Abstract
Rationale
Blood pressure (BP) usually decreases during nocturnal sleep and increases during daytime activities. Whether the endogenous circadian control system contributes to this daily BP variation has not been determined under appropriately controlled conditions.
Objective
To determine if there exists an endogenous circadian rhythm of BP in humans.
Methods and Results
In 28 normotensive adults (16 men), we assessed BP across three complementary, multi-day, in-laboratory protocols performed in dim light, throughout which behavioral and environmental influences were controlled and/or uniformly distributed across the circadian cycle via: (1) a 38-h ‘constant routine’, including continuous wakefulness; (2) a 196-h ‘forced desynchrony’ with seven recurring 28-h sleep/wake cycles; and (3) a 240-h ‘forced desynchrony’ with twelve recurring 20-h sleep/wake cycles. Circadian phases were derived from core body temperature. Each protocol revealed significant circadian rhythms in systolic and diastolic BP, with almost identical rhythm profiles among protocols. The peak-to-trough amplitudes were 3–6 mmHg for systolic BP and 2–3 mmHg for diastolic BP (always P<0.05). All six peaks (systolic and diastolic BP in three protocols) occurred at a circadian phase corresponding to ~9 PM. Based on substantial phase differences among circadian rhythms of BP and other variables, the rhythm in BP appeared to be unrelated to circadian rhythms in cortisol, catecholamines, cardiac vagal tone, heart rate, or urine flow.
Conclusions
There exists a robust endogenous circadian rhythm in BP. The highest BP occurred at the circadian time corresponding to ~9 PM, suggesting that the endogenous BP rhythm is unlikely to underlie the well-documented morning peak in adverse cardiovascular events.
Keywords: Circadian, Blood Pressure, Humans, Myocardial Infarction, Stroke
Numerous epidemiological studies reveal a profound morning increase in the incidence of adverse cardiovascular events, including sudden cardiac death, ventricular arrhythmia, stroke1 and myocardial infarction.2 The extent to which these peaks are caused by a day/night pattern of behaviors and/or endogenous circadian factors is unknown.3 The circadian timing system orchestrates endogenous circadian rhythms in physiology and behavior and is composed of the master circadian pacemaker located in the suprachiasmatic nucleus and circadian oscillators in peripheral tissues.4 The suprachiasmatic nucleus may influence the cardiovascular system via multi-synaptic neural projections to the heart, adrenal cortex, adrenal medulla, kidneys, and vasculature and resultant neural or endocrine effects.5 Moreover, recent animal investigations have also documented the actions of molecular circadian clocks in peripheral tissues that can affect BP,6 ischemia/reperfusion tolerance,7 and vascular remodeling.8 In contrast, mechanistic circadian studies of cardiovascular function in humans are sparse.
A primary risk factor for adverse cardiovascular events is elevated arterial blood pressure (BP).9 Countless studies have documented the day/night variation of BP in humans, which has been used to classify hypertensive patients into nocturnal ‘dippers’ (≥10% drop in BP overnight) and ‘non-dippers’, and it has been shown that ‘non-dippers’ are at increased risk for serious adverse events.10 However, no studies have adequately studied the relative contributions of sleep and the endogenous circadian cycle to this day/night BP variation. Thus, we tested the hypothesis that there exists an endogenous circadian rhythm in BP in humans.
Methods
Subjects
Subjects gave informed consent and the studies were approved by the local Human Research Committee. We studied 28 adults (16 men) who were normotensive, non-obese and healthy (other than mild asthma, n = 6), and who took no medications (other than oral contraceptives and β2-agonist rescue inhalers for asthma), and no caffeine, alcohol or nicotine products for 2–3 weeks immediately prior to and throughout the laboratory studies. The subjects with asthma participated in only one of three protocols and all data in the 4 h following any rescue inhaler use were excluded from analyses (see Supplemental Materials).
