Abstract
Self-injurious behavior (SIB) among individuals with intellectual and related neurodevelopmental disorders (IDD) is a clinical challenge and scientific puzzle. The physiological mechanisms regulating the sensory components of SIB remain a mystery with no clear understanding of the underlying pathophysiology. The central dogma regarding sensory processing in general and pain in particular among individuals with IDD and chronic SIB is that sensory processing is reduced and pain is absent or blunted. In this paper, recent findings challenging some of the conventional wisdom regarding pain and sensory function among individuals with IDD and SIB are reviewed. It seems that at least a subgroup of individuals with IDD and chronic SIB may be in a physiological state similar to neuropathic pain in which hyperalgesia is mediated by plasticity mechanisms regulating inflammatory, immune, and nociceptive systems. In response to repeated tissue damage associated with chronic self-injury, innate immune cells may be producing pro-inflammatory and pro-nociceptive cytokines that act on the brain to cause sickness-like behavior and sensitize primary sensory nerve afferents contributing to pain hypersensitivity (i.e., hyperalgesia).
Keywords: self-injury, developmental disorder, pain, immune
1.1. Overview of self-injurious behavior in intellectual and neurodevelopmental disabilities
1.1.1. Clinical significance of self-injury
Self-injurious behavior (SIB) among individuals with intellectual and developmental disabilities (IDD) is a clinically daunting and scientifically challenging behavior problem with profound implications for a person's general health and overall quality of life. Self-injury often leads to increased risk for institutionalization or reinstitutionalization, social stigmatization, and decreased learning opportunities. The National Institutes of Health Consensus Conference on Destructive Behavior in Developmental Disabilities generated a report estimating that the costs of care associated with the approximately 160,000 people in the United States with mental retardation and severe destructive behavior exceeded $3 billion (US) in 1989 (National Institutes of Health, 1991). There is no reason to believe the incidence or prevalence of SIB has changed in the past 20 years (Rojahn, et al. 2008). Because of the numerous deeply disturbing qualities associated with the behavior, there is little disagreement over its significance and the need for treatment. As a consequence, people with IDD who self-injure are treated with a wide array of behavioral techniques and psychotropic medications. Unfortunately, despite our best clinical efforts based on state-of-the-science interventions, a significant subgroup of individuals persist at injuring themselves with sufficient severity to produce permanent tissue damage and disfigurement with extreme instances resulting in brain damage and death. Little is known about self-injury’s developmental course or its underlying pathophysiology. Given the complexity of the disorder, a number of models and mechanisms have emerged to account for the development and maintenance of SIB (Schroeder et al. 2001).
1.1.2. Models and mechanisms of self-injury
SIB is a heterogeneous disorder and like other behaviorally-defined disorders it is reasonable to assume that SIB can be the consequence of a variety of etiologies which, in turn, involve a variety of environment-brain-behavior relationships. Consequently, a number of biological and behavioral models have emerged in the past two decades (Thompson & Schroeder, 1995). Neurobiological models of SIB initially identified roles for opioidergic (Sandman, 1988; 1990/1991), dopaminergic (Breese et al. 1995), and serotonergic (Cook, 1999) systems in the pathophysiology of SIB. In behavioral models of SIB, self-injury is conceptualized as a learned behavior (i.e., an operant) maintained by its reinforcing consequences (positive or negative). SIB may lead to inadvertent positive reinforcement in the form of parental or staff attention (Carr, 1977; Mace et al. 1992), or negative reinforcement such as termination of a demand following SIB (Carr et al. 1976; Fisher et al. 1993) or nonsocial positive reinforcement related to the production of sensory stimulation (Lovaas, et al. 1987). Despite the variety and breadth of models proposed, almost none formally integrate biology and behavior. The observed variability of SIB in response to different treatments and the continued presence of intractable cases suggest we have an incomplete understanding of the mechanisms of self-injury. Increasingly it appears that it may be more productive to focus on how biological and behavioral mechanisms work together to produce and maintain severe persistent self-injury.
1.1.3. Implications of self-injury characteristics: location, intensity, & chronicity
Common SIB forms include but are not limited to head banging, self-biting, self-scratching, self-hitting, gouging, and picking. For some individuals SIB is episodic, but for others chronic. Chronic SIB typically involves repeated, forceful stimulation to specific, localized body sites (e.g. hitting specific spot on head, picking an area on the leg, biting a specific part of the hand), rather than random patterns of SIB distributed to various points on the body (Symons & Thompson, 1997). Kinematic analyses reveal a high degree of cycle-to-cycle consistency in the qualitative dynamics of the limb movements, indicating that the motions involved in SIB are often highly repetitive in nature (Newell et al. 2002). The repetitive motions involved in some forms of SIB translate to repetitive localized stimulation to the targeted SIB site. The mean peak impact forces for some individuals with chronic SIB are well over 200 N (similar to the upper range of impact forces for heavy weight boxing punches and karate kicks). Coupling the impact forces with the high frequency of blows during a single bout of self-injury for some individuals would essentially be the equivalent of dropping a 48-oz (3-lb) hammer on your forehead every second for up to a half an hour. Recent but limited work to date (described below) has been motivated to empirically examine the biological consequences associated with the structural characteristics (frequency, intensity, and location) of chronic SIB in relation to the associated tissue damage it produces (Symons et al. 2001, 2003a, 2008).
1.1.4. Summary of SIB Overview
Tremendous advances have been made in the past 3 decades in our understanding of SIB. Theoretical perspectives have been sharpened and a robust behavioral assessment technology in the form of functional analysis exists designed to test environmentally-mediated reinforcement mechanisms maintaining an individual’s self-injury. In turn, functional analysis technology, based on the early conceptual work of Carr (1977) and the pioneering empirical work of Iwata et al (1982), have lead to efficacious direct behavioral interventions because they target the operant mechanism maintaining SIB. Notably, however, for a significant number of individuals social reinforcement mechanisms appear to be absent (or, at least, difficult to document) making it much more difficult to design targeted behavioral interventions. Pharmacologically, despite theoretically plausible predictions, we still do not have any proven tailoring variables to guide medication selection (although the compelling front runner is the novel work from Sandman’s lab showing that elevated levels of circulating beta-endorphin predict therapeutic response to opiate antagonist treatment [Sandman et al. 1999]). To date, however, the bulk of the SIB research has proceeded with little attention directed toward models of vulnerability and individual differences.
From a broader epidemiological perspective, SIB research seems stuck in the risk factor stage because so few studies use designs that are able to document causality or, at least, approximate it. The causal status of most risk factors for SIB (e.g., primary sensory impairment, severe/profound mental retardation, and some specific syndromes) is unknown. In some cases, we know what statistically predicts SIB, but not how or why. Considering the enormous costs associated with chronic SIB the cost of getting causation wrong is not trivial (Moffit et al. 2001 writing in a different context). Remaining frontiers critical to inform our scientific understanding of SIB include early developmental pathways, maintaining treatment effects, and, as argued in this paper, the physiological and sensory mechanisms regulating nociception. The nociceptive physiological and sensory mechanisms associated with and possibly mediating SIB are very poorly understood. One problem created by the paucity of scientific understanding about sensory variables is that a clinical vacuum has been created in which non-validated practice parameters become the default treatment technology for at least some forms of chronic SIB (e.g., sensory integration treatment) that claim to exert their effects by changing a variety of central nervous system pathways regulating sensation. Scientifically, there is little to no evidence for such claims. The approach adopted by our research group has been translational by addressing systematically the issue of whether and in what way sensory variables may be involved in regulating SIB.
