Summary
BMPs have been shown to play a role in neural tube development particularly as dorsalizing factors. To explore the possibility that BMP2 could play a role in the developing neural tube (NT) beyond the lethality of Bmp2 null embryos we created Bmp2 chimeras from Bmp2 null ES cells and WT blastocysts. Analysis of Bmp2 chimeras reveals NT defects at day 9.5 (E9.5). We found that exclusion of Bmp2 null ES cells from the dorsal NT did not always prevent defects. For further comparison, we used a Bmp2 mutant line in a mixed background. Phenotypes observed were similar to chimeras including open NT defects, postneurulation defects and abnormal neural ectoderm in heterozygous and homozygous null embryos demonstrating a pattern of dose dependent signaling. Our data exposes BMP2 as a unique player in the developing NT for dorsal patterning and identity and normal cephalic neural tube closure in a dose dependent manner.
Keywords: BMP2, neural tube defects, neurulation, neural tube closure, NTD, chimera
Introduction
Bone morphogenetic proteins (BMPs) were originally identified by their ability to cause bone differentiation more than 40 years ago (Urist, 1965). Together with growth and differentiation factors (GDFs), nodal and lefty, BMPs compose a large subgroup within the transforming growth factor-ß (TGF-ß) gene superfamily (Kingsley, 1994; Zhao, 2003). However, recent studies of several organisms demonstrate that several BMPs have other roles during embryogenesis, notably in dorsoventral (DV) and/or anterior–posterior (AP) axis formation and patterning (Hogan, 1996; Mishina, 2003; Whitman, 1998). Although BMPs and their signaling molecules are important factors in patterning in early embryonic development (Kishigami and Mishina, 2005), BMP2 function during early mouse development is poorly understood due to the early lethality of targeted null mutant embryos (Zhang and Bradley, 1996). Very recently, it has been reported that BMP2 has a unique functional role in the developing embryo for the migration but not induction of neural crest cells (Correia et al., 2007). Although, there are still many important questions unanswered as to the role of BMP2 in early organogenesis. A floxed mouse line for Bmp2 has recently become available and BMP2 function in later stages of development has begun to emerge (Ma et al., 2005; Tsuji et al., 2006). However, a limited number of attempts to reveal the function of BMP2 in early stages including neural tube development has been reported (Ybot-Gonzalez et al., 2002; Ybot-Gonzalez et al., 2007). During the 24 hours between E8.5 and E9.5, a mouse embryo goes through rapid and dramatic development (Kaufman et al., 1998). Developmental events at this stage include forelimbs budding, otic and optic pits invaginating, heart looping, along with embryonic turning and primitive gut evolution. Simultaneously, the neural tube (NT) closes and three distinct regions of the brain take shape. Closure in the mouse begins at 3 distinct points, the second of which varies among strains, and then the neural tube zips shut (Copp et al., 2003). While the process of neurulation or neural tube closure can be described its mechanisms and the players involved are still unclear. Dorsal-ventral (DV) patterning, cell shaping, cell proliferation, and cell movement are all actively involved in normal NT development and closure (Brook et al., 1991; Copp et al., 1988; Estibeiro et al., 1993; Padmanabhan, 2006), but the cephalic region has rarely been targeted experimentally (Mackay et al., 2006; Soo et al., 2002; Zohn et al., 2007), for its unique closure issues and differentiation requirements.
In mid-gestation, BMPs function in the progression, differentiation, and development of certain aspects of the brain. For example, BMP4 along with FGFs are involved in neural induction (Jessell and Sanes, 2000; Linker and Stern, 2004). BMP2 has established expression patterns in the dorsal midline of the brain, specifically the roof plate of the telencephalon (Furuta et al., 1997). BMP2 is also linked to glial or CNS fates and retinal patterning, neurogenesis, and astrogliogenesis as well as earlier stage events involving neural crest cells (Fukuda et al., 2007; Kanzler et al., 2000; Sailer et al., 2005; Sakuta et al., 2006; Timmer et al., 2002; Yanagisawa et al., 2001). Numbers of BMPs are expressed with distinctive patterns in the cephalic region prior to and during neural tube closure (Furuta et al., 1997). Notably, Bmp2 is expressed exclusively in the surface ectoderm adjacent to the dorsal neuroectoderm (Furuta et al., 1997) suggesting a unique role for BMP2 in the cephalic region. But is the expression of BMP2 in the surface ectoderm important only for roof plate formation and development after the closure of the neural tube? In this paper, we will describe how the level of BMP2 expression is inversely proportional to the severity of defects in the developing mouse embryo implicating a dose dependent requirement for development during early organogenesis. Furthermore, we propose that BMP2 signaling from and within the surface ectoderm propels NT closure regardless of the establishment of the dorsal identity and supports developing neural tissue in the dorsal portion of the NT.
Results
1. Embryos show cephalic neural tube defects
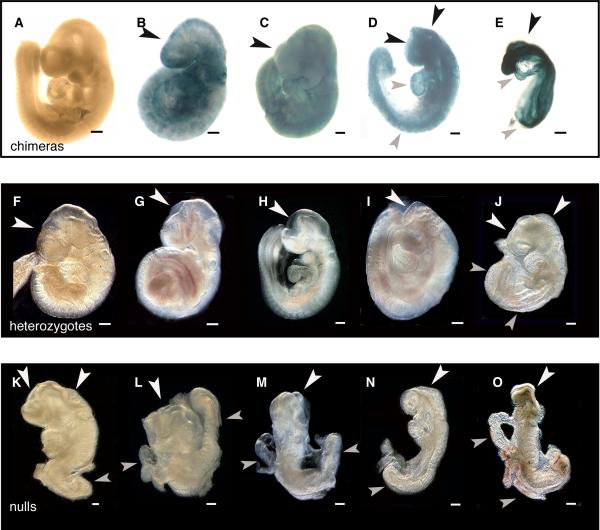
BMP2 chimeras generated from Bmp2 null ES cells and wild type (WT) blastocysts were harvested at E9.5. ES cell-derived cells in chimeras carrying one copy of ROSA26 (Soriano, 1999) were visible after X-gal staining. Embryos at every level of contribution of null ES cells verified that ES cells incorporate into all tissue types at this stage. In Figure 1(A), very low (less than 30%) contribution chimeras showed no overt abnormalities, whereas low to middle contribution chimeras showed slight abnormalities and postneurulation defects in the forebrain region (B); middle-high contribution chimeras show a more severe phenotype including open NT defects and some posterior defects (C); high contribution chimeras show open NT defects, failure to turn, posterior defects, failure to form limb buds, and heart looping defects (D). Very high contribution chimeras showed complete failure to develop beyond the E8.0-E8.5 stage (E). For some, there were no somites formed and no recognizable neural tissue. We used 4 independent Bmp2 -/- ES cell lines and all showed similar phenotypes. No abnormal embryos were recovered when wild type ES cells were used at any level of contribution (data not shown).
