometer sensors placed just above the eyes) were removed and the recordings were filtered with a band pass between 0.1 and 20 Hz, and subjected to an adaptive filtering procedure that is part of the 4-D neuroimaging signal analysis package. T1-weighted MRI (TR 13.6 ms; TE 4.8 ms; recording matrix 256 × 256 pixels, 1 excitation, 240-mm field of view, and 1.4-mm slice thickness) was obtained from six of the eight participants. In two cases, the subject’s own MRI was not available and substitute MRIs, which fit the head shape files of these two subjects, were substituted. Results reported below were not changed when these two subjects were removed; therefore they were retained in the study.
MRI and MEG data were coregistered using an automated coregistration routine within MNE, which aligns digitization points obtained while the subject is within the MEG scanner with the fiducial points demarcated on the outer skin surface reconstruction of the MRI. MEG source localization was performed using L1 minimum-norm estimation routines (Hamalainen and Ilmoniemi, 1994; Ilmoniemi, 1993) contained in MNE software (Hamalainen, 2006). The L1 estimation results in a current distribution with the smallest integral of the absolute value of the current density that could generate the measured magnetic field and provides a minimum-norm current estimate (Uutela et al., 1999) with location and strength information for the current sources at each time point. Estimated current sources were anatomically constrained by an MRI-derived surface model of each participant’s brain. This surface model was generated by a fully automated cortical surface reconstruction procedure using FreeSurfer software (Dale et al., 1999) for producing a detailed geometric description (e.g., regular tessellation of the cortical surface consisting of equilateral triangles known as vertices) of the gray-white matter boundary of the neocortical mantle and the mesial temporal lobe. Additionally, for computing the forward solution, the MNE software was used to construct a single compartment boundary element model using triangular tessellations to model each vertex as a potential current dipole perpendicular to the cortical surface during the forward calculations. The inverse solution was subsequently reduced to obtaining an estimate of the scalar distribution of dipole strength across current sources within orientation-specific cortical patches of vertices (Dale and Sereno, 1993).
Unlike equivalent current dipole (ECD) modeling, MNE requires no a priori information of the possible source configuration or restriction of the MEG channels included in the modeling (Uutela et al., 1999).
RESULTS
Regions of interest (ROIs) were chosen based on previous research and included lateral occipital cortex, fusiform gyrus, inferior parietal cortex, superior temporal gyrus, and areas within the inferior frontal gyrus including pars opercularis and pars triangularis bilaterally.
Average Current as a Function of Time in Each ROI
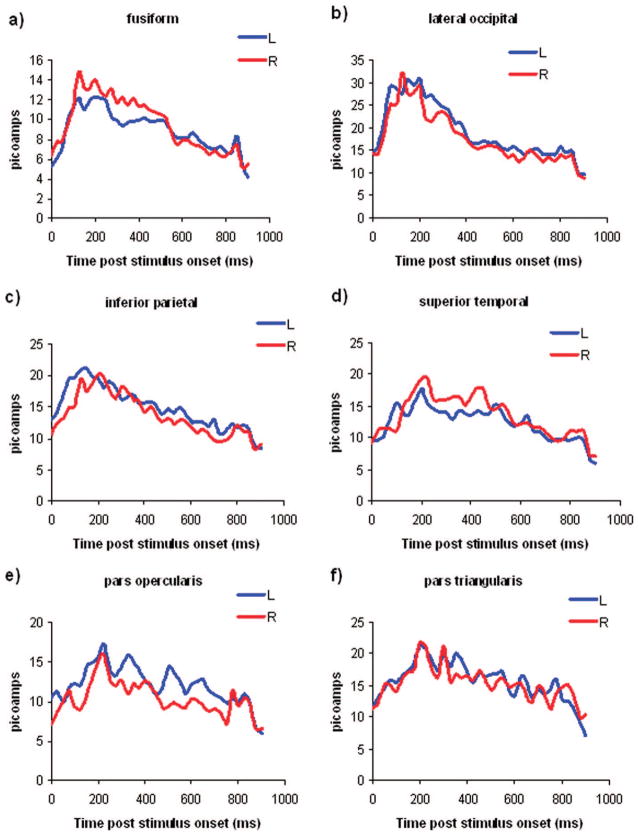
Estimated current was averaged across sessions for each source and then across sources within each ROI in 25-ms bins for each subject and then averaged across subjects. This data are presented in Fig. 1, a through f.
FIGURE 1.
Temporal evolution of activation in the fusiform gyrus (a); lateral occipital cortex (b); inferior parietal cortex (c); superior temporal gyrus (d); pars opercularis (e); and pars triangularis in the left (blue line) and right (red line) hemispheres (f).