2.2. SIB & Pain: SIB as Nociceptive Behavior
2.2.1. Sensory input & SIB
The abnormal sensory input associated with severe self-injury has not been studied systematically, nor has the relation between the peripheral and central mechanisms of pain transmission and regulation and the tissue damage associated with chronic SIB. It is not known in most cases whether pain is a cause or consequence of self-injury. Few models have incorporated mechanisms specific to pain transmission and regulation despite the fact that self-injury involves tissue damage and related injury. Although little consensus exists as to whether common mechanisms regulate aberrant pain responses and self-injury, a majority of study outcomes are consistent with a model in which self-injury is related to and regulated by sensory and pain processing (Symons, 2002). In this context, a useful bridge between behavior and biology may be found in the mechanisms regulating peripheral and central sensory transmission of painful or noxious stimuli.
Pain is a sensory experience normally generated by activation of a specific subset of high-threshold peripheral sensory neurons, the nociceptors (nociception is the detection of noxious or tissue-damaging stimuli). After repeated injury dramatic alterations in the somatosensory nervous system can occur amplifying responses and increasing sensitivity to peripheral stimuli so that pain can be activated by normally non-noxious or low-intensity stimuli (Woolf, 1993). In pre-clinical models of sensory dysfunction associated with chronic and neuropathic pain (i.e., pain induced by injury or dysfunction in the nervous system) the behavioral parameters used to indicate pain include autotomy (i.e., self-injury), allodynia (i.e., increased sensitivity to normally non-noxious stimuli), and hyperalgesia (i.e., increased pain sensitivity to noxious stimuli). The exact mechanisms regulating sensory dysfunction are unclear but it appears to depend on desensitization of afferent sensory fibers involving structural changes in the epidermal nerve fibers (ENF) mediated, in part, by substance P (SP) such that sensory dysfunction results, at least in part, from degeneration or reorganization of intracutaneous nerve fibers. Whether similar consequences are associated with chronic tissue-damaging SIB among individuals with neurodevelopmental disorders is not completely understood but the initial evidence from our work to date (reviewed below) is consistent with features of the pre-clinical models and clinical conditions associated with small-fiber neuropathy and neuropathic pain states.
2.2.2. Conceptualizing SIB as nociceptive behavior
Accordingly, the conceptual model begins by recognizing that chronic SIB typically involves repeated, forceful stimulation to specific, localized body sites (e.g. hitting specific spot on head, picking an area on the leg, biting a specific part of the hand), rather than random patterns of SIB distributed to various points on the body. Repeated tissue damage may result in chronic neuropathic-like pain characterized by increased sensitivity to painful stimuli (hyperalgesia), the perception of innocuous stimuli as painful (allodynia) and spontaneous pain. Animal models of peripheral neuropathic pain are now available in which the mechanisms underlying hyperalgesia and allodynia due to nerve injury or nerve inflammation can be analysed (Latremoliere & Woolf, 2009). Based on the pre-clinical work, it has also become clear that inflammatory and immune mechanisms both in the periphery and the central nervous system play an important role in neuropathic pain (see sections below). Damage to tissue or the nervous system leads to infiltration of inflammatory cells, as well as the activation of resident immune cells, subsequently leading to the production and secretion of various inflammatory mediators (Moalem & Tracey, 2006). These mediators promote neuroimmune activation and can sensitize primary afferent neurons and contribute to pain hypersensitivity. As a consequence, a subgroup of persons with chronic SIB and IDD may be in a neuropathic pain-like state with dysregulated pain capacities manifest in the form of altered pain sensory thresholds and/or impairments in pain expression and regulation.
In a nociceptive model of SIB, the self-inflicted stimulation associated with SIB and the following cascade of peripheral sensory and neurochemical events may be highly resistant to change. From this perspective, for some subgroup of individuals, SIB may be regulated in part by specific changes in the peripheral and central mechanisms mediating sensory and pain function. This conceptualization of SIB does not directly address etiology nor address whether pain is a cause or consequence of SIB, but it does provide a framework to test several predictions specific to behavioral and biological endpoints relevant to both SIB and pain that would be expected to differ between comparable individuals with IDD with and without SIB. Specifically, differences would be expected across multiple inter-related systems including (a) behavioral (social/adaptive behavior), b) sensory (altered pain expression), (c) peripheral physiology (altered morphology), and (d) neurochemistry (altered biochemistry of pain peptides).
2.2.3. SIB structural features relevant to nociception: Frequency, intensity, and location of injury
The region of the body stimulated plays a critical role in determining whether intense sensory stimulation, either through mechanical or electrical devices, is regulated by endogenous pain systems. In rats and humans the response to opiate antagonists is dependent, in part, on the area of the body being injured (Melzack & Wall, 1983; Westbrook & Greeley, 1990). For example, Watkins et al. (1982) showed that stress-induced analgesia produced by shock delivered to rat's forepaws can be blocked by naloxone but stress-induced analgesia produced by shocking all other body regions is not. In humans, our group (Symons et al. 1998) and others (Herman et al. 1987; Thompson et al. 1994) have reported that self-injury directed toward the head and face responded favorably to naltrexone while self-biting did not. In turn, our group has quantified SIB location and documented a relation between SIB localization and body sites known to be associated with analgesic functions when stimulated [acupuncture analgesia sites] (Symons & Thompson, 1997) and have found reliable group differences (Symons et al. 1999; Symons, 2002) for SIB body site locations between IDD syndromes.
Stimulus intensity has also received considerable attention in pain research (Woolf, 1993). Melzack (1994) suggested that the intensity of the stimulation is a crucial factor in stimulation-produced activity in nocieptive pathways. Different stimulus intensities are regulated, in part, by different sensory pathways and their associated neurochemical substrates. Stimulus intensity is also known to influence the effectiveness of analgesic drugs during testing (Thorn et al. 1994). The effectiveness of both mu and kappa opioid agonists is reduced in rats by increasing the intensity of electrical stimuli directed towards pain transmitting C-fibres. Thus, different intensities of peripheral noxious stimulation, in part, determine the analgesic effect (or non effect) of different opioid agonists. Although the degree of sensory stimulation resulting from self-injury is likely related to the frequency of the behavior, frequency per se is often poorly correlated with actual tissue damage and hence, nociceptive input (Iwata et al. 1990). Tissue damage, however, is a function of intensity. Unlike the controlled evaluations of stimulus intensity noted above for pain and analgesia research, relatively little attention has been directed towards the intensity of SIB. SIB intensity may be an important characteristic to differentiate subtypes of self-injury and provide an additional basis for treatment selection (e.g., intense self-injury may be more likely to have a neurochemical component and respond to pharmacologic treatment). Newell et al. (2002) were the first to apply kinematic analyses to reliably quantify the intensity of SIB among individuals with developmental disabilities. The significance of this approach is that it provides a method to translate basic work on discrete structural characteristics of SIB (i.e., intensity, location) into an empirically-based strategy for subgrouping cases into high/low intensity subgroups that may make it more likely to find nociceptive and related biomarkers leading to novel treatment approaches.