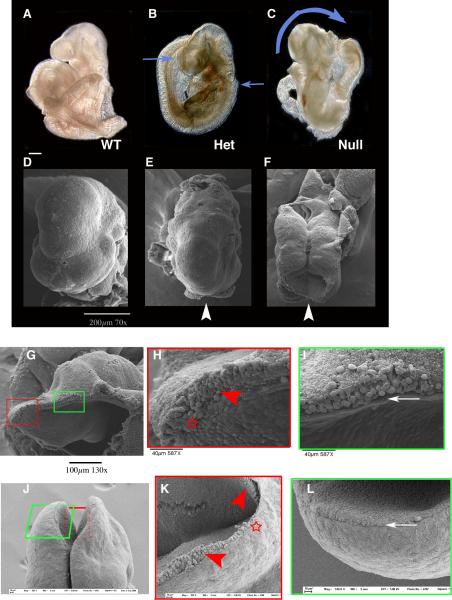
Fig. 1. Abnormalities found in chimeras, heterozygous, and homozygous null embryos.
BMP2 chimeras (A-E) were generated from null ES cells carrying the LacZ gene and wild type blastocysts and were harvested at E9.5. Shown are different levels of contribution of null ES cells to chimeric development in increasing amounts from A to E. X-gal stains null ES cells blue. Note that defects appear at fairly low levels of contribution. Black arrowheads show defects of the cephalic region and grey arrowheads show other stage-specific developmental defects. A. No contribution chimera shows no apparent defects. B. Low contribution chimera has an abnormal shaped head and fails to form telencephalic vesicles. C. Middle contribution chimera shows postneurulation (closed neural tube) defects in the forebrain, midbrain, and hindbrain regions of the developing embryo. The heart has not looped properly. D. High contribution chimera has open neural tube defects as well as stalled development in the anterior and posterior portions of the embryo. There is also abnormal heart looping, a failure to develop limb buds and failure of embryonic turning. E. Very high contribution chimera fails to develop any normal structures including somites. BMP2 Heterozygous and Homozygous Null Embryos, E9.5. White arrowheads show cephalic defects while grey arrowheads show other stage-specific developmental defects. Defects occur in some heterozygous embryos, (F-J) where postneurulation defects show abnormal head shape and lack of telencephalic vesicles (F) or embryos have open NT defects in the forebrain region (G, H) or forebrain-midbrain region, while some have completely open NT in the cephalic region (J). For homozygous nulls (K-O), defects in the developing embryo include failure to close the NT, abnormal heart development, lack of limb bud development and also failure to turn. Scale bar =1mm
We also transferred the targeted Bmp2 mouse line (Zhang and Bradley, 1996) from 129SvEv to a mixed 129/B6 background since mixed genetic backgrounds are generally less vulnerable to genetic defects and show less severe phenotypes than purely inbred strains. We collected litters at E9.5 and, as expected, the heterogeneity increased survival beyond E8.5. Homozygous mutant embryos for Bmp2 displayed phenotypes that mimic the phenotypes of the chimeras (Fig. 1, Table 1). Some heterozygous and all homozygous null embryos showed overt abnormal phenotypes similar in kind to chimeras with middle to very high contribution of null ES cells. In Figure 1 (F-J) heterozygous mice showed postneurulation (F) (category 2) and open NT defects (G-J) (category 3). A small number showed anterior and posterior NT defects, failure to turn and reduced somite count. Homozygous null embryos (Fig. 1 K-O) showed open NT defects, hypomorphism, failure to turn, failure to produce limb buds, heart looping defects and reduced somite count. Table 1 displays these phenotypes grouped into 4 categories based on abnormality. From 17 litters with normal Mendelian ratios we found 79% of heterozygotes and 100% of nulls showed some type of abnormality. Among these embryos, 3% of heterozygotes and 57% of nulls showed the most severe phenotype: no development beyond the 5-somite stage (category 4). The third category (category 3) is defined by phenotypes including reduced overall size, heart looping defects, an open NT in the cephalic region as well as a failure of the embryo to turn for 14% of heterozygous and 43% of homozygous null embryos respectively. The second category contains only heterozygous mice with a partially open cephalic NT that is usually found in the forebrain region and occasionally in the hindbrain region with closure occurring normally at closure point 2. The remaining heterozygotes were considered normal in nature and accounted for 21% of all collected heterozygous embryos. A high incidence of abnormalities found in the heterozygous embryos reflects a skew in the Mendelian ratio versus weaning stage ratios (WT:Het:mut = 58:81:0 from Het x Het 129/B6 background, WT/Het is 1.4 instead of 2.0). Simply, Bmp2 homozygous null, heterozygous and chimeric embryos showed comparable defects in cephalic neural tube closure. For clarification, we will focus for the remainder of this paper on cephalic neural tube development previously categorized in Table 1.
Table 1.
Data shows the various phenotypes of heterozygous and null embryos separated into four categories. Images in Figure 1 show heterozygous mice with phenotypes similar in kind to the chimeras with low to moderate range contribution while the nulls matched the high to very high contribution chimeras.
