Abstract
Although numerous EEG studies have shown that depression is associated with abnormal functional asymmetries in frontal cortex, fMRI and PET studies have largely failed to identify specific brain areas showing this effect. The present study tested the hypothesis that emotion processes are related to asymmetric patterns of fMRI activity, particularly within dorsolateral prefrontal cortex (DLPFC). Eleven depressed and 18 control participants identified the color in which pleasant, neutral, and unpleasant words were printed. Both groups showed a leftward lateralization for pleasant words in DLPFC. In a neighboring DLPFC area, the depression group showed more right-lateralized activation than controls, replicating EEG findings. These data confirm that emotional stimulus processing and trait depression are associated with asymmetric brain functions in distinct subregions of the DLPFC that may go undetected unless appropriate analytic procedures are used.
Introduction
The past decade has witnessed a rapid expansion of research on the neural correlates of emotion. The advance of hemodynamic imaging techniques has led to the refinement of longstanding theories about the structure of emotion and the biological correlates of mood disorders. However, in some instances it has proven difficult to reconcile findings from new techniques with established work. A prominent example of this problem has been the failure of hemodynamic imaging studies to replicate EEG findings on frontal asymmetries in emotion.
It was recently estimated that over 70 published EEG studies report emotion-modulated asymmetries in frontal cortex (Coan & Allen, 2004). These studies have established differential roles of left and right prefrontal cortex (PFC) for processing pleasant and unpleasant emotional information, respectively. Resting EEG measures figure prominently in this literature. In non-clinical samples, resting EEG asymmetries in PFC have been found subsequent to the induction of pleasant and unpleasant mood states (Ahern & Schwartz, 1985; Davidson, Ekman, Saron, Senulia, & Friesen, 1990; Jones & Fox 1992; Nitschke, Heller, Etienne, & Miller, 2004; Tucker, Stenslie, Roth, & Shearer, 1981).
In addition, numerous EEG studies have shown that depression is associated with a shift toward greater right than left PFC activity, consistent with findings for unpleasant emotion (for reviews, see Davidson et al., 2002; Heller & Nitschke, 1997; Miller & Cohen, 2001). Analyses of spectral activity across frontal electrodes indicate that left/right frontal activity differences in depression are relative rather than absolute (Bell, Schwartz, Hardin, Baldwin, & Kline, 1998; Bruder et al. 1997; Gotlib et al., 1998). It also appears that this asymmetry is superimposed on less bilateral activity in frontal cortex for depressed people in general (see Heller & Nitschke, 1998; Herrington et al., 2005). Studies have shown that these patterns can be altered through psychological interventions and pharmacotherapy (Davidson, Irwin, et al., 2003; Davidson, Kabat-Zinn, et al., 2003).
Nonetheless, few studies to date have reliably localized frontal EEG asymmetries to a specific area of prefrontal cortex. Furthermore, recent meta-analyses examining hemodynamic imaging studies of emotion have yielded few or no consistent findings of frontal asymmetries in emotion, leading some researchers to question the validity of the EEG results (Murphy, Nimmo-Smith, & Lawrence, 2003; Wager, Phan, Liberzon, & Taylor, 2003; examining 106 and 65 studies, respectively). The general conclusion reached by these meta-analyses was summarized by Murphy et al. (2003) who stated that hypotheses regarding frontal asymmetries “may be too coarse, in terms of both their neural underpinnings and the aspect of emotion under consideration” (p. 227).
Despite the conclusions of recent meta-analyses, existing evidence suggests that dorsolateral prefrontal cortex (DLPFC) may function asymmetrically for emotion processes, possibly driving the observed EEG asymmetries (Davidson, 2004; Herrington et al., 2005; Nitschke et al., 2004; see also Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Heller & Nitschke, 1997; Miller & Cohen, 2001). Evidence in favor of this view comes from numerous studies of depression, where decreased right DLPFC activity has been found superimposed on bilateral DLPFC decreases (for reviews, see Davidson, 1998; Heller & Nitschke, 1997; Nitschke & Heller, 2002). Importantly, these studies have relied on both resting state and task-based paradigms. In a review of what was then a 25-year history of frontal asymmetry research, Davidson (2004) wrote that “tasks that involve a response component will be more likely to show affect-related PFC activation asymmetry in the dorsolateral regions [of prefrontal cortex], and it is activity in these regions that are more likely to be reflected in scalp-recorded brain electrical signals” (p. 221). Conversely, Davidson pointed out that other frontal regions with clear roles in emotion processes – namely orbitofrontal cortex – are less likely candidates for observed frontal asymmetries. Data and conclusions from these articles suggest that the localization of emotion-related frontal asymmetries is likely to prove most fruitful in DLPFC.
To date DLPFC activity has been associated primarily with executive functions (Cohen, Braver, & O’Reilly, 1996; Fuster, 1989; Owen, Evans, & Petrides, 1996; Rajkowski & Goldman-Rakic, 1995; Stuss & Levine, 2002). However, a growing literature implicates DLPFC in various emotion processes, particularly those related to attentional maintenance and deployment (e.g., Compton et al., 2003; Gray et al., 2002; Herrington et al., 2005; Engels et al., 2007; Perlstein et al., 2002). Unfortunately, it is surprisingly rare for hemodynamic imaging studies to perform direct tests of asymmetry. Instead, considerations of functional asymmetries are generally based on the isolation of clusters that pass some predetermined activation threshold in one hemisphere and not the other. This approach is problematic, insofar as it does not test the reliability of the difference between regions and their contralateral homologues (Davidson & Irwin, 1999; Friston, 2003; Herrington et al., 2005). The virtual absence of robust tests of asymmetry among hemodynamic imaging studies leaves the role of regional specificity in emotion-modulated EEG asymmetries an open question.
The present study provided a direct investigation of lateralized activity by conducting per-voxel ANOVAs that include hemisphere as a factor. Higher-order effects within this ANOVA included the interaction of hemisphere with one of two manipulations involving emotion. The first consisted of a within-subject manipulation of stimulus valence (positive, neutral, negative) in an emotion-word Stroop paradigm. Previous studies using the emotion-word Stroop task have shown that activation in DLPFC varies as a function of the valence of a word (Compton et al., 2003; Herrington et al., 2005; Mohanty et al., 2005, 2007; Engels et al., 2007). The second was a between-subjects manipulation using trait negative affect (high, low) via selection of individuals based on self-rating of depressive symptoms. The primary substantive hypotheses were that asymmetric activity would be observed in DLPFC according to the valence of potent to-be-ignored emotional stimuli and that this asymmetry would be abnormal among individuals with depression. Furthermore, given theorizing about differential roles for subregions of the PFC, the design of the study allowed us to address potential distinctions between valence of the emotional stimuli and trait negative affect.
In order to effectively address the possibility that distinct brain regions in DLPFC are involved in distinct emotional functions, the present study implemented contrasts that reflect two distinct theoretical perspectives on the dimensional structure of emotion. The valence perspective holds that basic emotions (e.g., happiness, fear) follow a two-dimensional structure with axes representing valence (pleasant vs. unpleasant) and arousal (Bradley & Lang, 1994; Heller, 1990; Lang, 1980; Russell, 1980; Osgood, 1952) referred to as the circumplex model of emotion (Heller, 1993; Russell & Bullock, 1986). Studies have shown that the pleasant/unpleasant axis (valence) can be used to describe patterns of lateralized activity in frontal cortex among non-clinical samples and that abnormalities in these patterns are related to psychopathology, particularly depression and anxiety disorders (for review, see Coan & Allen, 2004). The second perspective, articulated by Watson and Tellegen (1985), holds that pleasant and unpleasant emotions do not belong on the same dimension (valence) and should be considered separately. Positive and negative affect (this perspective is henceforth referred to as PA/NA) are terms for the axes formed after a rotation of the circumplex axes that integrates valence and arousal dimensions (Watson & Tellegen, 1985; Zevon & Tellegen, 1982).
The valence and PA/NA perspectives are thus closely related but capture different aspects of variance related to emotion. Variance captured by the valence perspective is maximized when pleasant and unpleasant emotions function exclusively or are negatively correlated (Green, Goldman, & Salovey, 1993). The PA/NA perspective, on the other hand, better characterizes variance when positive and negative affect function in parallel, operating simultaneously but independently. The two models therefore reflect somewhat different aspects of the same underlying emotion structure. Considerable debate surrounds which of these two models is better suited to the study of emotion processes (Stewart, Levin-Silton, Sass, Heller, & Miller, 2008). One reason that this debate persists is that the two models cannot be readily contrasted with one another (such as in an ANOVA) due to their inherent overlap.