2.2.4. Pain, sensory expression, and SIB
In a general model of SIB as nociceptive behavior, individuals with chronic SIB and intellectual impairments would have dysregulated pain or nociceptive capacities (Sandman, 1988) manifest in the form of altered pain thresholds, impaired pain expression, and/or disrupted pain signaling. Unfortunately, most individuals with severe or profound intellectual disabilities are non-verbal or otherwise communicatively impaired making it difficult if not impossible to reliably ascertain pain status through conventional means (i.e., self-report). Because of this, there is no direct evidence characterizing whether pain function is altered among non-verbal individuals with chronic SIB, but our work has produced indirect evidence consistent with this notion including studies documenting differences in peripheral autonomic markers (Symons et al. 2001), nociceptive biochemistry (Symons et al. 2003b), and abnormal peripheral innervation (Symons et al. 2008) among subgroups of individuals with chronic SIB and mental retardation.
In other nonverbal or similarly compromised populations (e.g. neonates, infants, medically-frail elderly) objective measurement strategies have been used to identify sensory reactivity and pain expression based on facial coding of expression during known painful events such as vaccination, blood draw, or minor invasive procedures (Grunau & Craig, 1987; 1990; Izard et al. 1980). This work draws upon the large body of empirical evidence for objective, nonverbal signs of emotional expression based on changes in facial action units (Ekman & Friesen, 1978). Facial action units are anatomically based configurations of facial musculature that can be directly observed and reliably coded. Faces are highly plastic and can configure into a wide range of different displays in very short time spans. Work with facial action coding has identified reliable configurations of facial muscles that correspond to discrete emotional states and includes a ‘pain face’ configuration of facial action units (Craig et al. 1992). In studies of individuals with developmental disorders, facial action coding has been used to identify painful experiences during invasive procedures (LaChapelle et al. 1999; Oberlander et al. 1999). Results showed that facial actions associated with acute vaccine and venipuncture-related pain among nonverbal individuals with developmental disabilities corresponded to timing of injection. Importantly, the studies demonstrated that the magnitude of pain expression in nonverbal persons with severe cognitive disabilities can be reliably quantified. These studies of the objective measurement of pain expression in persons with severe IDD have consistently shown that while the sensitivity to painful stimuli may be lower in some persons with IDD, painful events do reliably occasion the forms of nonverbal pain expression seen in persons without developmental disabilities. In our work to date (Oberlander et al. 2003), we have successfully applied facial coding technology to chronic SIB cases and have extended this work to develop a sham-controlled modified quantitative sensory testing protocol to objectively evaluate reactivity to calibrated stimuli (Symons et al. 2010) in which we reliably found higher (not lower) levels of reactivity to an array of calibrated sensory stimuli for individuals with chronic SIB relative to age and gender matched controls.
Taking a different approach, we also examined pain/sensory expressive behavior in the form of symptoms via staff report during naturally occurring contexts (Symons et al. 2009a). The specific purpose was to test for any differences in pain expression in clinically rated pain symptoms in the context of daily living between a SIB and matched no-SIB group. Findings were consistent with our controlled sensory evaluations with the SIB group rated as more (not less) expressive on pain symptoms. For the SIB group, there was a positive association (r = 0.35, p < .05) between overall pain signs and overall severity of SIB. These findings are in line with a model of chronic SIB as nociceptive behavior and a neuropathic-like pain state. It is noteworthy that the SIB group also had elevated scores on the Eating/Sleeping subscale (indicating more problems), consistent with a ‘sickness’ behavior profile. Overall, these findings are consistent with a model of SIB as nociceptive-related behavior.
2.2.5. SIB, Sensory Experience, & Peripheral Innervation
The clinical presentation of chronic, high frequency, tissue-damaging acts leads to two broad sets of questions. First, what is the experience of pain like in persons who exhibit SIB? The difficulty with this question is that the majority of individuals in question have significant cognitive and language deficits that do not permit them to describe their pain experience in a conventional manner (Defrin et al. 2004). They cannot tell us (a) if they are in pain or experiencing some form of unusual and perhaps discomforting sensation and if so whether they engage in SIB in reaction to this uncomfortable sensory state, (b) if they are not in pain and do not feel pain or unusual sensory experiences following SIB, or (c) if they are not in pain but experience some type of desirable (for them) sensory experience following SIB. The second set of questions concern the physiological mechanisms involved in pain perception.
Because each of the subjective scenarios just described are plausible but not easily or readily testable in persons with severe intellectual impairment using conventional means (i.e., self-report), our goals have been to examine some of the structural and chemical features of sensory innervation that are known to be linked to peripheral sensory perception and experience. Our recent observation that the distribution of epidermal nerve fibers (ENFs; coefficient of variability) characterized by significantly more gaps between ENFs in chronic SIB cases relative to normal controls (Symons et al. 2008) raises the possibility that a once normal distribution of sensory fibers degenerated to the point of obvious ENF gaps in these cases (note that the samples were taken through skin punch biopsy from non-self injury body sites). Epidermal nerve fibers have been demonstrated to degenerate in a variety of painful neuropathic conditions (Kennedy et al. 2005). Altered or disrupted pain sensitivity is influenced, in part, by such morphological changes in cutaneous ENFs under normal and clinical pain conditions (Kennedy et al. 1996). This raises the possibility that functionally, persons with SIB with abnormal ENF innervation could experience atypical sensory experiences characterized by a relative insensitivity to pain. If so, pain insensitivity could plausibly either lead to or maintain persistent self-injury due at least in part to a failure of the typical pain-related feedback that would make self-injurious acts aversive. The importance of this hypothesis is that it would provide a putative biological marker (abnormal ENF innervation) for prospective longitudinal studies of the early development of SIB in at-risk populations.
Conversely, the morphological characteristics found in the present SIB cases could also be interpreted as consistent with increased pain sensitivity as previous work has found that altered ENF distribution and innervation is associated with clinical conditions with altered pain experience (Albrecht et al. 2006; Kennedy et al. 1996). One plausible functional consequence of this for persons with SIB is that abnormal ENF morphology could lead to abnormal sensory experiences including pain and hyperalgesia and SIB may develop in response to this aversive sensory state. Our additional preliminary finding that SP levels were higher in SIB cases is consistent with this interpretation and further suggests that a balance of peptidergic and nonpeptidergic innervation may be required for normal tactile perception and, consequently, integrity of a normal (not amplified) pain signal. Emerging work on the neuronal circuitry for the development and modulation of pain perception (Dan & Mooney, 2006) may be particularly relevant to prospective studies designed to understand sensory function in the context of neurologic impairment. The observation of altered ENF innervation and elevated SP positive (SP+) fibers along with apparent hyper-responsiveness to an array of tactile stimuli (at non-SIB body sites (Symons et al. 2009) is consistent with a model of SIB as nociceptive behavior.
It is also plausible that neurotrophic factors could play an important role in the pathophysiology underlying the observed ENF abnormalities. Neurotrophic factors (e.g. BDNF, NGF) are implicated in a variety of neurodegenerative disorders that are associated with severe cognitive impairments (e.g. Alzheimer's disease; Elliot & Ginzburg, 2006). There is an established link between neurotrophic factors and differential cutaneous innervation (Rice et al. 1998) that has been increasingly explored in relation to pathological conditions through animal models (Yamaoka et al. 2007) and human cutaneous disorders (Dou et al. 2006) suggesting that any comprehensive examination of ENF morphology should include close analyses of neurotrophic factors.