Protocols
Quantifying the effect of the endogenous circadian system on BP requires controlling all environmental factors and behaviors that can affect either BP or the circadian cycle (e.g., activity, posture, meals, sleep, room temperature, light) while measuring BP across the circadian cycle, and measuring an endogenous circadian phase marker that is relatively independent of behavior (e.g., core body temperature, rather than activity levels which has been used in most animal studies and greatly affects BP). This was achieved by measuring BP throughout three separate and complementary circadian protocols, as shown in Figure 1. To stabilize circadian rhythms, subjects maintained a regular sleep-wake schedule for 2–3 weeks before entering the laboratory, followed by two baseline days and nights in the laboratory (16-h scheduled wakefulness, 8-h scheduled sleep). Thereafter, to avoid resetting the phase of the circadian system, all laboratory protocols were performed in dim light (<4 lux).11 Subjects completed one of the three protocols: (1) 38-h ‘constant routine’ with continuous wakefulness, semi-recumbency, and 2-hourly isocaloric snacks (CR; Figure 1 top panel); (2) 196-h ‘forced desynchrony’ with seven recurring 28-h sleep/wake cycles (FD28; Figure 1 middle panel: 18-h 40-min wakefulness, 9-h 20-min sleep); or (3) 240-h ‘forced desynchrony’ with twelve recurring 20-h sleep/wake cycles (FD20; Figure 1 lower panel: 13-h 20-min wakefulness, 6-h 40-min sleep). In essence, the CR abolished sleep and minimized behaviors, whereas the FD28 and FD20 maintained a normal sleep:wake ratio of 1:2, and scheduled all activities so that they became uniformly distributed across the circadian cycle.
Figure 1. Three complementary protocols used to examine underlying circadian rhythmicity of blood pressure.
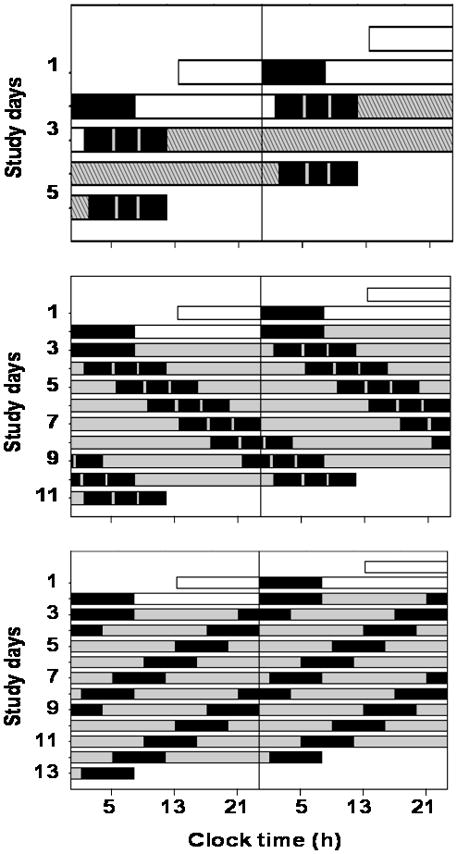
Three protocols designed to keep behaviors constant across the circadian cycle (top panel, 2 baseline days followed by 38-h ‘constant routine’ while semi-recumbent and awake) or to evenly distribute behaviors across all circadian phases (middle panel, 7 recurring 28-h behavioral cycles [28-h ‘forced-desynchrony’]; bottom panel, 12 recurring 20-h behavioral cycles [20-h forced desynchrony]). In each panel, subsequent days are ‘double-plotted’ to the right and below prior days to visually aid protocol continuity. X-axes: clock times for an example subject having an habitual wake time of 8 AM. Black boxes, scheduled sleep episodes in darkness; gray/hatched bars, scheduled wakefulness in dim light conditions (<4 lux) to avoid circadian rhythm resetting.11
Measurements and Analyses
Subjects wore a flexible rectal temperature sensor for measurement of core body temperature (CBT), which was used as the circadian phase marker.12 For each subject, the fitted CBT minimum was assigned as a reference phase marker of 0°. BP was measured repeatedly by automatic oscillometric cuff sphygmomanometer from an upper arm. Heart rate was also measured in each protocol. These measurements were made throughout periods of relaxed wakefulness during each protocol. Data were assigned a circadian phase and the existence of any circadian rhythms were tested by cosinor mixed-model analyses of variance. To assess whether the rhythms were robust, the phases of peaks, and the amplitudes of the circadian rhythms of BP and heart rate were compared across protocols using analyses of variance. To gain insight into control mechanisms across the circadian cycle, most of the following potentially related variables were measured throughout each protocol; cardiac vagal tone (estimated from high frequency power of inter-beat interval variability), plasma cortisol, urinary or plasma catecholamines and urine flow. The phases of peaks and troughs of these potentially related variables were compared to the circadian rhythm of BP using analyses of variance. Further details are provided in the Supplemental Materials and Online Table I and Online Figure I.