These models provided the framework for this experiment not only because of the interest and controversy surrounding them, but due to the imbalanced manner in which they are reflected in fMRI studies of emotion. A review of emotion paradigms used in hemodynamic imaging studies indicates that the vast majority of studies more directly fit the PA/NA perspective, in that most exclude pleasant stimuli and compare unpleasant emotional stimuli to neutral stimuli. The unpleasant-neutral comparison nicely captures the concept of negative affect but confounds valence and arousal. The present study employs separate analyses, following both valence and PA/NA perspectives, in order to maximize the probability of isolating asymmetry effects. In the context of this study, valence refers specifically to whether the emotional characteristics of the experimental stimuli were rated as pleasant versus unpleasant. Therefore, the research reported here does not attempt to take on the issue of whether the valence perspective or the motivation distinction of approach/withdrawal (for review, see Heller, Koven, & Miller, 2003) better accounts for frontal asymmetries associated with emotion.
Finally, controversy exists regarding whether the amygdala functions asymmetrically for emotion processes in general and depression in particular. Abercrombie et al. (1998) found a relationship between increased metabolic rate and depressive symptoms in right amygdala among individuals diagnosed with major depressive disorder (measured at rest). This is contrary to a meta-analysis by Baas, Aleman, and Kahn (2004), who concluded that left amygdala is generally more responsive to emotion manipulations than is right amygdala, regardless of the experimental paradigm. Such discrepancies are further complicated by the near absence of statistical analyses directly contrasting signal from left and right amygdala. The present study therefore reports direct tests of asymmetric amygdala function with respect to emotional stimuli and depressive symptoms.
Method
Participants and Selection Procedures
Participants were selected by screening large groups of undergraduates and inviting restricted subsets to participate in laboratory research. These subsets were chosen based on dimensional measures of depression and anxiety that have been shown to distinguish between these highly comorbid conditions (Nitschke, Heller, Palmieri, & Miller, 1999): the Anhedonic Depression and Anxious Arousal scales of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995) and the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990; Molina & Borkovec, 1994). Cutoff scores for group membership were assigned using 50th and 80th percentiles based on sample distributions for each measure. A large number of undergraduate volunteers were screened (1688) in order to obtain 90 participants who met experimental criteria for presence/absence of depression and anxiety. Seventy-six completed all components of the experiment (fMRI, EEG, and diagnostic interview; data from the latter two are reviewed elsewhere). Of these, 29 (11 females, mean age = 20.241, SD = 5.429) reported no more than moderate anxiety and could be classified as depressed (N = 11; 3 female) or control (N = 18; 8 female). (Behavioral performance data for two of 11 depressed participants with viable fMRI data were not collected due to computer error, leaving behavioral data Ns of nine for the depressed group and 18 for the control group.) All participants in the depressed and control groups scored below the 50th percentile on the PSWQ. The depressed group scored above the 80th percentile on an eight-item subscale of the MASQ Anhedonic Depression scale that has been shown to reflect depressed mood (Nitschke et al., 2001) and below the 50th percentile on the PSWQ and MASQ Anxious Arousal scales. The control group scored below the 50th percentile on the PSWQ and the MASQ Anxious Arousal and Anhedonic Depression subscales (see Table 1 for means and standard deviations for each group). The groups did not differ in age. All participants were right-handed, native speakers of English with self-reported normal color vision.
Table 1.
Questionnaire scores (mean (SD)) for each group at mass testing (time 1) and lab session (time 2)
| Group | PSWQ | MASQ-AA | MASQ-AD | |||
|---|---|---|---|---|---|---|
| Time1 | Time2 | Time1 | Time2 | Time1 | Time2 | |
| Depression | 34.20 (10.21) | 34.20 (10.21) | 21.27 (3.13) | 21.00 (2.93) | 23.91 (2.47) | 18.45 (3.36) |
| Control | 36.11 (9.04) | 34.39 (8.42) | 19.56 (2.87) | 20.17 (2.20) | 12.50 (2.77) | 12.67 (2.68) |
Note. At Time 2 (immediately prior to psychophysiological data collection), the two groups did not differ on PSWQ scores, t(27) = .813, ns, and MASQ Anxious Arousal scores, t(26) = .050, ns, but did differ on MASQ-Anhedonic Depression scores, t(27) = 4.851, p < .001.
The PSWQ and MASQ were administered again, this time individually, prior to the fMRI session (except for one participant, who received the MASQ but not the PSWQ). As expected, the two groups did not differ on PSWQ scores, t(26) = .050, n.s, or MASQ Anxious Arousal scores, t(27) = .813, n.s, but did differ appropriately in MASQ-Anhedonic Depression (depressive mood subscale) scores, t(27) = 4.851, p < .001. Gender x Group ANOVAs indicated that men and women did not differ in any of the three scores, nor were there any Gender x Group interactions.
Experimental Task
The participants completed two tasks, the emotion-word Stroop and the color-word Stroop. Only data from the emotion-word Stroop task are considered here, as a priori asymmetry predictions focused on emotion processes. The task was implemented as blocks of pleasant or unpleasant emotion words alternating with blocks of neutral words. Several studies have indicated that the most effective emotion-word Stroop designs use blocked stimuli (Compton, Heller, Banich, Palmieri, & Miller, 2000; Dalgleish, 1995; Holle, Neely, & Heimberg, 1997). Participants received 256 trials in 16 blocks (four pleasant, eight neutral, four unpleasant) of 16 trials, with a variable ITI (±225 ms) averaging 2000 ms between trial onsets. A trial began with the presentation of a word for 1500 ms, followed by a fixation cross for an average of 500 ms. Each trial consisted of one word presented in one of four ink colors (red, yellow, green, blue) with each color occurring equally often with each word type (pleasant, neutral, unpleasant). Each participant received one of eight orders that were designed to ensure that the emotional and neutral words preceded each other equally often. Familiarity was controlled by ensuring that no word was repeated throughout the experiment. Within a block, each color appeared four times, and trials were pseudorandomized such that no more than two trials featuring the same color appeared in a row. After every fourth block (except the last), there was a brief rest period. In addition to the 16 word blocks, there were four fixation blocks – one at the beginning, one at the end, and two in the middle of the session. In the fixation condition, instead of a word, a brighter fixation cross was presented for 1500 ms.
The 256 word stimuli included in the emotion-word Stroop task were selected from the Affective Norms for English Words (ANEW) set (see Table 2; Bradley & Lang, 1999). Sixty-four were pleasant (e.g., birthday, ecstasy, laughter), 64 were unpleasant (e.g., suicide, war, victim), and two sets of 64 were neutral (e.g., hydrant, moment, carpet). The words were carefully selected on the basis of established norms for valence, arousal, frequency of usage in the English language (Bradley & Lang, 1999; Toglia & Battig, 1978) and number of letters. Each word appeared centered on a black background. Participants responded with their middle and index fingers using left- and right-hand response boxes for both tasks.
Table 2.
Stimulus Characteristics
| Pleasant Words | Unpleasant Words | Neutral Words | |
|---|---|---|---|
| Average Valence | 7.83 | 2.47 | 5.21 |
| Average Arousal | 6.59 | 6.54 | 3.82 |
| Average Frequency | 52.39 | 60.03 | 60.00 |
| Average Word Length | 5.78 | 5.36 | 5.33 |
Note. Word stimuli were selected from the Affective Norms for English Words (ANEW) set (Bradley & Lang, 1999). Valence and arousal data from the ANEW set are represented on a scale ranging from 1 to 9, with 9 representing the most pleasant and most arousing ratings, respectively. Frequency information was collected from Toglia & Battig (1978).
fMRI Data Collection
A series of 370 fMRI images were acquired using a gradient-echo echo-planar pulse sequence (TR 2000 ms, TE 25 ms, flip angle 60°, FOV = 24 cm) on a 3T Siemens Allegra head-only scanner. Twenty contiguous oblique axial slices (slice thickness 7 mm, in-plane pixel size 3.75 × 3.75 mm) were acquired parallel to the anterior and posterior commissures. These scan parameters were selected to minimize susceptibility artifacts in orbitofrontal and medial temporal areas.
Prior to the fMRI sequence, a 20-slice T1-weighted structural scan was administered using the same slices as the functional acquisition (slice thickness 7 mm, in-plane pixel size .5 × .5 mm). After the fMRI sequence, a 128-slice MPRAGE structural sequence was acquired (slice thickness 1.3 mm, in-plane pixel size 1 × 1 mm). Both of these structural sequences were used to register the participant’s structural and functional data into standard space.