2.3. SIB and Immune-Mediated Pathological Pain: Effects on ‘Sickness’-Behavior and Adaptive Behavior
2.3.1. Structural features of SIB interact with biological plasticity
Sensory neurons are inherently plastic (Watkins & Maier, 2002). This biological feature combined with the clinical reality that chronic SIB produces continual tissue damage has led to our focus on peripheral innervation and skin. Recent work, however, shows that peripheral nerves do not act alone in isolation from their microenvironment. The same high intensity SIB that causes a primary sensory nerve to fire, also damages tissue leading to an inflammatory response. Thus, an additional feature of SIB correlated with at least three structural characteristics (frequency, intensity, and location) is that SIB activates inflammatory mechanisms (an inherent biological property associated with injury) (Moalem & Tracey, 2006) which, in turn, leads to an immune response; another biological reality (Dantzer et al. 2008). One plausible outcome, particularly if the SIB is poorly managed is a potentially potent and deleterious feed-forward cycle in which more SIB (see Sandman’s contagion model [Sandman et al. 2008]), leads to more tissue damage, leading to additional release of inflammatory and immune mediators. Inflammation and immune activation, in turn, lead to the release of mediators that produce pain. These are the type of events hypothesized to be regulating neuropathic pain (Zuo et al. 2003).
Under acute conditions, the physiological benefit of pain - so called ‘good’ pain – is tissue repair, healing, etc. (Melzack & Wall, 1983; Wall & Melzack, 1994). If, under pathological conditions (in the case of chronic SIB and repeated tissue damage), inflammation is chronic then there is a cascade of pathophysiological effects that result, in part, from a set of cytokines released at the injury site and in the brain that can mediate complex social and sensory behavior. More specifically, bi-directional interactions exist among inflammatory, immune, and nociceptive systems regulated, in part, by cytokines resulting in so-called sickness behavior (disrupted exploratory behavior and associated activity, disrupted social behavior, disrupted sleep) (Dantzer, 2008) as well as profound sensory consequences (altered sensory and pain thresholds, reactivity/expressivity) (Watkins et al. 1995) and, over time, altered neuronal properties by gene transcription modification (Woolf, 2004).
The conceptual basis for such a cascade was articulated by Dantzer’s et al. (2008) review paper on sickness behavior in conditions associated with chronically activated inflammatory and immune systems (e.g., cancer, autoimmune diseases) in which the pathological symptoms of sickness are clearly tied to cytokine activity. One implication based on this set of events is that, in the long run, effective treatment that leads to generalized and maintained effects is likely impossible for a subgroup of individuals with IDD and chronic SIB if their treatment does not directly address biomedical variables related to health and well being in general (i.e., ruling out/treating acute undiagnosed medical conditions) but also immune and inflammatory mechanisms in particular; systems which probably have long non-linear ‘half lives’ and which are likely quite capable once activated and shift from physiological (i.e., ‘good’ pain) to pathological (i.e., ‘bad’ pain) to continue to regulate the expression of SIB.
2.3.2. Local neuro-immune interaction (‘cross talk’) & SIB
The concept of neuro-immune interaction suggests that pathological conditions thought to be exclusively neural or immunological may be a joint product of the interaction between the two systems (Dines & Powell, 1997). Substance P (SP) released from small diameter sensory afferents, for example, has been shown to degranulate mast cells (one type of immune cell) under a variety of conditions associated with pain and stress (Kawana et al. 2006). Increases in SP positive (SP+) nerve fibers and contacts between SP+ nerve fibers and mast cells (Naukkarinen et al. 1996) have been found in the skin of patients with identified cutaneous disorders associated with scratch/itch and pain. Moreover, there is increasing evidence that stress-induced peripheral neurogenic inflammation is mediated by SP release from peripheral nerve endings resulting in mast cell degranulation (Arck et al. 2003; Singh et al. 1999). We have made preliminary observations of increased SP+ fibers in the skin of individuals with chronic SIB (Symons, et al. 2008; 2009b), notably sampling from non-SIB body locations suggesting the possibility of systemic pathophysiology which would be consistent with an immune-mediated neuropathic pain state among individuals with chronic SIB.
2.3.3. SIB & immune mediated hyperalgesia
There are a host of inflammatory chemicals that can affect primary afferent fibers (SP being one of them) that include bradykinin, nitric oxide, glutamate, NGF, NE, as well as a growing list of specific pro-inflammatory cytokines (interleukin (IL)-1, tumor necrosis factor (TNF)-alpha, IL-6, etc.) all of which are known to enhance nociception (Moalem & Tracey, 2006). In addition to their nociceptive effects, cytokines also mediate inflammatory and immune responses (Watkins & Maier, 2002). In the periphery, the sensory nerves in human skin contain a variety of bioactive neuropeptides related to nociceptive (Kennedy et al. 2005), inflammatory (Drummond, 2004) and immune (Paus et al. 2006) responses.
One proposed mechanism underlying peripheral inflammatory and immune response is mast cell degranulation. Initial evidence supporting mast cell involvement with sensory nerves came from structural observations that non-myelinated nerves exist in close morphological relationship with mast cells in both normal (Dines & Powell, 1997) and diseased (Naukkarinen et al. 1996) skin. There is a functional bi-directional relation (i.e., nerve-immune ‘cross-talk’) between mast cells and nerves in which the activation of small diameter afferent neurons results in the release of neuropeptides, like substance P, peripherally as well as centrally. This activates mast cells, which in turn release mediators increasing neuronal excitability (Ferry et al. 2002; Suzuki et al. 1999). Our most recent findings provide initial evidence of immune system hyperactivity in individuals with chronic self-injury and IDD. Specifically, we found mast cell degranulation was increased in the skin of individuals with chronic self-injury (Symons et al. 2009b). There were also significant epidermal nerve fiber innervation differences among the same individuals with chronic self-injury compared to non-self-injuring individuals with IDD. Although preliminary and not confirmatory of a model of altered nociception associated with self-injury, the results suggest that, at least in the periphery, ongoing neuro-immune interactions may, in part, be related to the as of yet poorly understood pathophysiology of chronic self-injury.
There are longstanding observations of elevated circulating blood levels of beta-endorphin associated with SIB (Sandman et al. 1990/1991). Although the opioid models of SIB involve centrally-mediated (i.e., brain-based) effects, it may be that the hyperalgesic responses and neuro-immune interactions described above are modulated by endogenous opioid peptides secreted in peripheral tissue by leukocytes, macrophages, keratinocytes, and sensory neurons (Mousa et al. 2004). Local release of beta-endorphin and met- and leu-enkephalin from these cells in response to various stimuli (i.e., cytokines, catecholamines, corticotropin releasing hormone, stress) can reverse inflammatory hyperalgesia via activation of peripheral opioid receptors (Cabot et al. 1997). Conversely, endogenous opioids can stimulate the immune system by promoting expression of cytokines, including interleukin-6 (IL-6) and IL-1 (Piva et al. 2005; Kowalski et al. 2000; Kowalski et al. 2002). In contrast to the other endogenous opioid peptides, dynorphin has both pro- and anti-nociceptive properties (Caudle & Mannes, 2000). Elevated dynorphin levels have been detected in neuropathic, inflammatory and malignant chronic pain states (Nahin et al. 1989) and a single intrathecal injection of dynorphin in rodents precipitates a long-lasting sensitivity to pain (Laughlin et al. 1997). In animal models, elevated dynorphin expression is thought to contribute to the maintenance of central sensitization, a persistent amplification in pain sensitivity that occurs because of changes in nociceptive spinal cord neurons (Vanderah et al. 2000).