| Categories | Heterozygotes | Homozygous Nulls |
|---|---|---|
| 1. Normal | 14 (21%) | 0 (0%) |
| 2. Postneurulation, partial open NT | 40 (62%) | 0 (0%) |
| 3. Hypomorphism, heart defects, open NT, failure to turn | 9 (14%) | 9 (43%) |
| 4. No development beyond the 5-somite stage | 2 (3%) | 12 (57%) |
2. BMP2 plays a role in the development of dorsal neural tissue
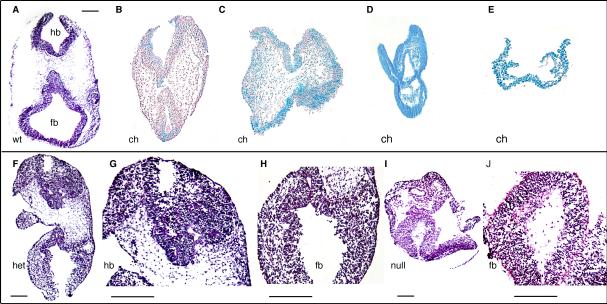
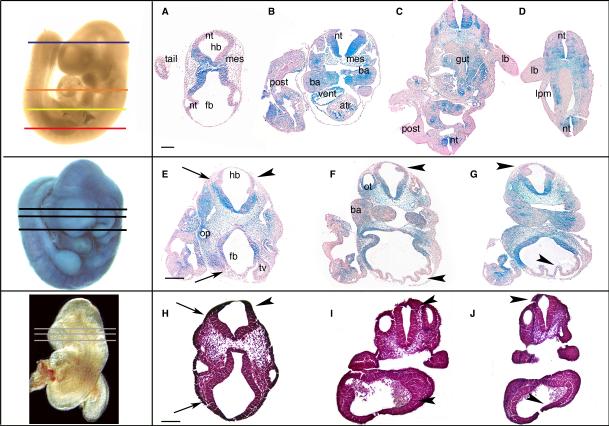
Histological sections of these embryos magnify the defects previously described and illuminated other unexpected abnormalities in tissue development (Fig. 2-4). A pattern emerged for exclusion of Bmp2 null ES cells from certain tissues in developing chimeric embryos. Based on available WT cells, null cells were excluded from the dorsal portion of the developing neural tube, the dorsal head mesenchyme, and the surface ectoderm as well as the developing limb buds, atria, posterior embryo, lateral plate mesoderm, primitive gut, and second branchial arch (Fig. 3A-D). With greater incorporation of Bmp2 null cells, as in higher contribution chimeras, those tissues either failed to develop or were severely impaired (Fig. 2D, E, Fig. 3E-G). Null cells could be excluded from limb buds, head mesenchyme and the dorsal portion of the NT until null cell incorporation reached the ‘high’ level. At the high and very high contribution levels there was not a pattern of exclusion and the few WT cells available were randomly distributed within the embryo and development was stalled (Fig. 1D, Fig. 2C-E). Exclusion from the dorsal portion of the NT was throughout the embryo even where the NT was properly closed in the trunk region of the embryo (Fig. 3A-D).
Fig. 2. Sections from the cephalic region.
Cephalic sections (A-J) show interesting patterns of development. Chimeric sections are paraffin sections while heterozygous and null sections are frozen sections. Chimeras (B-E) show an increase in severity of defects proportional to the incorporation of ES cells. Blue cells indicate ES cell-derived Bmp2 null cells. Sections from a low contribution chimera (B) show relatively normal development similar to WT (A). Sections from a high contribution chimera (C) show defects in the dorsal neural tube and head mesenchyme in the forebrain and hindbrain regions. Sections from heterozygous mice (F) show similar defects to high contribution chimeras. Magnified views (G and H) show disorganization and abnormal neural tissue in the dorsal portions of the neural tube. Sections from very high contribution chimeras (D, E) show severe abnormalities including an expanded and disorganized neural tube (D) or an absence of neural tissue altogether (E). Homozygous nulls show an abnormal neural tube (I). Magnified view (J) shows the neural mesenchymal like mixture. Ch, chimera. het, heterozygous mouse. hb, hindbrain. fb, forebrain. wt, wildtype. null, homozygous null. Scale bar = 1mm
Fig. 4. Serial sections of wild type and abnormal heterozygous mouse embryos.
Transverse frozen sections were made serially through the cephalic region. Serial sections through a heterozygous mouse show increasing disorganization especially in the forebrain region (D-F, arrowheads). Abnormal neural tissue and disorganization (star) pervade the anterior-most regions of the head. Mesenchyme appears evenly distributed and not condensed (D-F, arrows). The forebrain region is open (E, F) compared to WT (A-C) and neural tissue is disorganized. Blue boxes (in B, E) highlight areas of interest. Magnified images in corresponding blue boxes show disorganization and expansion of neuroectoderm and the lack of mesenchymal condensation in the forebrain region. Scale bar = 1mm
Fig. 3. Exclusion of Bmp2 null cells in chimeric embryos.
Sections from the head trunk and tail portion of various embryos (A-D) show a consistent pattern of exclusion of ES cell-derived Bmp2 null cells (blue) from these tissues in the developing embryo: the head mesenchyme, body wall, dorsal portion of the NT, the lateral plate mesoderm, ventricular chambers of the heart, dorsal portion of otic vesicles, limb buds, second branchial arch, and extraembryonic tissues. WT cells from host blastocysts stain pink. A moderate contribution chimera serial sectioned through the cephalic region (E-G) shows increased severity of neural tube defects in the hindbrain and the forebrain regions (arrowheads) when compared to the WT (H-J). The neural tube becomes disorganized and tissue appears abnormal. Exclusion of null cells is consistent. Mesenchyme appears normal and condensed in the forebrain regions but less dense in the hindbrain region (arrows E, H). The otic vesicle (a surface ectoderm derived tissue) is abnormal as is the optic vesicle (a NT derived tissue). Embryos shown before sectioning in the left boxes with approximate positions of sections (for E-J). Embryo left of A-D is only representing sectioning positions. Scale bar = 1mm
Sections from abnormal heterozygous embryos (category 2) showed abnormal neural tissue in the dorsal portion of the developing NT in the cephalic region including the developing eye (Fig. 2F, H). Abnormalities appeared in the most anterior sections (Fig. 4D) along with disorganization of the forebrain region (arrowheads, Fig. 4). Sections within the cephalic region showed an increasing amount of disorder from anterior to posterior and finally, an inability to close the NT in the forebrain region. The mesenchyme appeared greatly reduced surrounding the dorsal portions of the NT (arrows Fig. 4D, E). The developing eye, although formed, showed some irregular neural tissue but all other developing tissues including the surface ectoderm, appeared normal. Middle-high contribution chimeras showed the same anterior to posterior degeneration (Fig. 3E-G). Note that WT cells (pink) nearly exclusively populated the dorsal portion of the NT and dorsal head mesenchyme and still the neuroectoderm appears abnormal in size and shape (Fig. 3E-G, arrowheads) relative to WT (Fig. 3H-J, arrowheads). As with the heterozygous embryos, disorganization occurs in optic and otic vesicles and cells are abnormal in appearance (Fig.3E, F). Homozygous nulls showed open neural tube defects with abnormal neural tissue (Fig. 2I) or a complete failure to develop neural tissue (data not shown) similar to very high contribution chimeras (Fig. 2D, E respectively) as well as a lack of organization and delayed NT development. The ventral portion of the NT for most embryos appeared relatively normal.