Behavioral Performance Analysis
The primary hypothesis for behavioral performance was that depressed and control participants would take longer to respond to emotion than to neutral words (an arousal effect). It was also predicted that this difference would be greater for unpleasant than for pleasant words across groups (a valence effect). A third hypothesis was that individuals with depression would show a larger emotion-word Stroop effect for unpleasant words than would controls, i.e., longer reaction time for unpleasant than for neutral, as has been reported previously (see Mogg, Bradley, Williams, & Mathews, 1993).
Each of the three behavioral performance hypotheses was tested using a Group x Emotion ANOVA. The hypothesis of longer reaction time for high-arousal (pleasant and unpleasant) words was tested with all three emotion levels via the Emotion quadratic trend. Similarly, the hypothesis of longer reaction time for unpleasant versus pleasant words was tested via the Emotion linear trend. Finally, the prediction that the depression group would show a greater emotion-word Stroop effect for unpleasant words was tested via the Group x Emotion interaction, including just unpleasant and neutral levels of the Emotion factor (thus, a negative affect comparison).
fMRI Data Reduction and Analysis
Functional image processing and statistical analyses were implemented primarily using FEAT (FMRI Expert Analysis Tool, http://www.fmrib.ox.ac.uk/analysis/research/feat/), part of FMRIB’s Software Library (FSL) analysis package (http://www.fmrib.ox.ac.uk/fsl). Group-level analyses were carried out using locally written Matlab and C++ programs.
Prior to image processing, the first three time points of each dataset were discarded to allow the MR signal to reach a steady state. Each subject’s time series was motion-corrected via the FSL program MCFLIRT, intensity-normalized, and temporally filtered (nonlinear high-pass filter with a 1/256 Hz cutoff; Jenkinson & Smith, 2001; Jenkinson et al., 2002). A 3D Gaussian filter (FWHM = 7 mm) was applied to account for hemisphere and individual differences in brain morphology affecting image alignment and local variations in noise. A pre-coloring filter using a Tukey taper was applied to each voxel to account for serial autocorrelation in the time series. The autocorrelation structure introduced by the filter was then incorporated into analyses carried out for each voxel (see Woolrich, Ripley, Brady, & Smith, 2001).
Regression analyses were performed on each participant’s time series using FILM (FMRIB’s Improved Linear Model). Statistical maps were generated via multiple regression on each intracerebral voxel (Woolrich, Ripley, Brady, & Smith, 2001). An explanatory variable (EV) was created for each trial type (pleasant, neutral, unpleasant, and rest) and convolved with a gamma function to better approximate the temporal course of the blood-oxygen-dependent (BOLD) hemodynamic response (e.g., Aguirre, Zarahn, & D’Esposito, 1998; Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000). Each EV yielded a per-voxel effect-size parameter estimate (s) map representing the magnitude of activity associated with that EV. All functional activation maps and their corresponding structural MRI maps were transformed into a common stereotactic space (henceforth referred to as Talairach space or Talairach coordinates; Talairach & Tournoux, 1988) using FMRIB’s Linear Image Registration Tool (FLIRT).
Group-level ANOVAs addressed two different perspectives on emotion lateralization: valence (comparing pleasant to unpleasant words) and PA/NA (comparing pleasant or unpleasant words to neutral). The valence perspective was examined via a Valence × Hemisphere statistical map calculated in the context of a Group × Valence × Hemisphere ANOVA. The dependent measure for this ANOVA consisted of participant-wise s maps representing the contrasts of unpleasant to pleasant stimuli. The PA/NA perspective was examined via a Group × Hemisphere statistical map including only unpleasant versus neutral β maps as the dependent measure. The effect of hemisphere was examined on a per-voxel basis, contrasting each voxel in one hemisphere to its contralateral homologue. In practice, this entailed comparing each voxel to that with identical coordinates in the axial and coronal dimensions (y and z in MNI space), but with the sagittal coordinate multiplied by −1. (The same analysis was carried out using pleasant versus neutral β maps, but these data did not directly inform a priori depression hypotheses and are not reported here). Both of these ANOVAs included subject nested within group as a random effect (all other effects were fixed; for model specifications see Kirk, 1995, chapter 12). Family-wise Type I error was controlled via the simultaneous application of a per-voxel statistical threshold of p < .01 and a cluster size threshold determined from Monte-Carlo simulations (implemented via the program AlphaSim; Ward, 2000). Monte-Carlo simulations were run separately within DLPFC, amygdala, and non-DLPFC/amygdala areas, separately for each of the two statistical maps focused on in this paper (i.e., “partial-volume correction”; see Bencherif, Stumpf, Links, & Frost, 2004). All reported clusters were significant at a corrected p-value of < .05.
Results
Behavioral Performance Analyses
As predicted, reaction times were longer for high-arousal words than for neutral words, quadratic Emotion F(1, 50) = 3.986, p = .026 (see Figure 1). The two groups did not significantly differ in this effect, Group × quadratic Emotion F(1,50) = 1.108, n.s. The Depressed group responded more quickly overall, though this effect was only a statistical trend, F(1,79) = 3.337, p = .078.
Figure 1.
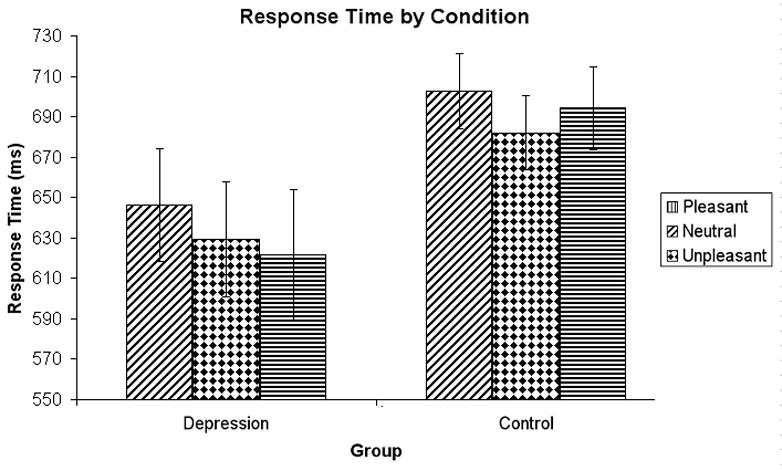
Response times for pleasant, neutral, and unpleasant words. Error bars represent 1 standard error above and below the mean.
Contrary to the valence hypothesis, reaction times for pleasant words were greater than for unpleasant words across groups, F(1,25) = 5.436, p = .028. This effect did not vary by group, Group × Valence F(1,25) = 1.350, n.s. For unpleasant words the depressed group did not show more Stroop interference than controls, Group × Emotion (unpleasant, neutral) F(1,25) = 1.714, n.s.
FMRI Analyses
Two ANOVAs were examined corresponding to valence and PA/NA emotion perspectives. Only those findings related to hypotheses within frontal cortex and amygdala are detailed below (significant activation clusters outside of these areas are listed in Table 3).
Table 3.
Summary of significant activation clusters
| Region | BA | Talairach coordinates* | Cluster size (# voxels) |
|---|---|---|---|
| Valence X Hemisphere Predicting Pleasant and Unpleasant Word Activation | |||
| Inferior Frontal Gyrus/DLPFC | 46 | ±36, 34, 16 | 31 |
| Superior Frontal Gyrus | 6 8 |
±41, 16, 49 | 41 |
| Amygdala/Superior Temporal Gyrus | 34 | ±30, 1, −16 | 24 |
| Inferior Parietal Lobule | 40 | ±41, −38, 51 | 70 |
| Group × Hemisphere Predicting Unpleasant Word Activation | |||
| Inferior Frontal Gyrus | 9 44 |
±44, 6, 30 | 242 |
| Amygdala/Superior Temporal Gyrus | 34 | ±29, 4, −18 | 63^ |
| Inferior Temporal Gyrus | 20 | ±37, −10, −32 | 69 |
| Hippocampus/Heschl’s Gyrus | 28 34 |
±20, −38, −7 | 111 |
| Precuneus | 7 | ±12, −55, 31 | 119 |
| Precuneus/Inferior Parietal Lobule | 7 | ±29, −53, 46 | 233 |
Note. The per-voxel threshold was p < .01 (corrected).
BA = Brodmann’s area
Because hemisphere is included as a factor in these analyses, x coordinates are both positive and negative.
^As amygdala encompasses a small area, a higher threshold of p < .025 was used, allowing for broader activation while still controlling family-wise error.