Results
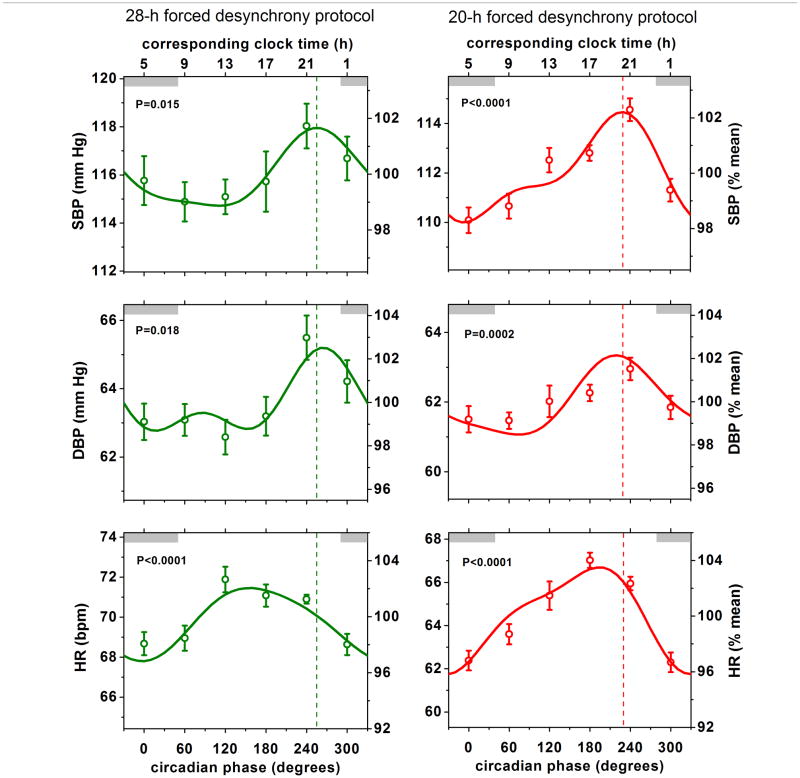
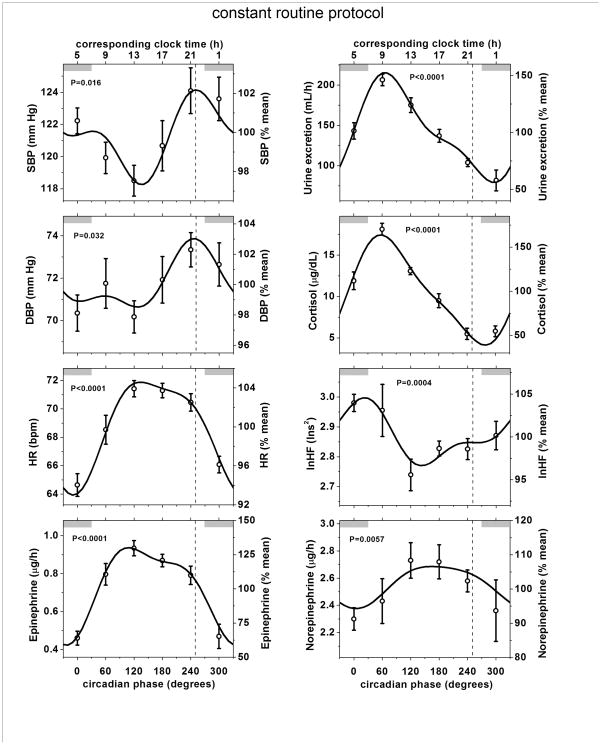
Each protocol revealed significant circadian rhythms in systolic and diastolic BP, with almost identical rhythm amplitudes and phase relationships among protocols (Figures 2 and 3; Supplemental Material Online Figure I and Online Table I). For the three protocols, the peak-to-trough amplitudes were 3–6 mmHg for systolic BP and 2–3 mmHg for diastolic BP (P always <0.05). All six peaks (systolic and diastolic BP in three protocols) occurred in the circadian phase range of 219–265°, with an average phase of 244°, equivalent to ~9 PM (Supplemental Material Online Table I). The average circadian phase of the peak in heart rate across the 3 protocols was 161°, which is ~6 h earlier than the average circadian peak in BP. During the constant routine protocol there were substantial and significant differences between the timing of the circadian peak in BP (systolic and diastolic) and all other potentially relevant circadian rhythms measured, including circadian peaks in heart rate, urinary catecholamines and plasma cortisol, and circadian troughs in urine flow and cardiac vagal tone (Figure 3 and Supplemental Material Online Table 1). Similarly, results for these variables during the two forced desynchrony protocols (where available), were highly consistent with the constant routine data (see Supplemental Material Online Table I and Online Figure I). Thus, the endogenous circadian rhythm in BP appeared to be unrelated to circadian rhythms in cortisol, catecholamines, cardiac vagal tone, heart rate, or urine flow.
Figure 2. Similar endogenous circadian variations in blood pressure across two complementary forced desynchrony protocols.