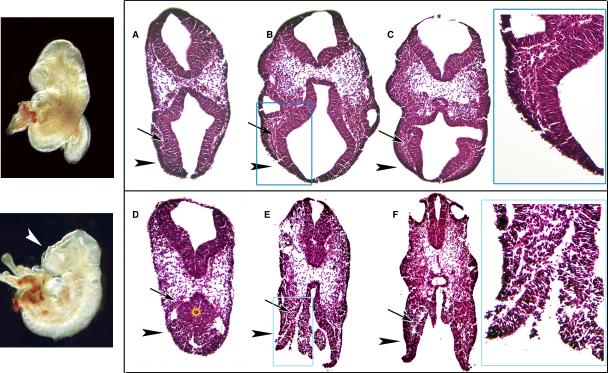
Scanning Electron Microscopy showed abnormalities in the heterozygous and homozygous null embryos. In the heterozygous embryo (category 2) (Fig. 5B) closure points 1, 2, and 3 appeared closed but tissue between those points (blue arrow Fig. 5B, arrowhead, Fig. 5E) was open and neural tissue was exposed. The overall shape of the head was irregular in contrast to the WT (Fig. 5A). The three regions of the brain that define this stage were unrecognizable. The null embryo (Fig. 5C) had a completely open neural tube (blue arrow) and although the otic vesicle (a surface ectoderm-derived tissue) appeared normal there was no neural tube closure or brain segmentation occurring in the cephalic region (Fig. 5C, F). Neural tissues that appeared columnar in the WT control (arrowheads, Fig. 5K) were round and mesenchymal in homozygous null embryos that showed closure defects (arrowhead, Fig. 5H). Where closure was occurring, the surface ectoderm appeared overwhelmed by abnormal neural tissue as closure point 3 attempted to zip toward closure point 2 (star, Fig. 5H) while in the WT the surface ectoderm contains the neuroectoderm within its boundaries as the NT and surface ectoderm zip shut (star, Fig. 5K). Near the midbrain-forebrain junction close to closure point 2 (green box, Figs.5 I. L), abnormal tissue remained but the surface ectoderm appeared normal and distinct. The surface ectoderm still met the leading edge of the NT, but appeared to be separate and distinct (arrow, Fig. 5I) whereas in the WT the two remain integrated (arrow, Fig. 5L).
Fig. 5. Scanning electron microscopy analyses of cephalic regions.
Scanning electron microscopy (SEM) of WT (A, D), heterozygous (B, E), and homozygous null embryos (C, F). Comparison of forebrain region for each embryo (white arrowheads) shows overt open neural tube defects (blue arrows). SEM analysis at higher magnification shows open neural tube defects. Note the abnormal shape of the neural tissue (red arrowhead H) compared with stage-matched WT (red arrowheads K) and the failure to close at any point of the developing neural tube. There is invagination at the optic pit, a medial/ventral NT derivative tissue. The surface ectoderm appears normal and aligned with the prospective dorsal portion of the NT (white arrow, I) but the border becomes obscure towards the forebrain region (star, H). Images of corresponding regions from a WT embryo before NT closure (E9.0) are shown for comparison (J-L). (G), dorso-lateral view, anterior is left, (J), dorsal view.
3. Expression of Pax3 is missing in the developing cephalic neural tube
Whole mount in situ hybridization (WMIS) using Bmp2 homozygous null embryos showed an absence of Pax3 (n=3), which was normally expressed in the dorsal-most portion of the neural tube in wild type embryos (Fig. 6A-E). However, we found normal expression patterns in the cephalic region for Pax6, Wnt1, Shh, Otx2, (data not shown, n=3 respectively) as well as Noggin and Bmp4 (supplemental figure 1, n=3 respectively). Although Wnt1 is considered a dorsal NT marker, in the cephalic region it is restricted to the caudal mesencephalon and not the forebrain regions (Guo et al., 2007) where Bmp2 heterozygous embryos showed abnormalities. Expression of Wnt1 in homozygous null embryos was normal even when the entire cephalic NT was open (data not shown and (Correia et al., 2007)).
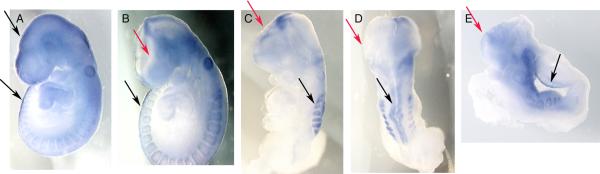
Fig. 6. Expression of Pax3.
WMIS for Pax3 expression is shown in A-E. WT embryos (A) show normal expression of Pax3 in the dorsal portion of the NT throughout the embryo. Heterozygous embryos with open NT defects (B) show normal expression in the trunk region (black arrows) but lack expression in the cephalic region (red arrows), as do homozygous null embryos (C-E). D is a dorsal view of the same embryo shown in C to further clarify the absence of Pax3 in the edge of unclosed neural tube (red arrows).
To determine the role of BMP2 signaling in the control of cell death versus proliferation, phosphohistone 3 staining and TUNEL analysis were performed. As shown in Fig. 7A-C, phosphohistone 3 (pH3) immunohistochemistry revealed no overt differences in proliferation in mesenchymal and neural tissue among wild type and Bmp2 heterozygous and null embryos. However, TUNEL assay demonstrated that heterozygous (category 2, 3) and homozygous null (category 3, 4) embryos displayed unique patterns of cell death in comparison to wild type embryos. Heterozygous embryos with postneurulation defects (category 2) showed cell death in the dorsal mesenchyme as well as within the neural tube and surface ectoderm in the hindbrain region (Fig. 8D-F). Apoptosis occurred mostly in the head mesenchyme (n=3) and in the surface ectoderm directly adjacent to the closing NT in category 3 heterozygous embryos with open NT defects (Fig. 8G-I). In category 3 Bmp2 null embryos, cell death occurred in the otic vesicles, surface ectoderm and head mesenchyme (Fig. 8K) but none was seen in the most anterior region of the head where no forebrain neuroectoderm was observed whereas the hindbrain contained abnormal neuroectoderm (Fig. 8J, n=3). For category 3 heterozygous embryos and category 4 null embryos where dorsal mesenchyme was greatly reduced, apoptosis (Fig. 8L and data not shown) occurred mostly in the remaining mesenchyme along the surface ectoderm. These results, together with histological observations, suggest that BMP2 plays unique and critical roles in maintaining proper identity of the neuroectoderm and prevention of apoptosis in the mesenchyme and surface ectoderm, especially in the dorsal portions of the cephalic region.
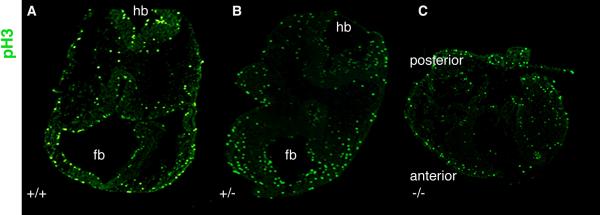
Fig. 7. Analysis of proliferation.
Analysis using phosphohistone 3 (pH3) immunohistochemistry of cephalic sections for proliferation show no overt differences among WT (A), heterozygous (B) and homozygous nulls (C).