Valence Analyses
A per-voxel statistical map was generated representing the interaction of Valence (pleasant versus unpleasant words) and Hemisphere in the context of a Group × Valence × Hemisphere ANOVA. The key a priori prediction was increased left-lateralized activity within DLPFC for pleasant words (Herrington et al., 2005), predominantly in the control group. Monte Carlo simulations based on this statistical map determined that contiguous clusters of greater than 18 voxels thresholded at p < .01 occurred randomly at a probability of p < .05 within the DLPFC mask, 10 voxels in the amygdala mask, and 67 voxels in the non-DLPFC/amygdala mask. Four significant clusters of activity were observed for the Valence X Hemisphere interaction (see Table 3).
Frontal lobe activation
Two Valence X Hemisphere interaction clusters were located in frontal cortex. The first cluster was in DLPFC, centered on ±36, 34, 16 (see Figure 2, top panel). The pattern of means in this cluster closely replicated that of the DLPFC cluster in Herrington et al. (2005), showing more left-lateralized activity for pleasant than for unpleasant words, Valence × Hemisphere F(1, 27) = 14.215, p < .001. Although in the same direction as Herrington et al. (2005), activation was not significantly greater for pleasant than for unpleasant words averaged across hemisphere when including both depressed and control groups, F(1, 27) = 1.673, n.s. However, pleasant words did show significantly more bilateral activity in this cluster when examining the control group alone [relevant because Herrington et al. (2005) used participants unselected for psychopathology], F(1, 17) = 4.446, p = .05. The control group was marginally more left-lateralized than the depressed group across pleasant and unpleasant conditions, F(1, 27) = 3.024, p = .093.
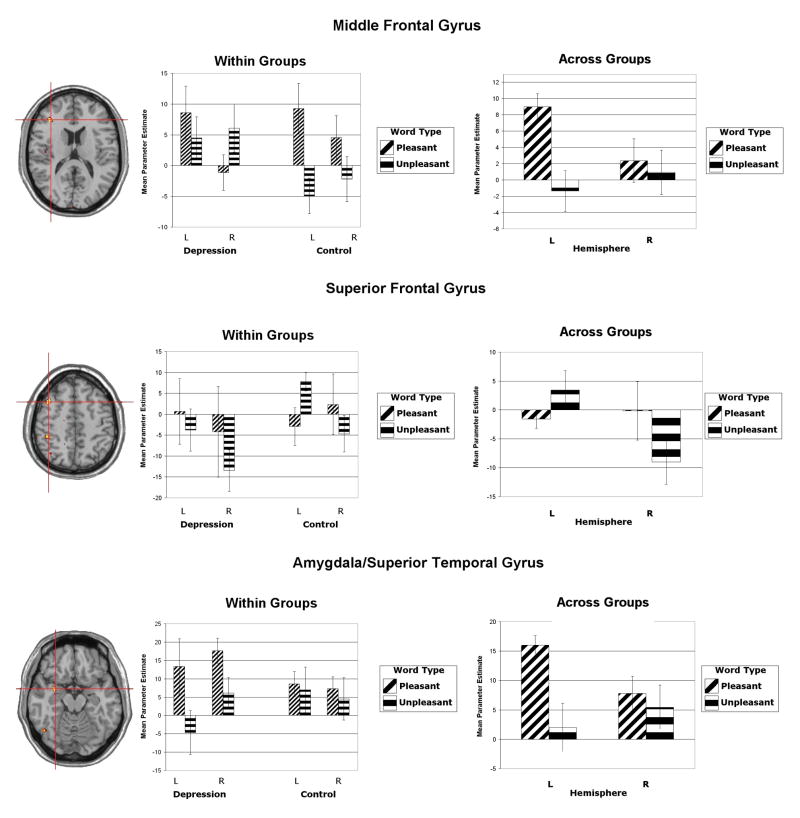
Figure 2.
Areas yielding a Significant Valence × Hemisphere interactions in middle (top) and superior (bottom) frontal gyri, and peri-amygdala. The left-hand column displays the clusters. Activation is arbitrarily overlaid on left-hemisphere anatomy, as hemisphere was included as a factor in the analysis. The red crosshairs are placed over the center of effect size for each cluster. The right column plots mean parameter estimates (i.e., the average of all voxel values within the cluster) for each level of valence and hemisphere averaged across both groups. Although these regions did not show a three-way interaction involving Group, the middle column contains plots of the average parameter estimates for each level of Group separately. Coordinates are centers of effect size in the stereotactic space of Talairach and Tournoux (1988). Error bars represent 1 standard error above and below the mean.
The second frontal cortex cluster (not tested in Herrington et al., 2005) showing a Valence × Hemisphere interaction was centered in superior frontal gyrus (within DLPFC), ±41, 16, 49 (see Figure 2, middle panel). The activation pattern in this region was opposite that of a priori hypotheses: activation for unpleasant words was more left-lateralized in this cluster, whereas activation for pleasant words was more right-lateralized, Valence × Hemisphere F(1, 27) = 12.212, p = .002. In simple-effects analyses, activation for unpleasant words was lateralized, F(1, 27) = 12.019, p = .002, whereas pleasant-word activation was not reliably lateralized. The unpleasant word lateralization was carried primarily by the control group, t(17) = 3.025, p = .004. Even though the depressed group showed the same trend, t(17) = 1.627, p = .067, the effects in that group were mainly driven by deactivation in this region. Accordingly, the Group × Valence × Hemisphere interaction was not significant.
Amygdala activation
A Valence (pleasant versus unpleasant words) × Hemisphere interaction cluster of activation was found in amygdala and adjacent superior temporal gyrus, centered on ±30, 1, −16 (see Figure 2, bottom panel). Activation for pleasant words was more left-lateralized than for unpleasant words, Valence x Hemisphere F(1, 27) = 15.592, p < .001. This interaction was driven primarily by less left hemisphere cluster activation for negative words in the depressed group, though the effect was only a trend, t(27) = 1.516, p = .071 (right hemisphere n.s.).
Negative Affect Analyses
The second statistical map represents the interaction of Group and Hemisphere in the context of a Group × Hemisphere ANOVA, where the dependent variable was unpleasant word activation (unpleasant minus neutral fMRI signal). The a priori prediction for this analysis was increased right-lateralized activation in the depression group. Monte Carlo simulations determined that contiguous clusters of greater than 16 voxels thresholded at p < .01 occurred randomly at a probability of p < .05 within the DLPFC mask, 31 voxels in the amygdala mask, and 63 voxels in the non-DLPFC/amygdala mask. This statistical map yielded six significant clusters of activity (see Table 3).
Frontal lobe activation
One Group × Hemisphere interaction cluster was found in the frontal lobes, centered on ±44, 6, 30 (see Figure 3, top panel). This cluster spanned middle and inferior frontal gyrus, Brodmann areas 9 and 44 (including DLPFC). Activation in this area was more right-lateralized for the depressed than the control group, F(1, 27) = 22.020, p < .001. This pattern was consistent with the key hypothesis, based on EEG findings, that depression would involve a greater asymmetry in favor of right frontal regions. This interaction was driven primarily by greater unpleasant word activation for the depressed group than for the control group in the right hemisphere, t(27) = 2.091, p = .004 (left hemisphere n.s.). In fact, unpleasant word activation was right-lateralized for the depressed group, t(10) = 3.219, p = .005, and left-lateralized for controls, t(17) = 2.974, p = .004.
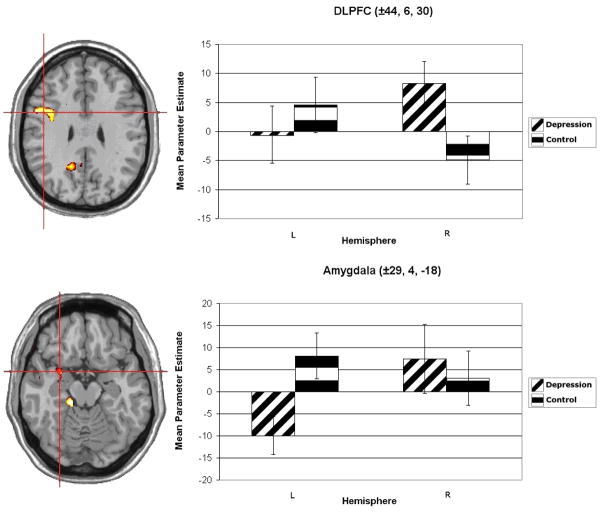
Figure 3.
Group × Hemisphere interaction for unpleasant word activation in dorsolateral prefrontal cortex (DLPFC). The left-hand column displays the DLPFC cluster. Activation is arbitrarily overlaid on left-hemisphere anatomy, as hemisphere was included as a factor in the analysis. The red crosshairs are placed over the center of effect size for the cluster. The right-hand column contains plots of the average parameter estimates for each level of Group (Depression and Control) and Hemisphere (with L and R labeling left and right hemisphere, respectively). Hemisphere main effects for both clusters were significant at p < .01. Coordinates are centers of effect size in the stereotactic space of Talairach and Tournoux (1988). Error bars represent 1 standard error above and below the mean.