Shown are (mean ± SEM) systolic BP (SBP), diastolic BP (DBP) and heart rate (HR) expressed in absolute units (left axes) and as percentages of individual averages (right axes). Data are aligned according to circadian phase (X-axis) and plotted in 60° bins (equivalent to ~4 h). Corresponding approximate clock time is shown on top X-axis. 0° represents core body temperature minimum (~5 AM in these subjects). Thin gray shaded bars are shown at the top of each panel to indicate the average equivalent clock time when subjects would normally sleep when at home (although all data were collected during wakefulness in the laboratory). Solid lines represent the Cosinor model fit and the p values indicate significance of circadian rhythmicity. The phases of the peak SBP are shown by dashed vertical lines in each protocol and occurred close to 240° (equivalent to ~9 PM), which was similar to the timing of the peaks in DBP, but quite distinct from the timing of the circadian peaks in HR.
Figure 3. Endogenous circadian variation in blood pressure during the constant routine protocol is out of phase with circadian variations in other measured variables.
Shown are data from the constant routine protocol. lnHF is a marker of cardiac vagal tone (see legend to Figure 2 for other abbreviations and explanations). The phase of peak SBP is shown in each panel by a dashed vertical line close to 251° which was similar to the timing of the circadian peak in DBP, but quite distinct from the circadian peaks or troughs in other relevant physiological variables shown above in the other panels. Very similar circadian rhythms occurred in the two forced desynchrony protocols (Figure 2; Supplemental Material Online Figure I and Online Table I).