Peak Activation as a Function of Time in Each ROI
The latency of peak activation in each hemisphere for each ROI is presented as in Fig. 2. Error bars represent the standard deviation. Although activation progresses temporally from posterior to anterior there is substantial variability among subjects and overlap between ROIs. As expected, activation generally progresses from posterior, primary sensory areas to more anterior areas, although there is a good deal of intersubject variance.
FIGURE 2.
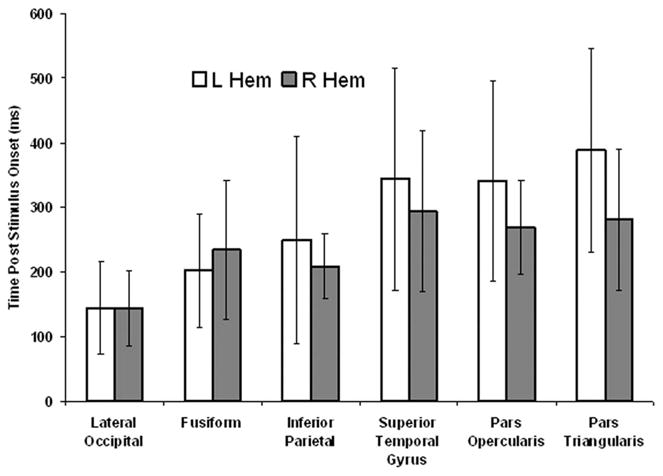
Latency of peak activation in each of the ROIs in the left (open bars) and right (gray bars) hemispheres.
Lateralization of Activation in Each ROI
Laterality was evaluated by forming an asymmetry index as (total right hemisphere amperage – total left hemisphere amperage) in each of three time windows: early (0 ms to 200 ms post stimulus onset), middle (201 ms to 400 ms post stimulus onset), and late (400 to 850 ms after stimulus onset) for each ROI. Whether this asymmetry index differed significantly from 0 (indicating lateralized activation) during each time window was determined using a one-sample t-test and a critical p value of 0.0167 (0.05/3). Only the index for the pars opercularis (M = −1.7 picoamps, SD = 1.42 picoamps) during the late time window was significant [t (1, 7) = −3.28, P < 0.013], suggesting left hemisphere lateralization of activation during this late time window in this area of the inferior frontal gyrus. All eight participants exhibited greater left as compared with right hemisphere activation in the pars opercularis during the late time window.
MEG-MRI coregistered data for one of the participants is presented in Fig. 3. Activation has been integrated over a time period of 50 ms within the a) early (centered at 175 ms after stimulus onset), b) middle (centered at 275 ms post stimulus onset), and c) late (centered at 675 ms after stimulus onset) time periods within each hemisphere. Consistent with the averaged data, during the early time period strong activation within occipital cortex is observed bilaterally, during the middle time period activation shifts anteriorly, including temporal and frontal areas, whereas during the late time period focal activation within left posterior superior temporal and inferior frontal gyrus is evident, particularly within the left hemisphere.
FIGURE 3.
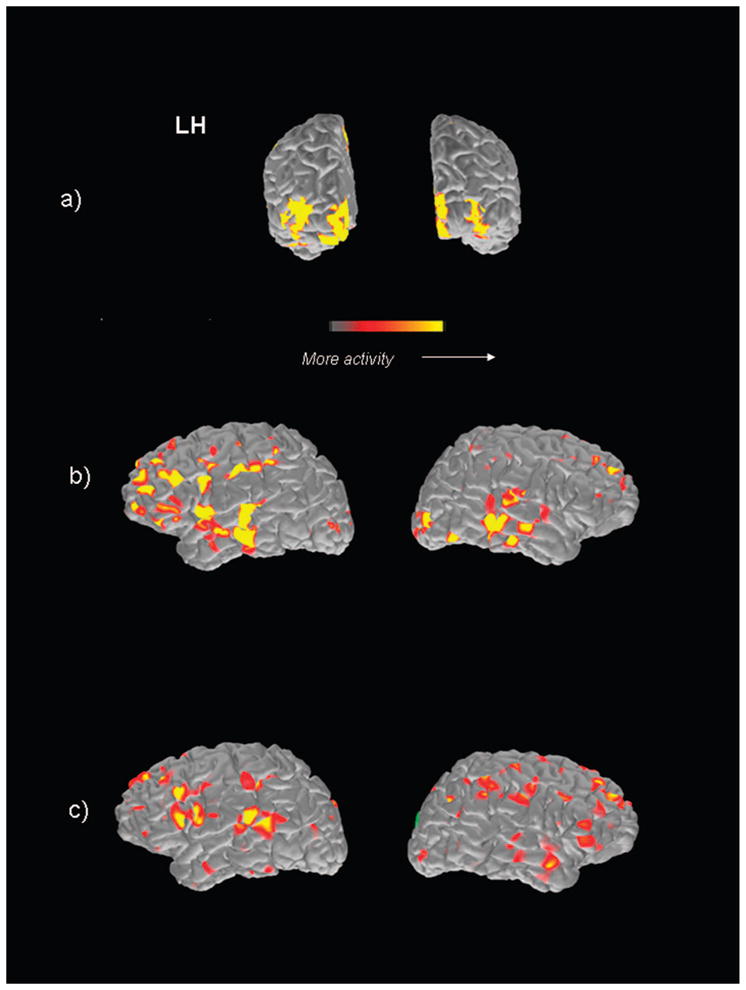
MEG-MRI coregistered data for one participant. Activation has been integrated over a time period of 50 ms within the a, early (centered at 175 ms after stimulus onset); b, middle (centered at 275 ms after stimulus onset); and c, late (centered at 675 ms post stimulus onset) time periods within each hemisphere.