3. Summary & Conclusions
One distinct clinical implication of this conceptual model (and although not directly addressing etiology) is that if SIB develops, is not treated early, and reliably produces tissue damage, there will be a cascade of nociceptive effects. The net result could be to create a chronic pain like state physiologically with predictable effects on sensory (hyperalgesia) and sicknesslike behavior (sleep/appetite/social) which could cumulatively create a feed-forward process with SIB maintained by both its social but also its biological consequences. From our prior work (Hartman et al. 2008) and that of others (O’Reilly, 1997), we know that pain can lead to SIB in this population but we know almost nothing about whether SIB leads to pain and the resulting neuron-immune cascade of effects.
SIB and altered pain experiences are also associated with a wide spectrum of neurological (acquired sensory neuropathies; Roach, et al. 1985), psychiatric (borderline personality disorder, Herpertz, 1995), and developmental (autism, Bodfish et al. 2000) disorders. In autism and related pervasive developmental disorders, for example, it has long been recognized that many individuals have reduced sensation or responses to presumably painful stimuli (Ornitz, 1976). This observation takes on added significance considering an estimated prevalence of self-injury as high as 40–50% in persons with autism (Bartak & Rutter, 1976; Bodfish et al. 2000). Similarly, in many IDD-specific syndromes, SIB is a prominent feature of their phenotype including Lesch-Nyhan (Nyhan, 2002), fragile X (Symons et al., 2003a), Prader-Willi (Symons et al. 1999), Cornelia de Lange (Hall et al., 2008), and many others (Rojahn, et al., 2008). At the same time, there appear to be significant subgroups of individuals with IDD-specific syndromes in which normal sensory function is impaired or there is at the very least a strong index of suspicion (Symons, 2002).
There is also an emerging evidence base from studies of individuals without IDD but with SIB or ‘nonsuicidal self-injury’ (NSSI) that is of relevance to models of SIB and altered nociceptive mechanisms (Ballard, Bosk, & Pao, 2010). Nock and Mendes (2008) showed that an NSSI group had higher levels of physiological reactivity (indexed by skin conductance) during a distressing task, more difficulties tolerating task-related distress, and social problem-solving deficits. These findings are important because they provide a direct examination of physiological arousal among individuals with and without NSSI and complement prior work demonstrating that imagining engaging in NSSI leads to decreased physiological arousal (Haines, Williams, Brain, & Wilson, 1995). Earlier, Coid, Allolio, and Rees (1983) reported that plasma met-enkephalin levels were raised in inpatient psychiatric patients who habitually mutilated themselves. Subsequently, Russ et al (1992) found evidence for possible NSSI subgroups related to pain present or absent during self-injury based on sensory threshold testing (cold pressor test). Taken together, despite the limited database directly related to SIB and IDD, the emerging evidence from other clinical populations with SIB (NSSI) as well as pre-clinical animal models of SIB (Novak, 2003) suggests that the role of autonomic arousal, sensory regulation, and associated neurochemical and physiological mechanisms should be more fully explored in relation to SIB and pain among individuals with SIB and IDD.
When considered from the breadth of conditions in which SIB and altered nociception may be interacting, the model being suggesting may have broad applicability to account for multiple developmental pathways by using general principles related to nociception, immune response, and environmental context as a guide for recognizing commonalities of underlying processes despite phenotypic differences. As a first step in identifying candidate markers for the role of underlying nociceptive processes, we began by studying peripheral sensory factors in relation to repeated self-injury, and have examined the specific body site locations of self-injury (Symons & Thompson, 1997; Symons et al. 1993; Symons et al. 2003b) and physiological correlates at those sites (Symons, et al., 2001). Now there is initial evidence of altered peripheral innervation (Symons et al. 2008) and possible neuro-immune ‘cross talk’ (Symons et al. 2009b) at non self-injury sites. At the same time, there is behavioral evidence of generalized sensory and nociceptive hypersensitivity for subgroups of individuals with chronic SIB (Oberlander et al. 2003; Symons et al. 2009a; Symons et al. 2010). Considering this possibility, our overall goal going forward is to use the variables for which reliable differences between SIB and no-SIB controls as well as within SIB group comparisons as endpoint markers for systems hypothesized to be involved pathologically with chronic SIB.
Further work appears warranted to (a) conduct confirmatory tests of differences in peripheral innervation in relation to SIB, (b) determine the degree to which inflammatory and immune mediators are elevated, and (c) understand the functional significance of the pathological differences in nociceptive innervation and inflammatory/immune activity for sensory and adaptive behavior between individuals with neurodevelopmental disorders with and without SIB. The tremendous human costs associated with the disorder demand new scientific approaches to solve an old clinical problem
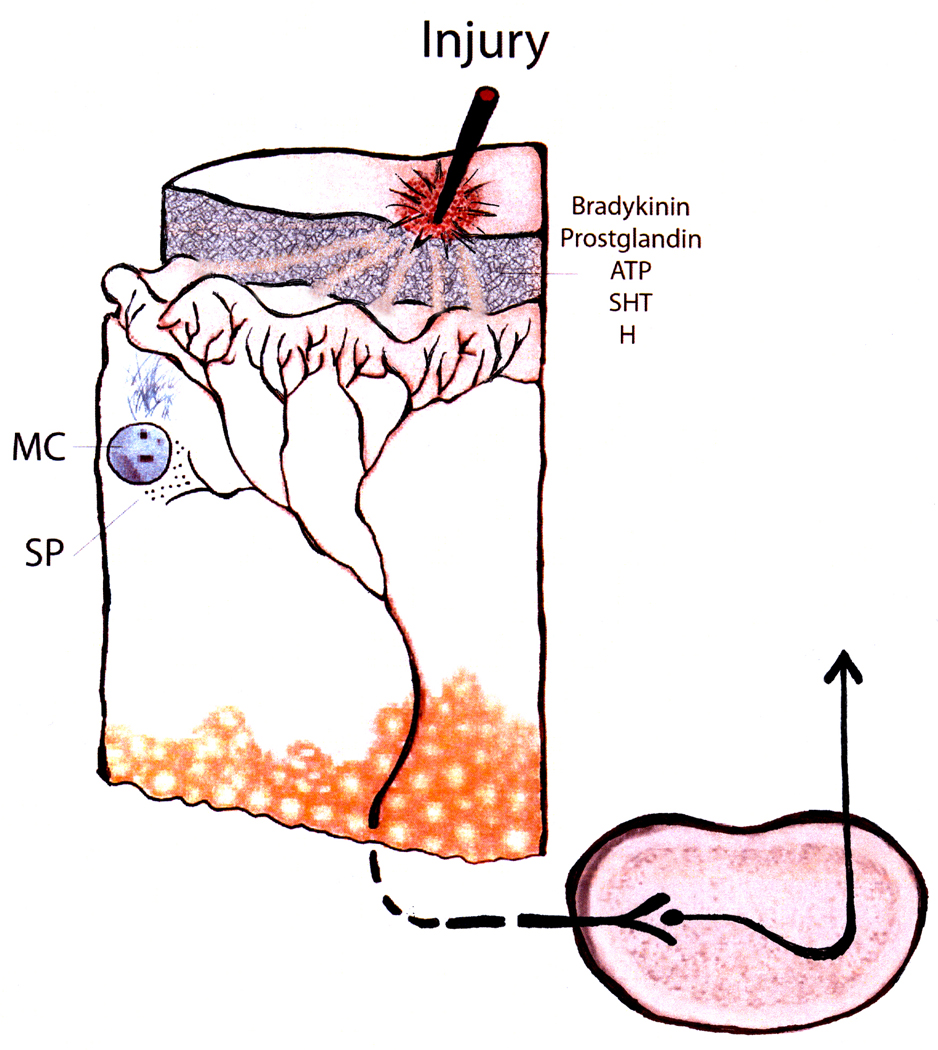
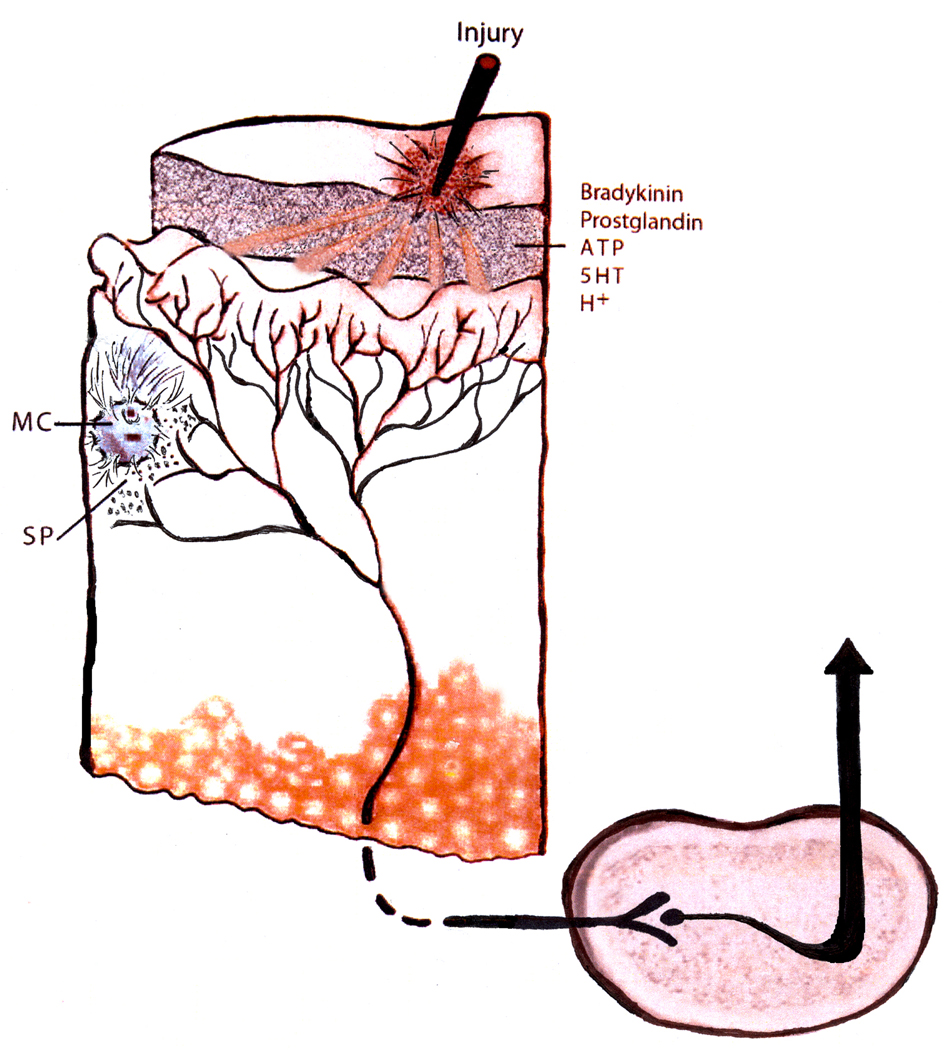
Figure 1.