Discussion
The current study has revealed an endogenous circadian rhythm of BP. This study yielded robust results that were almost identical in three groups of subjects during three different protocols. This study was performed because of the possible relevance of endogenous circadian BP rhythms to the day/night pattern of adverse cardiovascular events. Our results are perhaps unexpected because the timing of the endogenous circadian peak in BP occurs in the evening – whereas the lowest circadian BP occurs around the most vulnerable time for adverse cardiovascular events. Thus, our data suggest that the morning peaks in adverse cardiovascular events are not caused by circadian rhythm-related increases in BP. Presumably, endogenous circadian rhythms in other cardiovascular variables (e.g. platelet function, sympathovagal balance, and endothelial function), and/or physiological responses to day/night patterns of behavioral changes are more important in this regard. We note that waking in the evening, as can occur with shift work, jet lag and sleep disorders, may result in an exaggerated BP surge due to summed behavioral- and circadian-related increases in BP, perhaps helping to explain the curious secondary evening peak in incidence of myocardial infarction in vulnerable individuals2. Moreover, there may be more than simple summation of these effects, whereby the circadian system modulates the BP response to a standardized stress.13,14,15
This study was performed in healthy subjects and it remains to be seen whether the endogenous circadian morning trough in BP that we observed could increase the risk of ischemic stroke at that time in people with existing carotid or cerebral artery stenoses, and whether the amplitudes or phases of the endogenous circadian BP rhythms are abnormal in more vulnerable groups.
There are many studies that refer to a ‘circadian rhythm’ of BP in humans and other animals without clarifying whether this is an endogenous rhythm, as almost all such studies made BP measurements across the 24-h period while permitting sleep and altered activity across the day and night, such that these behaviors rather than endogenous circadian rhythmicity could cause much of these observed day/night pattern in BP. Our study overcame many such limitations and quantified the independent endogenous circadian rhythmicity of BP, and ought to help clarify some misperceptions in the field due to such methodological and interpretative problems (see discussion in Supplemental Materials).
Regarding mechanisms, there were notable circadian rhythms in numerous variables that would be expected to correlate in the short term with acute changes in BP, including cortisol, catecholamines, cardiac vagal tone, heart rate, and urine flow (likely inversely related to blood volume). However, based on the substantial phase differences among these rhythms, the endogenous circadian evening peaks in systolic and diastolic BP did not appear to be mechanistically linked with the endogenous ~24 rhythmicity of these other variables. This is an intriguing result and points to different control mechanisms, which have yet to be elucidated, that must regulate BP across the circadian cycle.
Supplementary Material
Acknowledgments
Sources of Funding: NIH grants R01 HL64815, K24 HL76446, P30 HL101299 and GCRC M01 RR02635.
Non-standard Abbreviations
- BP
Blood pressure
- CR
Constant routine protocol
- FD20
Forced desynchrony protocol with 12 recurring 20-h sleep/wake cycles
- FD28
Forced desynchrony protocol with 7 recurring 28-h sleep/wake cycles
- CBT
Core body temperature
Footnotes
Disclosures: None
References
- 1.Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, Hier DB, Wolf PA, Caplan LR, Foulkes MA. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476. doi: 10.1161/01.str.20.4.473. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 3.Shea SA, Hilton MF, Muller JE. Day/Night Patterns of Myocardial Infarction and Sudden Cardiac Death: Interacting Roles of the Endogenous Circadian System and Behavioral Triggers. In: White WB, editor. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Humana Press; 2007. pp. 251–289. [Google Scholar]
- 4.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 5.Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 6.Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res. 2009;105:1047–1061. doi: 10.1161/CIRCRESAHA.109.206201. [DOI] [PubMed] [Google Scholar]
- 7.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen WB, Vestbo J, Jensen GB. Isolated systolic hypertension as a major risk factor for stroke and myocardial infarction and an unexploited source of cardiovascular prevention: a prospective population-based study. J Hum Hypertens. 1995;9:175–180. [PubMed] [Google Scholar]
- 10.Fagard RH Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645–653. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 11.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeisler CA Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 13.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–5. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise and their interaction on cardiovascular function. Proc Nat Acad Sci U S A. 2010;107:20541–6. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu K, Scheer AJL, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.943019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.