DISCUSSION
The results of this study are consistent with previous functional imaging studies involving naming paradigms using PET (Damasio et al., 2001), fMRI (Saccuman et al., 2006), and MEG (Bowyer et al., 2004; Kober et al., 2001), in indicating consistent left hemisphere lateralization of activation in areas of IFG during an action naming task. In our study, as in others that use verb generation to pictorial stimuli as a task, this activity was mainly in pars opercularis, which is an area just anterior to the face area of the motor strip, and did not extend to pars triangularis, an area of the IFG just anterior to pars opercularis.
Although there was significant individual variance in the latency of peak activation in ROIs and overlap between timing of activation among areas was common, as has been reported previously (Levelt et al., 1998; Salmelin et al., 1994; Vihla et al., 2006), averaged data were consistent with a progression of activation that generally proceeded from posterior to anterior cortical areas temporally. Initial activation in primary sensory areas of occipital cortex was followed by activation in basal temporal areas (fusiform gyrus), inferior parietal areas and then by areas within the inferior frontal gyrus (pars opercularis and pars triangularis) associated with location of the classical Broca’s area and a variety of linguistic functions. This is consistent with other MEG studies (e.g., Soros et al., 2003; Vihla et al., 2006). Interestingly, superior temporal activity occurred in the same time frame as the more frontal activation. This may be related to participation of this latter area in lexical access and/or access to phonological representations and interactions with more frontal areas, even though the task itself was subvocal. A functional subdivision has been proposed for left IFG, with area 45, or pars triangularis, more involved in semantic aspects of language processing, whereas area 44, or pars opercularis may be more involved in speech production per se (Amunts et al., 2004). The current study used a silent action naming task. Although subjects may automatically begin the process of speech production during such a task, it clearly requires semantic processing as well. Interestingly, we found only laterality effects for pars opercularis, not pars triangularis. Although this does not mean that the latter was not involved in the task the reason for lack of laterality effects in an area involved in semantic processing of verbal material is not clear. One hypothesis is that the pictorial nature of the stimuli activated homotopic areas of the right hemisphere involved in nonverbal aspects of the task to an extent similar to the left hemisphere. Although the differences in the spatiotemporal parameters of activation between areas within the IFG supports the hypothesis of functional subdivisions within this areas, further research would be necessary to establish the cognitive components underlying the observed neurophysiological differences.
The identification of the semantic content of a visual stimulus is a relatively complex cognitive process involving a number of specialized, spatially distributed areas in the brain that likely overlap in their temporal pattern of activation. Use of methodologies such as MNE have an advantage in imaging the activation associated with such tasks over those that rely on the identification of surface maps that reflect the presence of compact cortical sources in allowing for detection of concurrent, spatially distributed activation that may more accurately reflect pattern of activation. This may have allowed the detection in the current study of consistently left hemisphere lateralization of activation in IFG, specifically in pars opercularis, in the current study as in other studies that have used distributed source methodologies (e.g., Bowyer et al., 2004; Kober et al., 2001), whereas those that have attempted to account for the surface distribution in terms of equivalent current dipoles have had more limited success (e.g., (Bowyer et al., 2004; Levelt et al., 1998; Salmelin et al., 1994; Soros et al., 2003).
There was a wide range of ages among the subjects in the current study. Although there were no significant relationships between age and any of the variables analyzed in the data presented above, this is a small study, and a more systematic exploration of age differences in the parameters of brain activation during cognitive tasks might well produce significant effects of age.