Amygdala activation
For unpleasant words, significant group differences in lateralized activation were observed in a cluster encompassing amygdala and immediately adjacent superior temporal gyrus (see Figure 3, bottom panel). As amygdala encompasses such a small region, a higher per-voxel threshold of p < .025 was used for Monte-Carlo simulation, allowing for broader areas of activity. This simulation yielded a 51-voxel threshold, which was exceeded by the present amygdala cluster (centered on ±29, 4, −18). This indicates that activation in the cluster lacks a “peak” of sufficient size to exceed the initial cluster threshold but is nevertheless statistically reliable as a whole.
Consistent with previous lateralized amygdala findings (Glascher & Adolphs, 2003; Markowitsch, 1998), activation for the depressed group was more right-lateralized than for the control group, F(1,27) = 9.180, p = .005. The depressed group showed a rightward lateralization for unpleasant words in the amygdala cluster, t(10) = 2.436, p = .018, whereas the control group showed a nonsignificant leftward lateralization, t(17) = 1.145, n.s. The interaction was driven primarily by less left amygdala activation in the depression group, t(17) = 2.012, p = .027 (right hemisphere n.s.). Activation in this cluster was right-lateralized, though not significant, F(1,27) = 2.781, p = .108.
Discussion
DLPFC asymmetry
The present fMRI study supports and extends EEG findings of asymmetric brain activation in frontal brain regions in response to emotional information. In this experiment, participants were asked to name the color in which a series of words were presented. Although participants were instructed to ignore word meaning, a manipulation of the emotional valence of the words elicited asymmetric activation consistent with decades of research on frontal lobe contributions to emotion and depression.
In particular, a region of DLPFC in Brodmann’s area 46 showed differential activation depending on whether the information to be ignored was of positive or negative valence, replicating Herrington et al. (2005) and consistent with EEG findings. The pattern of activity for this region did not distinguish depressed and non-depressed participants. Responding to the valence manipulation, this DLPFC cluster showed more activity in the context of pleasant but not unpleasant words. Furthermore, this area showed less activity in the context of unpleasant than neutral words. These two findings indicate that this area of DLPFC functions differentially across the valence spectrum – up-regulated for pleasant emotional stimuli and down-regulated for unpleasant emotional stimuli. The greater sensitivity of this left-hemisphere DLPFC region to pleasant and unpleasant emotional stimuli resulted in the observed functional asymmetry (e.g., an interaction between valence and hemisphere, illustrated in Figure 2, top panel). Although predicted by EEG studies (see Nitschke et al., 2004), this experiment and its predecessors (Herrington et al., 2005; Engels et al., 2007)1 are apparently the first fMRI studies to confirm directly that DLPFC functions asymmetrically while processing emotional stimuli2.
Furthermore, the present study is the first to explicitly demonstrate abnormal DLPFC asymmetries with symptoms of depression. Methods used emotional stimulus manipulations conforming to two perspectives on the structure of emotion, referred to here as valence and PA/NA. Although not created to describe brain function per se, these perspectives have provided a framework for numerous EEG and hemodynamic imaging studies of and theoretical papers on brain mechanisms in emotion (e.g., Davidson, 1992; Heller, 1990, 1993; Heller & Nitschke, 1998; Keller, Hicks, & Miller, 2000; Nitschke et al., 1997, 1999, 2001, 1999; Wacker, Heldmann, & Stemmler, 2003; Williams, Watts, MacLeod, & Mathews, 1997).
A more posterior DLPFC cluster that revealed an asymmetry consistent with EEG findings for depression emerged only when comparing unpleasant trials to neutral trials, in line with the PA/NA perspective. Although activation in this cluster was greater for pleasant than unpleasant words (suggesting some sensitivity to valence), depression was characterized by an abnormal asymmetry for unpleasant but not pleasant words. This second DLPFC asymmetry arose from a group difference in which hemisphere showed the larger response to unpleasant words (right for depression, left for control).
In addition to confirming the existence of two asymmetries for processing emotional stimuli and depression, the fMRI paradigm allowed for further localization of different regions in DLPFC for state versus trait emotional processes. The more anterior location of the region in left DLPFC has now been found in several studies to be more active in the context of pleasant stimuli, showing more activity even when valence is to be ignored (Herrington et al., 2005; Engels et al., 2007). In contrast, trait affect was associated with less activity in a more posterior region of DLPFC. Although it remains to be confirmed that state versus trait emotion is associated with distinct DLPFC regions, the results are consistent with a model of cognitive control suggesting that mid-DLPFC is more involved in selecting and maintaining task-relevant representations of stimuli (Milham, Banich, & Barad, 2003), whereas regions of posterior DLPFC are more involved in biasing or steering posterior brain regions such as parietal cortex toward task-relevant processing, such as ink color identification (in the case of the Stroop task) and away from task-irrelevant processing (such as word reading). In this model, the more anterior mid-DLPFC region is stimulus-driven and involves more transient aspects of attentional control, whereas the more posterior DLPFC region is driven by top-down processes and may represent more static aspects of attentional control.
With regard to emotion-modulated attention, activity in DLPFC may therefore be signaling an “affective set” comparable to the hypothesized “cognitive set” often attributed to this region. Mid-DLPFC may signal more stimulus-driven aspects of the “affective set” whereas activity in posterior DLPFC may be reflecting more persistent, internalized, and trait-related aspects of the “affective set.”
These considerations lead to the hypothesis that activity in the left mid-DLPFC region signals the presence of a safe, pleasant, and affiliative context, in which threat is minimal, and in which left-hemisphere functions such as talking, reading, reflecting, and writing (behaviors that require selective attention to detail or internal focus, highly incompatible with scanning the environment for danger) are promoted. Thus, the affective dimensions of positive affect, pleasant valence, and approach motivation that have been associated with left prefrontal activity support efficient processing of the cognitive functions implemented by distributed left hemisphere brain regions. Trait-related activity associated with the more posterior region in DLPFC is likely to interact with that of the more anterior region to determine the net impact and nature of the overall “affective set.”
In contrast, we have argued that a distinct set of cognitive characteristics is implemented in various right hemisphere regions that taken together are ideally suited for a threat-response system (for review see Nitschke, Heller, & Miller, 2000). For example, parietally mediated visual and spatial abilities allow for rapid processing of threat-relevant information (e.g., facial expression, attentional monitoring of space), and frontally mediated mechanisms inhibit ongoing behavior and interrupt ongoing activity to stop and take stock of a situation. Thus, depression-related activity in more posterior right DLPFC signals the presence of threat and is associated with negative affect, avoidance or withdrawal behavior, anxious arousal, and other behavioral manifestations indicative of a threat response.
Another brain region located in a more posterior region of DLPFC, in superior frontal gyrus, showed the opposite asymmetry, in which activation for unpleasant words was left-lateralized. This region did not distinguish the groups, thus reflecting a more stimulus-driven pattern. The role of this brain region in a Stroop task is unknown, but it is similar to asymmetries that have been reported for orbitofrontal cortex (OFC).
Some hemodynamic imaging studies have indicated that OFC may respond asymmetrically to emotion, particularly when elicited via olfaction (Anderson et al., 2003, Zald & Pardo, 2000). Although seldom explicitly tested, these studies generally localize processing of pleasant stimuli to right OFC and unpleasant stimuli to left OFC. As this OFC pattern was not observed in the present study, it is possible that the OFC emotion asymmetries are to some degree limited to olfaction or to an aspect of emotion that is tightly coupled with olfactory processing. One important difference between olfactory stimuli and emotional words is that the former are more likely to invoke an immediate change in mood state (e.g., disgust or fear). The paradigm used in this study is not likely as effective in triggering such a change.
Indeed, OFC activation prompted by emotionally valenced information, identified in a variety of studies examining, among other things, olfaction, taste, and somatosensory processing (for review see O’Doherty, 2004), seems to track the affective relevance of highly situation-specific stimuli. For example, OFC activity in response to appetizing food increases and decreases with satiety (Kringelbach, O’Doherty, Rolls, & Andrews, 2003). DLPFC, in contrast, may be more involved in producing a more general “affective set”, as suggested above, reflecting both stimulus-driven and trait-related emotional factors. OFC-mediated responses to smells, tastes, and other stimuli with immediate and rapidly-changing reward value would then be superimposed on this more general affective backdrop. A related distinction has been suggested by Pizzagalli, Sherwood, Henriques, and Davidson (2003), who argued for DLPFC-mediated goal representation and OFC-mediated learning of stimulus-incentive associations.