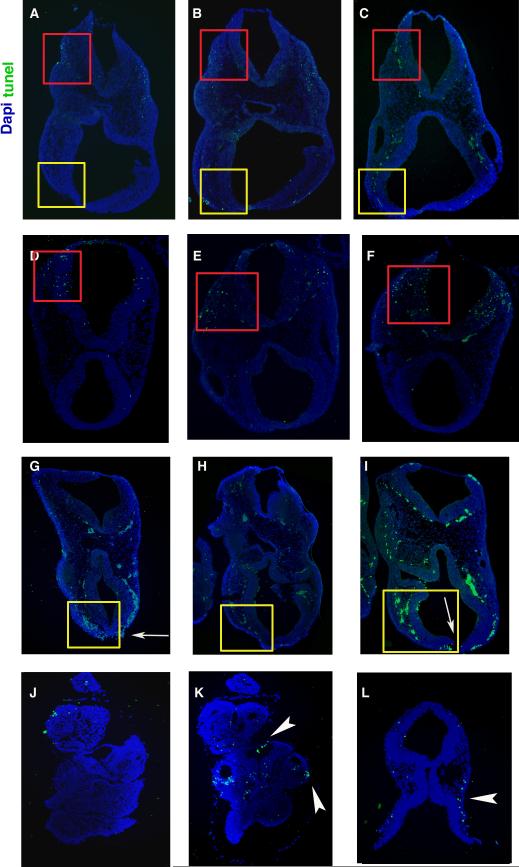
Fig. 8. Analysis of Apoptosis.
TUNEL assay as performed on WT (A-C), Category 2 Heterozygous (D-F) Category 3 heterozygous (G-I) and homozygous null embryos (J-L). Sections from left to right are from anterior to posterior within the cephalic region for each set. Apoptosis (green) was increased in heterozygous mice with both open NT defects (yellow boxes G-I) and postneurulation defects (red boxes, D-F) when compared to WT (respective boxes, A-C). Homozygous nulls with abnormal neural tissue (arrowheads K,L) show an increase in apoptosis but nulls with no recognizable neural tissue (J) in the forebrain region show no apoptosis in the cephalic region. Nuclei were stained with DAPI (blue). Red box emphasizes the hindbrain region, while the yellow box emphasizes the forebrain region.
4. Does p53-dependent apoptosis affect neural tube closure in BMP2 embryos?
Because of the increase in apoptosis in BMP2 embryos we considered whether it played a role in the failure of the NT to close. Recently, it has been reported (Jones et al., 2008; Pani et al., 2002) that p53 affects apoptosis during neural tube development. Enhanced apoptosis in the neuroectoderm is found in the Pax3 (Phelan et al., 1997) and Tcof1 (Dixon et al., 2006) mutants and these were rescued (Jones et al., 2008; Pani et al., 2002) by pifithrin-α, an inhibitor of p53. We considered whether we could also rescue apoptosis and ultimately NT defects found in Bmp2 embryos by using pifithrin-α. We treated pregnant females at stages appropriate to NT closure. Overall, we found no rescue (Table 2) for homozygous null embryos regardless of treatment with most classified as category 4. We found partial rescue for heterozygous embryos with fewer category 2 as well as category 3 in the pifithrin-α treated embryos versus the DMSO treated embryos. There were also more category 1 (normal) heterozygous embryos recovered.
Table 2.
Data shows the various phenotypes of heterozygous and null embryos separated into four categories. Using pifithrin-α treatment on pregnant females we were able to find a partial rescue for heterozygous mice but none for homozygous nulls.
| Categories: | Heterozygotes: | Homozygous Nulls: | ||
|---|---|---|---|---|
| PFT treated | DMSO treated | PFT treated | DMSO treated | |
| 1. Normal | 27(79%) | 18(46%) | 0(0%) | 0(0%) |
| 2. Postneurulation, partial open NT | 6(18%) | 15(38%) | 0(0%) | 0(0%) |
| 3. Hypomorphism, heart defects, open NT, failure to turn | 1(3%) | 5(13%) | 1(14%) | 0(0%) |
| 4. No development beyond E8.5 stage | 0(0%) | 1(3%) | 6(86%) | 7(100%) |
Discussion
The severity of defects in BMP2 null and heterozygous embryos is dose dependent
Chimeric embryos and heterozygous and homozygous null embryos show comparable phenotypes in the developing neural tube and in progressive severity based on the available amount of BMP2 signal. Chimeras show not only a predisposition to exclude null cells from specific tissues of the developing embryo but they also show abnormal phenotypes at a fairly low level of contribution. Beginning with abnormal neural tissue and the failure to invaginate telencephalic vesicles, chimeras with increasing contribution of null cells then fail to maintain neural tissue in the cephalic region or fail to close the NT altogether. Chimeras did not show cephalic NT closure defects specifically in the forebrain or midbrain region as heterozygous mice did probably because they have the ability to exclude null cells and, therefore, prevent those defects. Those chimeras that show postneurulation defects demonstrate that surface ectoderm closure takes precedence over NT closure itself. We never observed embryos with a closed neural tube while the surface ectoderm remained open. Chimeric embryos with NT closure defects also show defects in dorsal neural tissue development including disorganization and dissolution, as do abnormal heterozygous and homozygous null embryos. Higher contribution chimeras fail to exclude and have cephalic regions that contain either reduced mesenchyme and disorganized neural tissue or increased mesenchyme and the absence of neural tissue, the same as homozygous null embryos.