In summary, the current study supports the use of MEG in conjunction with MNE analysis for the purpose of lateralizing and localizing language-specific activation in frontal areas as well as the study of the spatiotemporal parameters of brain activation associated with cognitive tasks that are likely to be accompanied by spatially distributed and temporally overlapping activation. In addition, findings support the hypothesis that left IFG contains areas specialized for different aspects of linguistic processing.
Acknowledgments
This work was supported by NI H/NINDS Grant P51-NS046588 to A.C. Papanicolaou.
References
- Amunts K, Weiss PH, Mohlberg H, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space: the roles of Brodmann areas 44 and 45. Neuroimage. 2004;1:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Baciu M, Rubin C, Decorps M, Segebarth C. Hemispheric language dominance testing by means of fMRI. J Neuroimaging. 1999;4:246–257. doi: 10.1111/jon199994246. [DOI] [PubMed] [Google Scholar]
- Blacker D, Byrnes ML, Mastaglia FL, Thickbroom GW. Differential activation of frontal lobe areas by lexical and semantic language tasks: a functional magnetic resonance imaging study. J Clin Neurosci. 2006;1:91–95. doi: 10.1016/j.jocn.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bowyer SM, Moran JE, Mason KM, et al. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology. 2004;12:2247–2255. doi: 10.1212/01.wnl.0000130385.21160.7a. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain Lang. 2004;3:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siège de la facultédu langage articulé, suives d’une observation d’aphemie. Bull Soc Anat. 1861:330–357. [Google Scholar]
- Costafreda SG, Fu CH, Lee L, et al. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;10:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, et al. Neural correlates of naming actions and of naming spatial relations. Neuroimage. 2001;6:1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;1:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Hamalainen M. MNE Software Users Guide. Version 2.5. Charlestown, MA: 2006. [Google Scholar]
- Hamalainen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;1:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Harrington GS, Buonocore MH, Farias ST. Intrasubject reproducibility of functional MR imaging activation in language tasks. AJNR Am J Neuroradiol. 2006;4:938–944. [PMC free article] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD. Distributed cortical networks for syntax processing: Broca’s area as the common denominator. Brain Lang. 2003;3:402–408. doi: 10.1016/s0093-934x(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;4:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ. Models of source currents in the brain. Brain Topogr. 1993;4:331–336. doi: 10.1007/BF01128686. [DOI] [PubMed] [Google Scholar]
- Kamada K, Sawamura Y, Takeuchi F, et al. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;2:296–305. doi: 10.1227/01.NEU.0000249262.03451.0E. [DOI] [PubMed] [Google Scholar]
- Kober H, Moller M, Nimsky C, et al. New approach to localize speech relevant brain areas and hemispheric dominance using spatially filtered magnetoencephalography. Hum Brain Mapp. 2001;4:236–250. doi: 10.1002/hbm.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Praamstra P, Meyer AS, et al. An MEG study of picture naming. J Cogn Neurosci. 1998;5:553–567. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;2:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Obler LK, Albert ML. The Action Naming Test. Boston: VA Medical Center; 1979. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;1:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti M, et al. Word and picture matching: a PET study of semantic category effects. Neuropsychologia. 1999;3:293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Saccuman MC, Cappa SF, Bates EA, et al. The impact of semantic reference on word class: an fMRI study of action and object naming. Neuroimage. 2006;4:1865–1878. doi: 10.1016/j.neuroimage.2006.04.179. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R, Lounasmaa OV, Sams M. Dynamics of brain activation during picture naming. Nature. 1994;6470:463–465. doi: 10.1038/368463a0. [DOI] [PubMed] [Google Scholar]
- Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol. 1987;1:11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- Soros P, Cornelissen K, Laine M, Salmelin R. Naming actions and objects: cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. Neuroimage. 2003;4:1787–1801. doi: 10.1016/s1053-8119(03)00217-9. [DOI] [PubMed] [Google Scholar]
- Thierry G, Boulanouar K, Kherif F, et al. Temporal sorting of neural components underlying phonological processing. NeuroReport. 1999;12:2599–2603. doi: 10.1097/00001756-199908200-00029. [DOI] [PubMed] [Google Scholar]
- Uutela K, Hamalainen M, Somersalo E. Visualization of magnetoencephalographic data using minimum current estimates. Neuroimage. 1999;2:173–180. doi: 10.1006/nimg.1999.0454. [DOI] [PubMed] [Google Scholar]
- Vihla M, Laine M, Salmelin R. Cortical dynamics of visual/semantic vs phonological analysis in picture confrontation. Neuroimage. 2006;2:732–738. doi: 10.1016/j.neuroimage.2006.06.040. [DOI] [PubMed] [Google Scholar]