Cartoon representing (a). Tissue damage results in local cells releasing a variety of bioactive molecules with multiple effects (nociceptive fiber excitation, mast cell degranulation, vasodilation) collectively changing the sensitivity of nociceptive fibers to subsequent stimulation. (b). Repeated tissue damage associated with chronic self-injury may be associated with an amplified set of effects leading to increased ascending nociceptive signaling. Evidence, to date, for this speculation are (i) observations of altered epidermal nerve fiber innervations density (Symons et al., 2008); (ii) increased substance P positive fibers (Symons et al, 2008, 2009b); (iii) extensive mast cell degranulation (Symons et al., 2009b); (iv) increased pain symptoms among individuals with chronic self-injury (Symons et al, 2009a); and (v) enhanced behavioral response to calibrated sensory stimuli (Symons et al., 2010). Note: the histological and biochemical findings to date are based on tissue sampled at non-self injury body sites from individuals with chronic self-injury suggesting the possibility of a systemic rather than localized sensory problem.
Acknowledgments
Funding for this work was provided, in part, by the University of Minnesota in the form of a McKnight Land-Grant Professorship and Minnesota Futures award as well as NICHD Grant No. 44763 & 447201; the NICHD had no further role in the work; in the collection, analysis and interpretation of data appearing herein; in the writing of the report; and in the decision to submit the paper for publication. I am grateful to the insightful comments tirelessly provided by Jim Bodfish on this line of thinking, the constructive critiques of Jennifer McComas and John Hoch, as well as more recent conversations with Lois Kehl, Alice Larson, Gwen Wendelschafer-Crabb, Bill Kennedy, and George Wilcox.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finaly DR, Connolly MK, Pare M, Davar G, Rice FL. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EMJ, Peter AS, Hagen E, Fischer A, Klapp BF, Paus R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollikular inflammatory events via neuropeptide substance-P dependent pathways. Am. J. Path. 2003;162:803–814. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard E, Bosk A, Pao M. Understanding brain mechanisms of pain processing in adolescents’ non-suicidal self-injury. J. Youth Adol. 2010;39:327–334. doi: 10.1007/s10964-009-9457-1. [DOI] [PubMed] [Google Scholar]
- Bartak L, Rutter M. Differences between mentally retarded and normally intelligent autistic children. J of Aut Child Schizo. 1976;6:109–120. doi: 10.1007/BF01538054. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparison to mental retardation. J Aut Dev Dis. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Lewis MH. Self-injury and comorbid behavior in developmental, neurological, psychiatric, and genetic disorders. In: Schroeder S, Oster-Granite M, Thompsons T, editors. Self-injurious Behavior: Gene-Brain-Behavior Relationships. Washington, DC: American Psychological Association Press; 2002. [Google Scholar]
- Breese GR, Criswell HE, Duncan GE, Moy Ss, Johnson KB, Wong DR, Mueller RA. Model for reduced brain dopamine in Lesch-Nyhan Syndrome and the mentally retarded: Neurobiology of neonatal-6-hydroxydopamine lesioned rats. Mentl Retard Dev Dis Res Rev. 1995;1:111–119. [Google Scholar]
- Cabot PJ, et al. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100:142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EG. The motivation of self-injurious behavior: A review of some hypotheses. Psych Bul. 1977;84:800–816. [PubMed] [Google Scholar]
- Carr EG, Newsom CD, Binkoff JA. Stimulus control of self-destructive behavior in a psychotic child. J Abn Ch Psych. 1976;4:139–153. doi: 10.1007/BF00916518. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Coid J, Allolio B, Rees LH. Raised plasma metenkaphlin in patients who habitually mutilate themselves. Lancet. 1983:545–546. doi: 10.1016/s0140-6736(83)90572-x. [DOI] [PubMed] [Google Scholar]
- Cook EH. Serotonergic mechanisms in self-injurious behavior and related disorders; Paper presented at NICHD Conference on SIB; December 6–7; Rockville, MD. 1999. [Google Scholar]
- Craig KD, Prkachin KM, Grunau RVE. The facial expression of pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. NY: Guilford Press; 1992. pp. 257–276. [Google Scholar]
- Dan Y, Mooney RD. Sensory systems. Curr Op Neurobio. 2006;16:359–362. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Neuroscience. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin R, Pick CG, Peretz C, Carmeli E. A quantitative somatosensory testing of pain threshold in individuals with mental retardation. Pain. 2004;108:58–66. doi: 10.1016/j.pain.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Dines KC, Powell HC. Mast cell interactions with the nervous system: Relationship to mechanisms of disease. J. Neuropath. Exper. Neurol. 1997;56:627–640. [PubMed] [Google Scholar]
- Dou YC, Hagstromer L, Emtestam L, Johannson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006;296:31–37. doi: 10.1007/s00403-006-0657-1. [DOI] [PubMed] [Google Scholar]
- Drummond PD. The effects of cutaneous mast cell degranulation on sensitivity to heat. Inflam. Res. 2004;53:309–315. doi: 10.1007/s00011-004-1263-3. [DOI] [PubMed] [Google Scholar]
- Eckman P, Friesen WV. Investigator’s guide to the Facial Action Coding System. Palo Alto: Consulting Psychologists Press; 1978. [Google Scholar]
- Elliot E, Ginzburg I. The role of neurotrophins and insulin on tau pathology in Alzheimer's disease. Rev Neurosci. 2006;17:635–642. doi: 10.1515/revneuro.2006.17.6.635. [DOI] [PubMed] [Google Scholar]
- Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cells by peptides and basic secretagogues. Peptides. 2002;23:1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Fisher WW, Piazza C, Cataldo M, Harrell R, Jefferson G, Conner R. Functional communication training with and without extinction and punishment. J Appl Beh Analy. 1993;26:23–36. doi: 10.1901/jaba.1993.26-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RVE, Craig KD. Facial activity as a measure of neonatal pain expression. In: Tyler DC, Crane EJ, editors. Advances in pain research and therapy. Vol. 15. NY: Raven Press; 1990. pp. 147–156. [Google Scholar]