A leftward DLPFC asymmetry in the same task as a rightward asymmetry for OFC (and also perhaps for the region identified in superior frontal gyrus) may indicate simply that there are two separate systems recruited. The systems could be independent, reciprocal, or in some more complex way interactive. For example, when activity in the critical regions of left DLPFC is low, signaling a low positive affect context, left OFC may become more active when confronted with an unpleasant stimulus. Chronic low activity (e.g., as in depression) in left DLPFC could lead to chronic over-activity in left OFC. Conversely, high activity in left DLPFC could modulate the degree to which left OFC becomes active when confronted with an unpleasant stimulus, accounting for a pattern of resilience in some individuals as well as findings that positive affect leads to superior emotional coping, greater generosity in social situations, and higher accuracy in processing social and cognitive information (for review see Isen, in press).
The present study obtained support for both valence and PA/NA models of emotion, involving distinct frontal regions, with the PA/NA model better capturing abnormal DLPFC asymmetries in depression. Given the likely functional diversity of frontal subregions, the two models need not be mutually exclusive. With such regional differentiation, not yet accomplished in the EEG literature, the considerable evidence in favor of prefrontal asymmetry for emotional valence is easily reconciled with the more recent evidence in favor of prefrontal asymmetry for PA/NA and approach motivation.
Amygdala asymmetry
Observing rightward amygdala asymmetry for the depressed group is consistent with the findings of least one other study. Abercrombie et al. (1998) reported a relationship between increased metabolic rate and depressive symptoms among individuals with depression (but not a control group), measured at rest. The present study extends this finding to activation during an emotion provocation task.
Present data may be relevant to some theories of amygdala function. For example, Glascher and Adolphs (2003) showed decreased electrodermal activity in response to pleasant and unpleasant emotional pictures among individuals with damage to the right amygdala. They concluded that right amygdala function is related to general physiological arousal, independent of valence. Sabatinelli, Bradley, Fitzsimmons, and Lang (2005) reached the same conclusion in an fMRI assessment of amygdala response to diverse emotional pictures. Although depression may be associated with low levels of sympathetic arousal, it is also associated with heightened responsiveness to arousing unpleasant information (Clark & Watson, 1991; Koster, De Raedt, Goeleven, Franck, & Crombez, 2005), which may in turn be associated with more rightward amygdala lateralization. This proposal fits with present data and those of Glascher and Adolphs. However, present data are somewhat inconsistent with Glascher and Adolphs in a different respect. If amygdala functioned according to Glascher and Adolph’s proposal, one would expect an overall rightward lateralization of amygdala function for both emotion conditions across both groups. This pattern did not emerge here.
The amygdala activation pattern was also somewhat consistent with Markowitsch’s (1998) proposal implicating right amygdala in the rapid processing of emotion-related material. Markowitsch based his conclusions in part on studies by Morris, Ohman, and Dolan (1998 1999) showing enhanced right amygdala activity during the perception of masked emotional faces. Present amygdala findings may therefore reflect hyperactivation in depression of a rapid response system for processing emotional information. However, as noted above, this conclusion would be more strongly supported if a more general rightward lateralization were observed in amygdala across groups and emotion word conditions. In particular, it is possible that the emotion words in the present study were not associated with sufficient arousal to engage the amygdala in such a way that asymmetry effects are detectable; these may emerge only in the context of trait depression coupled with unpleasant valence. This interpretation is consistent with Glascher and Adolphs (2003), insofar as they used emotional pictures that are likely more arousing than words.
Behavioral performance
Reaction-time data served the dual purpose of confirming the effectiveness of the emotion manipulation and evaluating possible group differences in overt performance in the face of emotional challenge. Results supported the hypothesis that emotionally arousing words slow performance, replicating work from our lab and elsewhere (Compton et al., 2003; Koven, Heller, Banich, & Miller, 2003; Stormack, Nordby, & Hugdahl, 1995; Williams, Mathews, & MacLeod, 1996). Thus, in the present study, behavioral performance data were more consistent with a PA/NA than a valence perspective, in that pleasant and unpleasant words differed from neutral but not from one another. The observed emotion-word Stroop effect indicates that the emotional content of the words was being processed (and not ignored), despite being irrelevant to the color naming task. These data support the assertion that observed asymmetries in brain activation were driven by the emotional content of the words.
The individual difference manipulation (depressed versus control group contrast) did not increase the emotional Stroop effect for unpleasant words. The overall trend towards decreased response times in the depression group was somewhat unexpected, given the association between depression and diminished attentional processes. Nevertheless, this result is consistent with depression studies that have failed to show reliable performance biases for tasks involving automatic attentional processes (see Mogg et al., 1993). Thus, depressed and control groups responded to emotional stimuli differently (as detected by the fMRI data) but in a manner that is not necessarily evident when measuring rapid behavioral responses. As suggested by Mogg et al. (1993), the behavioral manifestations of attentional biases in depression may emerge only during tasks that demand more attentional resources than the emotion-word Stroop task. The trend toward decreased response time in the depression group is also unexpected given the psychomotor delays that often accompany a major depressive disorder diagnosis (American Psychiatric Association, 2000; Levin, Heller, Mohanty, Herrington, & Miller, 2007). Conceivably, the observed increase in right DLPFC activity for depressed participants provided an alternative approach to cognitive control on the task (e.g., by boosting inhibition of response to negative words as opposed to the more normative top-down attentional emphasis on task set — respond to color — mediated by the left DLPFC). If the observed response time decrease proves more robust in future emotional Stroop studies, this issue could be more systematically assessed via additional tests of psychomotor functions and paradigms that manipulate aspects of cognitive control and executive function .
Heterogeneity of DLPFC function
The present differentiation of brain areas responsive to emotional stimulus characteristics and depressive symptoms may also help to explain the failure of hemodynamic imaging studies to replicate the substantial EEG literature. In fact, just such a replication failure emerged in a preliminary analysis using these data (not reported here). A statistical map representing the main effect of Hemisphere in a Group x Valence x Hemisphere ANOVA did not yield a significant DLPFC cluster, as was predicted by EEG findings. Stated another way, DLPFC clusters emerged only by 1) contrasting the levels of either the Valence or Group factors via Valence × Hemisphere or Group × Hemisphere interactions, or 2) examining pleasant and unpleasant word conditions separately. Thus, lateralized DLPFC activation conforms to both valence and PA/NA perspectives and to group manipulations but effects may not be robust enough to emerge in typical fMRI studies with typical group sizes. Present findings avoid the failure of many relevant hemodynamic imaging studies to replicate the EEG literature on frontal lateralization in emotion and underscore the need for appropriate analytic strategies to advance this literature.
In addition to careful selection of emotion conditions, methodological advances will likely permit more detailed considerations of DLPFC heterogeneity. Although this study demonstrates the potential of testing asymmetry hypotheses via direct comparisons of per-voxel contralateral homologues, it is important to note that left and right hemispheres are not morphological mirror images of one another. There seems little reason to conclude that the amount of error induced by finding a simple geometric homologue is any greater than that inherent in typical registration procedures, or that this error is likely to be additive. Nevertheless, as with studies using traditional within-hemisphere analyses, the localization of signal to specific cortical areas will only improve as registration procedures become more robust (particular those procedures that account for variations in sulcal/gyral boundaries).
Generalizability of Findings to Other Paradigms and Stimuli
The emotional Stroop task is somewhat unique in that the emotional manipulation is implicit, insofar as it is orthogonal to the required response (i.e., naming the ink color). However, it appears likely that task completion nevertheless requires attentional selection and is therefore not distinctly preattentive (Dalgleish, 2005; Mohanty et al., 2007). Therefore, these findings are likely to generalize to other tasks (and stimuli) where attention to emotion is more explicit (such as judging the valence of a facial expression). This hypothesis can be addressed in future studies using explicit emotion paradigms, as well as in studies where competition between emotional and non-emotional responses are systematically varied.
Conclusion
This study indicates that, despite the conclusions of the Murphy et al. (2003) and Wager et al. (2003) meta-analyses of hemodynamic neuroimaging studies, specific brain areas within and outside prefrontal cortex function asymmetrically in emotion processing – including DLPFC. In addition, DLPFC may be critically involved in the emotional modulation of attention and cognition, with subregions of DLPFC displaying functional distinctions that mirror those that have been found for non-emotional, cognitive processes. These areas will likely be found only when using analytic approaches that explicitly address functional lateralization.