It is interesting to consider why phenotypes found in both heterozygous and homozygous mutant embryos for Bmp2 varied so much (table 1). Since these mice come from a mixed background, we cannot exclude the influence of genetic modifiers as one of the possible reasons. As mentioned in the results section, we experienced a lower number of heterozygous pups at weaning stage from heterozygous intercross (WT/Het is 1.4 instead of 2.0, proportion of Het is 58% instead of 67%). With over 5 years of breeding, we experienced similar phenomena, but rates of reduction were varied based on their genetic background (WT/Het is 1.5 for 129SvEv inbred, 1.4 for Swiss Webster, 0.84 for C57BL6/J congenic). These facts suggest that genetic modifiers would influence frequency and severity of the phenotypes described in this study. We also experienced a reduced number of heterozygous pups from WT (female) x heterozygous (male) breeding. For the mixed background we used in this study, the proportion of heterozygotes for Bmp2 at weaning stage is nearly 50% as expected. However, this ratio varies depending on their genetic background, 38% for 129SvEv inbred, 42% for Swiss Webster, and 35% for C57BL6/J congenic. These numbers again suggest that the severity of phenotypes and penetrance may be subject to the genetic background. Another potential explanation for phenotypic variation is maternal contribution since, as stated above, survivability of the heterozygous pups was lower when mothers were heterozygous for Bmp2. We also discovered that among heterozygous intercrosses when severe phenotypes were found in homozygous null embryos heterozygous littermates usually had NT defects as well. When homozygous nulls were less severe heterozygous embryos from the same mother had no phenotype or only mild postneurulation defects. Since all pregnant females were heterozygotes we have confidence her contribution affects embryonic development. Recently, we generated a mouse with a conditional allele for Bmp2. The floxed allele includes a neomycin selection cassette in the Bmp2 locus (floxed-neo allele, abbreviated ‘fn’) that can transcribe only 10 % of the transcript when compared with the wild type locus (Singh et al., 2008). Embryos with genotype ‘fn/-’ show closure defects in the cephalic neural tube similar to the ones described in this study, and its frequency varies depending on the genotype of mother, more frequent, if mother is ‘fn/-’ resulting in lower expression levels of Bmp2 in the decidua and uterus (Singh et al., 2008). These facts further support the idea that a maternal influence including expression levels of Bmp2 may affect the severity of the embryonic phenotypes shown in Table 1 especially when expression levels in Bmp2 embryos is decreased. Maternal influence, genetic background and other unknown genetic modifiers may all contribute to phenotypic variation in Bmp2 heterozygous and homozygous null embryos which is ultimately controlled by the available amount of BMP2. Clearly, a minimal reduction of BMP2 signaling will negatively affect embryonic development at this stage. We, therefore, believe that the severity of defects among chimeras, heterozygous and homozygous null embryos is inversely proportional to the amount of BMP2 signaling.
BMP2 may play a role in dorsal identity of the cephalic neural tube
Since other BMPs, especially BMP4 and BMP7, are also expressed in the developing cephalic region (Furuta et al., 1997) of the E8.5-E9.5 embryo it is important to note that the pattern of expression for each BMP is unique with potentially overlapping functions. Our histological data show that chimeras exclude Bmp2 null cells when possible. Specifically, in the cephalic region, exclusion of Bmp2 null cells may illuminate the requirement of BMP2 for NT closure in the cephalic regions of heterozygous and homozygous null embryos. These NT closure defects occurring in the presence of wild type cells for middle to high contribution chimeras and for heterozygous embryos (with one functional copy of Bmp2) demonstrate not only a dosage requirement of BMP2 but also a failure of other BMPs to rescue normal NT development and tissue identity. While exclusion of Bmp2 nulls ES cells from specific tissues including the surface ectoderm implies a cell autonomous function for BMP2 (Morin-Kensicki et al., 2001) exclusion from tissues that do not express BMP2, such as the dorsal neuroectoderm, suggests a non cell autonomous function for BMP2 in those tissues. Since Bmp2 is a signaling molecule availability of BMP2 from neighboring WT cells may be a determining factor for contribution to those tissues that do not express BMP2. These activities suggest that BMP2 has unique and non-overlapping functions in the cephalic region for NT development. Since dorsal identity issues have been investigated mainly in the trunk region (Dietrich et al., 1993; Goulding et al., 1993b; Meyer and Roelink, 2003) the cephalic region may be regulated differently. The loss of Pax3 expression in the dorsal cephalic NT of these embryos implies that BMP2 is necessary for dorsal identity of the cephalic NT. And although the loss of a single marker does not definitively eliminate other possibilities the lack of Pax3 expression and the failure of other BMPs to rescue the NT defect strongly supports the idea that BMP2plays a role in dorsal identity of the cephalic NT.
BMP2 is required for normal cephalic neural tube closure
Another important aspect of neural tube closure is physical kinetics including cell movement, cell proliferation, and cell shaping (Padmanabhan, 2006). Closure defects in the cephalic region for heterozygous embryos for Bmp2 ranges from completely open to specific opening in the midbrain and forebrain, and then, the least severe case, open only in the forebrain region of the NT. These openings may reflect the faulty physical kinetics of NT closure in embryos lacking BMP2 expression. It is important to note that for the cephalic region the forebrain is the most expansive region required for closure. It would require the most proliferation of mesenchyme, neural ectoderm, and surface ectoderm to close. Closure point 2 in the mouse is found near the midbrain region and from this point the NT zips in opposite directions toward the forebrain to meet closure point 3 or toward the hindbrain to meet closure point 1 (Copp et al., 2003). Curly tail (ct) mutation causes NT closure defect in the caudal region (Copp et al., 1988). It is speculated that alteration in the physical torque of the tail would be a cause of the NT defect because of a lack of proliferation in the posterior portion of the embryo. It is notable that none of the Bmp2 heterozygous embryos show open NT defects in the hindbrain, if the forebrain region is closed. These facts suggest the possibility that the forebrain region because of its physical size and shape requires more BMP2 to support the proliferation of mesenchymal tissues and surface ectoderm.
It is believed that morphological changes of the neural plate to form paired dorsolateral hinge points (DLHPs) are necessary for NT closure at the spinal level (Copp et al., 2003). Recently it has been reported that the antagonism of BMP2 stimulates formation of DLHPs in the spinal region of the NT (Ybot-Gonzalez et al., 2007). However, in the cephalic region, formation of DLHPs is not required (Copp, 2005; Ybot-Gonzalez et al., 2002). These reports support the role of BMP2 in neural tube closure including the need for regulated and organized expression for normal neural tube closure.
Apoptosis plays a role in NT defects for many mouse models (Padmanabhan, 2006; Pani et al., 2002). Pax-3 deficient embryos (Phelan et al., 1997) suffer from NTDs and apoptosis. However, these embryos were rescued (Pani et al., 2002) by down-regulating p53 protein using pifithrin-α treatment or germline mutation. Since we also found a lack of Pax3 expression along with NTDs in the Bmp2 mutant embryos, it was reasonable to speculate that the loss of Pax3 would have induced p53-dependent apoptosis and subsequent NTD. However, our results show a failure to rescue the NTDs in Bmp2 homozygous null embryos. These facts suggest that p53 dependent apoptosis is not directly involved in NT closure defects for BMP2 embryos, and the loss of Pax3 may not be a direct cause of those NTDs, but rather, a result of the NTDs. Future experiments to elucidate mechanistic insight of apoptosis in the mesenchyme may help to understand the direct cause(s) of the NTD in the cephalic region. It would be interesting to include colony development using this treatment to show a possible increase in survival for heterozygous mice, and a potentially important future clinical application.