- Grunau RVE, Craig KD. Pain expression in neonates: Facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- Haines J, Williams CL, Brain KL, Wilson GV. The psychophysiology of self-mutilation. J Abnormal Psych. 1995;104:471–489. doi: 10.1037//0021-843x.104.3.471. [DOI] [PubMed] [Google Scholar]
- Hall SS, Arron K, Sloneem J, Oliver C. Health and sleep problems in Cornelia de Lange syndrome: A case control study. J Int Dis Res. 2008;52:458–468. doi: 10.1111/j.1365-2788.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- Hartman EC, Gilles E, McComas JJ, Danov SE, Symons FJ. Clinical observation of self-injurious behavior correlated with changes in scalp morphology in a child with congenital hydrocephalus. J Child Neur. 2008;9:1062–1065. doi: 10.1177/0883073808314155. [DOI] [PubMed] [Google Scholar]
- Herman BH, Hammock MK, Arthur-Smith A, Egan J, Chatoor I, Wernes A, Zelnik N. Naltrexone decreases self-injurious behavior. Ann Neuro. 1987;22:550–552. doi: 10.1002/ana.410220419. [DOI] [PubMed] [Google Scholar]
- Herpertz S. Self-injurious behavior: Psychopathological and nosological characteristics in subtypes of self-injurers. Acta Psychiatrica Scandinavica. 1995;91:57–68. doi: 10.1111/j.1600-0447.1995.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Iwata Ba, Dorsey MF, Slifer KJ, Bauman KE, Richman GS. Toward a functional analysis of self-injury. Analy Int Dev Dis. 1982;2:3–20. doi: 10.1901/jaba.1994.27-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata BA, Pace GM, Kissel RC, Nau PA, Farber JM. The self-injury trauma (SIT) scale: A method for quantifying surface tissue damage caused by self-injurious behavior. J Appl Beh Analy. 1990;23:99–110. doi: 10.1901/jaba.1990.23-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE, Huebner RR, Risser D, McGinnes GC, Dougherty LM. The young infant’s ability to produce discrete emotional expressions. Dev Psych. 1980;16:132–140. [Google Scholar]
- Kawana S, Liang Z, Nagano M, Suzuki H. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J. Dermatolog. Sci. 2006;42:47–54. doi: 10.1016/j.jdermsci.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wedelschafer-Crabb G, Johnson T. Quantification of epidermal nerves in diabetic neuropathy. Neuro. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur J. Pathology and quantitation of cutaneous nerves. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th ed. Philadelphia: Saunders; 2005. pp. 869–896. [Google Scholar]
- Kowalski J, Gabryel B, Labuzek K, Herman Z. Methionin-enkephalin and leucine-enkephalin increase interleukin-1beta release in mixed glia cultures. Neuropep. 2002;36:401–406. doi: 10.1016/s0143-4179(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Kowalski J, Makowiecka K, Belowski D, Herman ZS. Augmenting effect of methionine-enkephalin on interleukin-6 production by cytokine-stimulated murine macrophages. Neuropep. 2000;34:187–192. doi: 10.1054/npep.2000.0812. [DOI] [PubMed] [Google Scholar]
- LaChapelle DL, Hadjistavropoulos T, Craig KD. Pain measurement in persons with intellectual disabilities. Clin J Pain. 1999;15:13–23. doi: 10.1097/00002508-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin TM, et al. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Lovaas I, Newsom C, Hickman C. Self-stimulatory behavior and perceptual reinforcement. J Appl Beh Analy. 1987;20:45–68. doi: 10.1901/jaba.1987.20-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace FC, Lalli JS, Shea MC. Functional analysis of self-injury. In: Luiselli J, Matson J, Singh N, editors. Assessment, analysis, and treatment of self-injury. NY: Springer-Verlag; 1992. pp. 122–152. [Google Scholar]
- Melzack R. Folk medicine and the sensory stimulation of pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd. ed. New York: Churchill Livingstone; 1994. pp. 1209–1217. [Google Scholar]
- Melzack R, Wall PD. The Challenge of Pain. NY: Basic Books, Inc; 1983. [Google Scholar]
- Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Rutter M, Silva RP. Sex differences in anti-social behavior, conduct disorders, delinquency, and violence in the Dunedin longitudinal study. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Mousa SA, Shakibaei M, Sitte N, Schafer M, Stein C. Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinol. 2004;145:1331–1341. doi: 10.1210/en.2003-1287. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Hylden JL, Iadarola MJ, Dubner R. Peripheral inflammation is associated with increased dynorphin immunoreactivity in both projection and local circuit neurons in the superficial dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 1989;96:247–252. doi: 10.1016/0304-3940(89)90386-8. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Treatment of destructive behaviors in persons with developmental disabilities. 1991. (NIH Publication No. 91-2410) [PubMed] [Google Scholar]
- Newell KM, Challis JH, Boros RL, Bodfish JW. Further evidence on the dynamics of self-injurious behaviors: Impact forces and limb motions. Am J Ment Ret. 2002;107:60–68. doi: 10.1352/0895-8017(2002)107<0060:FEOTDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nock MK, Mendes WB. Physiological arousal, distress tolerance, and social problem-solving deficits among adolescent self-injurers. J Consult Clin Psych. 2008;76 doi: 10.1037/0022-006X.76.1.28. 28-28. [DOI] [PubMed] [Google Scholar]
- Novak M. Self-injurious behavior in rhesus monkeys: New insights into it etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Nyhan WL. Lessons from Lesch-Nyhan syndrome. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-injurious behavior: Gene-brain-behavior relationships. Washington, DC: American Psychological Association; 2002. pp. 251–267. [Google Scholar]