When interpreting the present findings, it is important to consider that depression is a broad construct with many manifestations. The varied aspects of depression touched upon by different researchers may lead to distinct conclusions, each potentially valid, none exhaustive. One perspective on depression that was not considered in the present study was that provided by the Diagnostic and Statistical Manual-Fourth Edition (DSM-IV-TR; American Psychiatric Association, 2000) – specifically, the diagnosis of Major Depressive Disorder. The present reliance on self-report measures of emotional function rather than diagnostic interview information reflects extensive research relating these measures to established models of depression (e.g., Nitschke, Heller, Imig, McDonald, & Miller, 2001) as well as their track record in isolating important aspects of brain function in depression using EEG. Furthermore, DSM-IV diagnostic criteria for depression, often used in isolation, lack the specificity required to distinguish depression and other diagnoses (primarily anxiety disorder diagnoses, which are often comorbid), as is often required to establish the specificity of relevant research findings (Heller, 1993; Heller, Etienne, & Miller, 1995; Heller & Nitschke, 1998; Heller, Nitschke, Etienne, & Miller, 1997; Nitschke et al., 2001). However, a consideration of diagnostic status can be important in studies examining other aspects of depression. For example, although the dimensional measures used in this study would be quite appropriate for treatment studies examining asymmetries, a consideration of diagnostic status may facilitate the direct translation of results from these studies to clinical practice (for example, see Davidson, Irwin, Anderle, & Kalin, 2003).
Present DLPFC findings have implications for understanding the clinical presentation of individuals with depression. Decades of human and non-human primate research have established DLPFC as a key constituent in attention, executive function, and working memory (Rajkowska & Goldman-Rakic, 1995; Davidson & Irwin, 1999). One need look no further than the DSM-IV criteria to realize that attentional processes are central to the conceptualization of mood disorders. A cardinal symptom of Major Depressive Disorder is an “inability to concentrate.” However, much more detail is needed regarding the nature and degree of executive dysfunction in depression (for review, see Levin et al., 2007). Present findings underscore the importance of further research to understand the neural correlates of executive function deficits in depression.
Furthermore, such research has implications for the application of therapeutic techniques in the treatment of depression. Treatment approaches that target executive dysfunction may be particularly effective for at least certain types of depression. For example, Strauman and colleagues have developed a therapeutic approach called self-system therapy that targets individual differences in the ability to attain promotion goals, in other words, the ability to make good things happen. Individuals whose self-reported socialization history lacked an emphasis on pursuing promotion goals showed reduced left prefrontal activation as measured by fMRI when their promotion goals were activated (Merrill et al., 2006). Similarly, depressed patients displayed a deficit in left prefrontal cortical activation after priming of their individual promotion goals.
Other novel treatments for depression also appear to target prefrontal functions. Behavioral activation treatment (Dimidjian et al., 2006) appears to be as effective as cognitive therapy and pharmacological therapy in more severely depressed individuals. This treatment approach emphasizes the use of behavioral strategies, such as goal-setting, self-monitoring, activity scheduling, and problem-solving, that promote the executive function and could plausibly address deficits in left prefrontal function. Furthermore, behavioral activation therapy explicitly addresses the avoidance or withdrawal component of depression, consistent with abnormal rightward lateralization of function. This treatment approach may therefore target dysfunctional prefrontal activity in depression directly through the modification of behavior relevant to both left and right hemispheres. It remains to be seen whether such interventions are reflected in normalization of brain activity.
In summary, key hypotheses regarding asymmetric emotion processes in DLPFC were strongly supported by present data. Furthermore, the analytic technique used here isolated asymmetries in other regions for which little valid or reliable data exist. These findings argue that distinguishable asymmetries exist across numerous nodes in the network of brain areas involved in emotion.
Footnotes
The present control sample was also used by Engels et al. (2007). None of the present depression sample was used in the Engels et al. study. None of present samples or the Engels samples overlapped with the Herrington et al. (2005) sample.
Although Brody et al. (2001) reported that they included hemisphere as a factor in some analyses involving DLPFC, they apparently did not report findings from such analyses. A few studies (e.g., Dolcos et al., 2004; Grimm et al., 2006) have reported significant clusters in left and right prefrontal cortex during presentation of pleasant or unpleasant pictures, respectively. However, to our knowledge, none of these directly tested whether activation in these areas was asymmetric.
Contributor Information
John D. Herrington, Children’s Hospital of Philadelphia
Wendy Heller, University of Illinois at Urbana-Champaign.
Aprajita Mohanty, Northwestern University.
Anna S. Engels, Pennsylvania State University
Marie T. Banich, University of Colorado-Boulder
Andrew G. Webb, Pennsylvania State University
Gregory A. Miller, University of Illinois at Urbana-Champaign
References
- Abercrombie H, Schaefer S, Larson C, Oakes T, Lindgren K, Holden J, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9(14):3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8(4):360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris C, O’Connor E, Breiter H. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Ahern G, Schwartz G. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23(6):745–755. doi: 10.1016/0028-3932(85)90081-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fourth edition, Text revision. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn R. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell I, Schwartz G, Hardin E, Baldwin C, Kline J. Differential resting quantitative electroencephalographic alpha patterns in women with environmental chemical intolerance, depressives, and normals. Biological Psychiatry. 1998;43(5):376–388. doi: 10.1016/s0006-3223(97)00245-x. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Stumpf MJ, Links JM, Frost JJ. Application of MRI-based partial-volume correction to the analysis of PET images of mu-opioid receptors using statistical parametric mapping. Journal of Nuclear Medicine. 2004;45(3):402–408. [PubMed] [Google Scholar]
- Bradley M, Lang P. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang P. Affective norms for English words (ANEW) Gainesville, FL: The NIMH Center for the Study of Emotion and Attention, University of Florida; 1998. [Google Scholar]
- Bruder G, Stewart J, Mercier M, Agosti V, Leite P, Donovan S, et al. Outcome of cognitive-behavioral therapy for depression: relation to hemispheric dominance for verbal processing. Journal of Abnormal Psychology. 1997;106(1):138–144. doi: 10.1037//0021-843x.106.1.138. [DOI] [PubMed] [Google Scholar]
- Clark L, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Coan J, Allen J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen J, Braver T, O’Reilly R. A computational approach to prefrontal cortex, cognitive control and schizophrenia: recent developments and current challenges. Philosophical Transactions of the Royal Society of London B: Biological Science. 1996;351(1346):1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Compton R, Banich M, Mohanty A, Milham M, Herrington J, Miller G, et al. Paying attention to emotion: An fMRI investigation of cognitive an emotional stroop tasks. Cognitive, Affective and Behavioral Neuroscience. 2003;3(2):81–98. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Compton R, Heller W, Banich M, Palmieri P, Miller G. Responding to threat: Hemispheric asymmetries and interhemispheric division of input. Neuropsychology. 2000;14(2):254–264. doi: 10.1037//0894-4105.14.2.254. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional Stroop task in groups of anxious, expert, and control subjects: A comparison of computer and card presentation formats. Cognition & Emotion. 1995;9(4):341–362. [Google Scholar]
- Dalgleish T. Putting some feeling into it--the conceptual and empirical relationships between the classic and emotional Stroop tasks: comment on Algom, Chajut, and Lev (2004) Journal of Experimental Psychology - General. 2005;134(4):585–591. doi: 10.1037/0096-3445.134.4.585. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affect, cognition, and hemispheric specialization. In: Izard C, Kagan J, Zajonc R, editors. Emotion, Cognition, and Behavior. New York, NY: Cambridge University Press; 2004. [Google Scholar]
- Davidson R. Prolegomenon to the structure of emotion: Gleanings from neuropsychology. Cognition & Emotion. 1992;6:245–268. [Google Scholar]
- Davidson R. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12(3):307–330. [Google Scholar]
- Davidson R. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67(1–2):219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson R, Ekman P, Saron C, Senulis J, Friesen W. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. Journal of Personality & Social Psychology. 1990;58(2):330–341. [PubMed] [Google Scholar]
- Davidson R, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson R, Irwin W, Anderle M, Kalin N. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160(1):64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Davidson R, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli S, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Davidson R, Lewis D, Alloy L, Amaral D, Bush G, Cohen J, et al. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Davidson R, Pizzagalli D, Nitschke J, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53(1):545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–70. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage. 2004;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Drevets W, Raichle M. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes. Cognition & Emotion. 1998;12(3):353–358. [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, et al. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44(3):352–63. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex. New York, NY: Raven; 1989. [Google Scholar]
- Garavan H, Pendergrass J, Ross T, Stein E, Risinger R. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12(12):2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. The Journal of Neuroscience. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, Ranganath C, Rosenfeld P. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion. 1998;12:449–478. [Google Scholar]