Cephalic mesenchyme STRUGGLES without BMP2 signaling
Null embryos for Bmp2 that survive beyond gastrulation stages still suffer in the next stage of development. From E8.5 to E9.5 the anterior neural ridge needs to proliferate, close and differentiate into 3 distinct regions – forebrain, midbrain, and hindbrain. Mesenchymal tissue in the head that surrounds the developing NT shows an interesting pattern of development with condensation occurring over time from the center of the head near the ventral portions of the NT outward toward the surface ectoderm and the dorsal portions of the NT. Of the numerous mouse models with neural tube defects (Brook et al., 1991; Juriloff and Harris, 2000; Soo et al., 2002; Stumpo et al., 1995; Zohn et al., 2005) the impact of normal development of the head mesenchyme on neural tube closure is highly relevant (Soo et al., 2002; Zhao et al., 1996; Zohn et al., 2007). Changes in proliferation and apoptosis have also impacted the head mesenchyme of mouse models but in different ways. Homozygous mice for Twist or Cart1 show a decrease in proliferation and an increase in apoptosis in head mesenchyme (Soo et al., 2002; Zhao et al., 1996). The opm mutants show no change in these parameters but suggest an actual movement of mesenchyme to support the growing forebrain region at the expense of the midbrain region and NT closure (Ybot-Gonzalez et al., 2002; Zohn et al., 2007). Both Bmp2 heterozygous and homozygous null embryos, however, show normal proliferation with an increase in apoptosis targeted specifically to the mesenchyme near the surface ectoderm and dorsal portion of the NT, and within the surface ectoderm itself. Overall the mesenchyme within these embryos was reduced. In very high contribution chimeras and severely affected homozygous nulls, the cephalic region is either entirely neural tissue or entirely mesenchymal-like tissue, implying that without BMP2 signaling to the proliferating mesenchyme the boundary between mesenchyme and neuroepithelium is lost resulting in disorganization, loss of identity and, ultimately, failure to close the neural tube. Since neural crest cells migrate through the head mesenchyme during this stage it is important to recognize their migration may be affected by abnormal mesenchymal development and neural tube closure. However, Correia et al. reported that BMP2 is important for neural crest cell migration but not induction (Correia et al., 2007). It would be an important future endeavor to address the function of BMP2 in migrating neural crest cells and whether or not it impacts neural tube closure.
Our results show through SEM and histological analyses that the development, organization and amount of head mesenchyme are all crucial for neural tube closure. As we revealed, the mesenchyme does not condense or is poorly condensed surrounding the dorsal portion of the NT in Bmp2 null embryos and in chimeric embryos. In abnormal heterozygous mice the mesenchyme condenses relatively normally in the midbrain and hindbrain, but less so in the forebrain in mice with open NT defects. Moreover, we never observed embryos with a closed neural tube while the surface ectoderm remained open. Therefore, we believe that BMP2 from the surface ectoderm signals to the mesenchyme for proper condensation and the prevention of apoptosis allowing for normal NT closure.
Materials and Methods
Generation and analysis of Bmp2 chimeras and Bmp2 mutant embryos
Bmp2 heterozygous mice were kindly provided by Allan Bradley (Zhang and Bradley, 1996). These mice were maintained in a mixed background of C57Bl/6 and 129SvEv. ES cell lines were created from blastocysts of crosses between Bmp2 heterozygous males containing one copy of a ROSA26 reporter after Cre recombination and Bmp2 heterozygous females. Wild type blastocysts from CD1 females were injected with five to fifteen Bmp2 -/- ES cells and reimplanted into pseudopregnant females to generate Bmp2 -/-(<->) +/+ chimeras. Chimeras were harvested at E9.5 and stained for ß-galactosidase activity (X-gal staining hereafter) as described previously (Kishigami et al., 2004). Stained embryos were post-fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin (Kishigami et al., 2004). 7μm thick sections were counterstained with Eosin Y such that blastocyst-derived cells stained pink and ES-derived cells stained blue. Bmp2 heterozygous mice in a mixed background were intercrossed to obtain homozygous and heterozygous embryos for Bmp2. Plugged females were scored as 0.5 at noon of plug date. All animal procedures were approved by the Institutional Animal Care and Use Committee at the National Institute of Environmental Health Sciences, NIH.
In situ hybridization
Embryos were harvested at E9.5 and fixed in 4% paraformaldehyde in PBS for 2h and then dehydrated and stored at -20C in 100% methanol. Whole mount in situ hybridization was performed as described (Wilkinson and Nieto, 1993) using the following probes: Shh (Echelard et al., 1993) and Wnt1 (Wilkinson et al., 1987) provided by Andrew McMahon, Otx2 (Ang et al., 1996) provided by Janet Rossant, Noggin (McMahon et al., 1998) provided by Richard Harland, Pax3 (Goulding et al., 1993a) and Pax6 (Walther and Gruss, 1991) provided by Peter Gruss and, lastly, Bmp2 provided by Yas Furuta and Brigid Hogan (Furuta et al., 1997).
Immunohistochemistry
Embryos were collected at E9.5 and fixed for 1h with 4% paraformaldehyde and then placed in 30% sucrose overnight. Embryos were embedded in OCT and stored at -80C. Embryos were then sectioned at 10μm and immunohistochemistry was performed as described (Caspary et al., 2002). Primary antibodies were purchased from the Developmental Studies Hybridoma Bank at the University of Iowa (dshb.biology.uiowa.edu/). Alexa 488 and 568 (Molecular Probes) were used as secondary antibodies for fluorescent detection. Images were captured using an Olympus DP70 camera.
Pifithrin-α Treatment
Pifithrin-α (Calbiochem) was dissolved in 50% DMSO in PBS, diluted with PBS and administered to pregnant females by interperitoneal injection of 2.2mg/kg at day 8 and 9. Embryos were harvested at day E10.5. (Correia et al., 2007; Pani et al., 2002)
Scanning Electron Microscopy
Embryos were harvested at E9.5 then fixed with 2% paraformaldehyde, 2.5% glutaraldehyde in 0.15M sodium phosphate buffer, pH 7.4. Following three buffer rinses (0.15M sodium phosphate buffer, pH7.4) samples were postfixed in 1% buffered osmium tetroxide for 1h. Then samples were gradually dehydrated with ethanol. Samples were dried to the critical point using carbon dioxide as the transition solvent (Balzers Union Ltd., Principality of Liechtenstein). Embryos were mounted on aluminum SEM stubs with colloidal silver paste and sputter-coated with gold:palladium alloy 60:40 to a 20nm thickness using a Hummer X Sputter Coater (Anatech Ltd., Alexandria VA). Samples were examined with a scanning electron microscope using an acceleration voltage of 20kV and a working distance of 20mm (Cambridge Stereoscan S200 scanning electron microscope, LEO Electron Microscopy, Inc., Thornwood, NY).
Supplementary Material
Supplemental Figure Legend
Supplemental Figure 1: Expression in the dorsal neural tube region.
Whole mount in situ for Noggin (A-B) and Bmp4 (C-F) expressions are shown. WT embryos show normal expression of Noggin in the dorsal portion of the NT throughout the embryo (A). Heterozygous embryos with open NT defects (A) also show normal expression in the trunk region as well as in the cephalic region (arrows), as do homozygous null embryos (B). Bmp4 expression was detected in the dorsal portion of the cephalic neural tube of WT embryo (D), heterozygous embryo with open NT defects (D), and two homozygous null embryos (E-F) (arrowheads).
Acknowledgements
We dedicate this work to the memory of our good friend and colleague, Dr. Deborah L. Swope. We gratefully thank Drs. Hongbing Zhang and Allan Bradley for Bmp2 mice, Drs. Peter Gruss, Richard Harland, Andrew McMahon, Janet Rossant, Yasuhide Furuta and Brigid L. M. Hogan for in situ probes, Ms. Victoria Madden, Microscopy Services Laboratory, Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill for technical support, Drs. Satoshi Kishigami and Yoshihiro Komatsu for technical support and advice, Dr. Deborah Stumpo, Ms. Diane Spencer and Dr. E. Mitch Eddy for critical reading of the manuscript, Ms. Tonya Simmons for mouse husbandry, and members of LRDT/MDBG, as well as CRD, JJC2, EMC, and RDS for advice and encouragement. This work was supported by the Intramural Research Program of the NIEHS/NIH (ES071003-10) and as a gift from RIKEN Brain Science Institute to Y. M.
References
- Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development. 1996;122:243–52. doi: 10.1242/dev.122.1.243. [DOI] [PubMed] [Google Scholar]
- Brook FA, Shum AS, Van Straaten HW, Copp AJ. Curvature of the caudal region is responsible for failure of neural tube closure in the curly tail (ct) mouse embryo. Development. 1991;113:671–8. doi: 10.1242/dev.113.2.671. [DOI] [PubMed] [Google Scholar]
- Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, Alcorn HL, Anderson KV. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol. 2002;12:1628–32. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Neurulation in the cranial region--normal and abnormal. J Anat. 2005;207:623–35. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Brook FA, Roberts HJ. A cell-type-specific abnormality of cell proliferation in mutant (curly tail) mouse embryos developing spinal neural tube defects. Development. 1988;104:285–95. doi: 10.1242/dev.104.2.285. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Gruss P. Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech Dev. 1993;44:189–207. doi: 10.1016/0925-4773(93)90067-8. [DOI] [PubMed] [Google Scholar]
- Dixon J, Jones NC, Sandell LL, Jayasinghe SM, Crane J, Rey JP, Dixon MJ, Trainor PA. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci U S A. 2006;103:13403–8. doi: 10.1073/pnas.0603730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Estibeiro JP, Brook FA, Copp AJ. Interaction between splotch (Sp) and curly tail (ct) mouse mutants in the embryonic development of neural tube defects. Development. 1993;119:113–21. doi: 10.1242/dev.119.1.113. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Abematsu M, Mori H, Yanagisawa M, Kagawa T, Nakashima K, Yoshimura A, Taga T. Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-Smad signaling in neural stem cells. Mol Cell Biol. 2007;27:4931–7. doi: 10.1128/MCB.02435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–12. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Goulding M, Sterrer S, Fleming J, Balling R, Nadeau J, Moore KJ, Brown SD, Steel KP, Gruss P. Analysis of the Pax-3 gene in the mouse mutant splotch. Genomics. 1993a;17:355–63. doi: 10.1006/geno.1993.1332. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993b;117:1001–16. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Guo C, Qiu HY, Huang Y, Chen H, Yang RQ, Chen SD, Johnson RL, Chen ZF, Ding YQ. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134:317–25. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–8. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–33. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. Mouse models for neural tube closure defects. Hum Mol Genet. 2000;9:993–1000. doi: 10.1093/hmg/9.6.993. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Brune RM, Davidson DR, Baldock RA. Computer-generated three-dimensional reconstructions of serially sectioned mouse embryos. J Anat. 1998;193(Pt 3):323–36. doi: 10.1046/j.1469-7580.1998.19330323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–46. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–93. doi: 10.1016/j.ydbio.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–81. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–11. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mackay DR, Hu M, Li B, Rheaume C, Dai X. The mouse Ovol2 gene is required for cranial neural tube development. Dev Biol. 2006;291:38–52. doi: 10.1016/j.ydbio.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NP, Roelink H. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev Biol. 2003;257:343–55. doi: 10.1016/s0012-1606(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–69. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Faust C, LaMantia C, Magnuson T. Cell and tissue requirements for the gene eed during mouse gastrulation and organogenesis. Genesis. 2001;31:142–6. doi: 10.1002/gene.10017. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto) 2006;46:55–67. doi: 10.1111/j.1741-4520.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3-dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–97. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- Sailer MH, Hazel TG, Panchision DM, Hoeppner DJ, Schwab ME, McKay RD. BMP2 and FGF2 cooperate to induce neural-crest-like fates from fetal and adult CNS stem cells. J Cell Sci. 2005;118:5849–60. doi: 10.1242/jcs.02708. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Takahashi H, Shintani T, Etani K, Aoshima A, Noda M. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci. 2006;26:10868–78. doi: 10.1523/JNEUROSCI.3027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev. 2008;2:134–41. doi: 10.1159/000143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K, O'Rourke MP, Khoo PL, Steiner KA, Wong N, Behringer RR, Tam PP. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251–70. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995;92:944–8. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–72. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–49. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–62. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bailes JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–73. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takeda K, Ochiai W, Takizawa T, Ueno M, Takizawa M, Shibuya H, Taga T. Inhibition of BMP2-induced, TAK1 kinase-mediated neurite outgrowth by Smad6 and Smad7. Genes Cells. 2001;6:1091–9. doi: 10.1046/j.1365-2443.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–17. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13:275–83. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Anderson KV, Niswander L. Using genomewide mutagenesis screens to identify the genes required for neural tube closure in the mouse. Birth Defects Res A Clin Mol Teratol. 2005;73:583–90. doi: 10.1002/bdra.20164. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev Biol. 2007;306:208–21. doi: 10.1016/j.ydbio.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legend
Supplemental Figure 1: Expression in the dorsal neural tube region.
Whole mount in situ for Noggin (A-B) and Bmp4 (C-F) expressions are shown. WT embryos show normal expression of Noggin in the dorsal portion of the NT throughout the embryo (A). Heterozygous embryos with open NT defects (A) also show normal expression in the trunk region as well as in the cephalic region (arrows), as do homozygous null embryos (B). Bmp4 expression was detected in the dorsal portion of the cephalic neural tube of WT embryo (D), heterozygous embryo with open NT defects (D), and two homozygous null embryos (E-F) (arrowheads).