- Gray J, Braver T, Raichle M. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences. 2002;99(6):4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Goldman S, Salovey P. Measurement error masks bipolarity in affect ratings. Journal of Personality & Social Psychology. 1993;64(6):1029–1041. doi: 10.1037//0022-3514.64.6.1029. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. NeuroImage. 2006;30(1):325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hamann S, Ely T, Hoffman J, Kilts C. Ecstasy and agony: Activation of human amygdala in positive and negative emotion. Psychological Science. 2002;3(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Heller W. The neurospychology of emotion: Developmental patterns and implications for psychopathology. In: Stein NL, Leventhal B, Trabasso T, editors. Psychological and Biological Approaches to Emotion. Hillsdale, NJ: Lawrence Erlbaum & Associates; 1990. [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Heller W, Etienne M, Miller G. Patterns of perceptual asymmetry in depression and anxiety: inplications for neuropsychological models of emotion and psychopathology. Journal of Abnormal Psychology. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke J. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition & Emotion. 1997;11(5–6):637–661. [Google Scholar]
- Heller W, Nitschke J. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition & Emotion. 1998;12(3):421–447. [Google Scholar]
- Heller W, Nitschke J, Etienne M, Miller G. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106(3):376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke J, Miller G. Lateralization in emotion and emotional disorders. Current Directions in Psychological Science. 1998;7(1):26–32. [Google Scholar]
- Heller W, Koven N, Miller GA. Regional brain activity in anxiety and depression, cognition/emotion interaction, and emotion regulation. In: Hugdahl K, Davidson RJ, editors. Brain Asymmetry. 2. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Herrington J, Mohanty A, Koven N, Fisher J, Stewart J, Banich M, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5(2):200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Holle C, Neely J, Heimberg R. The effects of blocked versus random presentation and semantic relatedness of stimulus words on response to a modified Stroop Task among social phobics. Cognitive Therapy & Research. 1997;21(6):681–697. [Google Scholar]
- Hommer D, Knutson B, Fong G, Bennett S, Adams C, Varnera J. Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Annals of the New York Academy of Science. 2003;985:476–478. doi: 10.1111/j.1749-6632.2003.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Irwin W, Anderle M, Abercrombie H, Schaefer S, Kalin N, Davidson R. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. NeuroImage. 2004;21(2):674–686. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karama S, Lecours A, Leroux J, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16(1):1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Hicks B, Miller G. Psychophysiology in the study of psychopathology. In: Caccioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2000. pp. 719–721. [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Monterey, CA: Brooks/Cole Publishing Company; 1995. [Google Scholar]
- Knutson B, Fong G, Adams C, Varner J, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Koster E, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion. 2005;5(4):446–455. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Koven NS, Heller W, Banich MT, Miller GA. Relationships of distinct affective dimensions to performance on an emotional Stroop task. Cognitive Therapy and Research. 2003;27(6):671–680. [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LaBar K, Gitelman D, Parrish T, Kim Y, Nobre A, Mesulam M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lane R, Reiman E, Ahern G, Schwartz G, Davidson R. Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry. 1997;157(7):926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski J, Johnson J, Williams T, editors. Technology in Mental Health Care Delivery Systems. Norwood, NJ: Ablex Publishing; 1980. [Google Scholar]
- Lang P. Presidential address, 1978. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16(6):495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang P. The varieties of emotional experience: a meditation on James-Lange theory. Psychological Review. 1994;101(2):211–221. doi: 10.1037/0033-295x.101.2.211. [DOI] [PubMed] [Google Scholar]
- Levin R, Heller W, Mohanty A, Herrington J, Miller G. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. [Google Scholar]
- Markowitsch H. Differential contribution of right and left amygdala to affective information processing. Behavioural Neurology. 1998;11:233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Meyer T, Miller M, Metzger R, Borkovec T. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miezin F, Maccotta L, Ollinger J, Petersen S, Buckner R. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative thinking. NeuroImage. 2000;11:735–739. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller G, Keller J. Psychology and neuroscience: Making peace. Current Directions in Psychological Science. 2000;9:212–215. [Google Scholar]
- Miller G, Cohen S. Psychological interventions and the immune system: A meta-analytic review and critique. Health Psychology. 2001;20(1):47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley B, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102(2):304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Herrington J, Koven N, Fisher J, Wenzel E, Webb A, et al. Neural mechanisms of affective interference in schizotypy. Journal of Abnormal Psychology. 2005;114(1):16–27. doi: 10.1037/0021-843X.114.1.16. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MR, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec T. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment and treatment. Chichester, UK: John Wiley and Sons; 1994. pp. 265–283. [Google Scholar]
- Morris J, Ohman A, Dolan R. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Murphy F, Nimmo-Smith I, Lawrence A. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, and Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Newman S. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Science. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W. The neuropsychology of anxiety disorders: Affect, cognition, and neural circuitry. In: D’Haenen H, den Boer JA, Willner P, editors. Biological Psychiatry. Chichester, UK: John Wiley & Sons; 2002. pp. 975–988. [Google Scholar]
- Nitschke J, Heller W, Etienne M, Miller G. Prefrontal cortex activity differentiates processes affecting memory in depression. Biological Psychology. 2004;67:125–143. doi: 10.1016/j.biopsycho.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nitschke J, Heller W, Imig J, McDonald R, Miller G. Distinguishing dimensions of anxiety and depression. Cognitive Therapy & Research. 2001;25(1):1–22. [Google Scholar]
- Nitschke J, Heller W, Miller G. The neuropsychology of emotion. In: Borod Joan C., editor. Series in Affective Science. 2000. pp. 298–319. [Google Scholar]
- Nitschke J, Heller W, Palmieri P, Miller G. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36(5):628–637. [PubMed] [Google Scholar]
- O’Doherty J, Rolls E, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Deichmann R, Critchley H, Dolan R. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Osgood C. The nature and measurement of meaning. Psychological Bulletin. 1952;49(3):197–237. doi: 10.1037/h0055737. [DOI] [PubMed] [Google Scholar]
- Owen A, Evans A, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cerebral Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Perlstein W, Elbert T, Stenger V. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Science U S A. 2002;99(3):1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E, O’Connor K, Gatenby J, Gore J, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychological Science: A Journal of the American Psychological Society/APS. 2005;16(10):805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Prochaska J, Norcross J. Stages of change. Psychotherapy. 2001;38(4):443–448. [Google Scholar]
- Rajkowska G, Goldman-Rakic P. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995;5(4):323–37. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Russell J. A circumplex model of affect. Journal of Personality & Social Psychology. 1980;39:1161–1178. [Google Scholar]
- Russell JA, Bullock M. Fuzzy concepts and the perception of emotion in facial expressions. Social Cognition. 1986;4(3):309–341. [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage. 2005;24(4):1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. NeuroImage. 2004;22(1):409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Shafritz K, Collins S, Blumberg H. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31(1):468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Levin-Silton R, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. 2008;8(5):701–713. doi: 10.1037/a0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormack K, Nordby H, Hugdahl K. Attentional shifts to emotionally charged cues: Behavioral and ERP data. Cognition and Emotion. 1995;9(5):507. [Google Scholar]
- Stuss D, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53(1):401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tornoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Theime; 1988. [Google Scholar]
- Toglia M, Battig W. Handbook of semantic word norms. New York, NY: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Tucker D, Stenslie C, Roth R, Shearer S. Right frontal lobe activation and right hemisphere performance. Decrement during a depressed mood. Archives of General Psychiatry. 1981;38(2):169–174. doi: 10.1001/archpsyc.1981.01780270055007. [DOI] [PubMed] [Google Scholar]
- Wacker J, Heldmann M, Stemmler G. Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion. 2003;3(2):167–193. doi: 10.1037/1528-3542.3.2.167. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for fMRI data. 2006 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. Retrieved from http://afni/nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Watson D, Clark L, Weber K, Assenheimer J, Strauss M, McCormick R. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Wager T, Phan K, Liberzon I, Taylor S. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98(2):219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Williams J, Watts F, MacLeod C, Mathews A. Cognitive Psychology and Emotional Disorders. Chichester, UK: John Wiley and Sons; 1997. [Google Scholar]
- Williams J, Mathews A, MacLeod C. The emotional stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2000;36(2):165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Zald D. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zevon MA, Tellegen A. The structure of mood change: An idiographic/nomothetic analysis. Journal of Personality and Social Psychology. 1982;43:111–122. [Google Scholar]