- Oberlander TF, Gilbert CA, Chambers CT, O’Donnell ME, Craig KD. Biobehavioral responses to acute pain in adolescents with a significant neurologic impairment. Clin J Pain. 1999;15:201–209. doi: 10.1097/00002508-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Symons FJ, van Dongen K, Abu-Saad HH. Pain in individuals with developmental disabilities: Challenges for the future. In: Dorstrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain: Progress in pain research and management. Seattle, WA: IASP Press; 2003. pp. 705–723. [Google Scholar]
- O'Reilly MF. Functional analysis of episodic self-injury correlated with recurrent otitis media. J Appl Beh Analy. 1997;30:165–167. doi: 10.1901/jaba.1997.30-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz EM. The modulation of sensory input and motor output in autistic children. In: Schopler E, Reichler RJ, editors. Psychopathology & child development. New York: Plenum Press; 1976. pp. 115–133. [Google Scholar]
- Paus R, Theoharides, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends in Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Piva M, Moreno JI, Jenkins FS, Smith JK, Thomas JL, Montgomery C, Wilcons CB, Sizemore RC. In vitro modulation of cytokine expression by enkephalin-derived peptides. Neuroimmunomod. 2005;12:339–347. doi: 10.1159/000091127. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophics, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Roach ES, Abramson JS, Lawless MR. Self-injurious behavior in acquired sensory neuropathy. Neuropediatrics. 1985;16:159–161. doi: 10.1055/s-2008-1059532. [DOI] [PubMed] [Google Scholar]
- Rojahn J, Schroeder SR, Hoch TA. Self-injurious behavior in intellectual disabilities. Oxford, UK: Elsevier Ltd; 2008. [Google Scholar]
- Russ MJ, Roth SD, Lerman A, Kakuma T, Harrison K, Shindledecker RD, Hull J, Mattis S. Pain perception in self-injurious patients with borderline personality disorder. Biol Psychiatry. 1992;32:501–511. doi: 10.1016/0006-3223(92)90218-o. [DOI] [PubMed] [Google Scholar]
- Sandman CA. Beta-endorphin dysregulation in autistic and self-injurious behavior: A neurodevelopmental hypothesis. Synapse. 1988;2:193–199. doi: 10.1002/syn.890020304. [DOI] [PubMed] [Google Scholar]
- Sandman CA. The opiate hypothesis in autism and self-injury. J Child Adoles Psychopharm. 1990/1991;1:237–248. [Google Scholar]
- Sandman CA, Spence MA, Smith M. Proopiomelanocortin (POMC) disregulation and response to opiate blockers. Ment Retard Dev Dis Res Rev. 1999;5:314–321. [Google Scholar]
- Sandman CA, Touchette PE, Marion SD, Chicz-DeMet A. The role of proopiomelanocortin (POMC) in sequentially dependent self-injurious behavior. Dev Psychobio. 2008;50:680–689. doi: 10.1002/dev.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SR, Oster-Granite ML, Berkson G, Bodfish JW, Breese GR, Cataldo MF, Cook EH, Crnic LS, DeLeon I, Fisher W, Harris JC, Horner RH, Iwata B, Jinnah HA, King BH, Lauder JM, Lewis MH, Kewell K, Nyhan WL, Rojahn J, Sackett GP, Sandman C, Symons FJ, Tessel RE, Thompson T, Wong DF. Self-injurious behavior: Gene-brain-behavior relationships. Ment Retard Dev Dis Res Rev. 2001;7:3–12. doi: 10.1002/1098-2779(200102)7:1<3::AID-MRDD1002>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via cotricotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain, Beh. Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Symons FJ. Pain and self-injury: Mechanisms and models. In: Schroeder S, Thompson T, Oster-Granite ML, editors. Self-injurious behavior: Genes, brain, and behavior. American Psychological Association; 2002. [Google Scholar]
- Symons FJ, Butler MG, Sanders MD, Feurer ID, Thompson T. Self-injurious behavior and Prader-Willi syndrome: Behavioral forms and body locations. Am J Ment Retard. 1999;104:260–269. doi: 10.1352/0895-8017(1999)104<0260:SBAPSB>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB. Self-injurious behavior in young boys with fragile X syndrome. Am J Med Gen. 2003a;118:115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Fox ND, Thompson T. Functional communication training and naltrexone treatment of self-injurious behavior: An experimental case report. J Appl Res Int Dis. 1998;11:273–292. [Google Scholar]
- Symons FJ, Harper VH, Breau L, McGrath PJ, Bodfish JW. Evidence of increased non-verbal behavioral signs of pain in adults with neurodevelopmental disorders and chronic self-injury. Res Dev Dis. 2009a;30:521–528. doi: 10.1016/j.ridd.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Sutton KA, Bodfish JW. A preliminary study of altered skin temperature at body sites associated with self-injurious behavior in adults with developmental disabilities. Am J Ment Retard. 2001;106:336–343. doi: 10.1352/0895-8017(2001)106<0336:PSOAST>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Sutton KA, Walker C, Bodfish JW. Altered diurnal pattern of salivary substance-P in adults with developmental disabilities and chronic self-injury. Am J Ment Retard. 2003b;108:13–18. doi: 10.1352/0895-8017(2003)108<0013:ADPOSS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Tapp J, Wulfsberg A, Sutton KA, Heeth WL, Bodfish JW. Sequential analysis of the effects of naltrexone on the environmental mediation of self-injurious behavior. Exp Clinic Psychopharm. 2001;9:269–276. doi: 10.1037//1064-1297.9.3.269. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Shinde S, Clary J, Harper V, Bodfish JW. A sham-controlled sensory testing protocol for nonverbal individuals with intellectual and developmental disabilities: Self-injury and gender effects. J of Pain. 2010;11:773–781. doi: 10.1016/j.jpain.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Thompson T. Self-injurious behavior and body site preference. J Int Dis Res. 1997;41:456–468. doi: 10.1111/j.1365-2788.1997.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Wendelschafer-Crabb G, Kennedy W, Hardrict R, Dahl N, Bodfish JW. Evidence of altered epidermal nerve fiber morphology in adults with self-injurious behavior and neurodevelopmental disorders. Pain. 2008;134:232–237. doi: 10.1016/j.pain.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Wendelschafer-Crabb G, Kennedy W, Heeth W, Bodfish JW. Degranulated mast cells in the skin of adults with self-injurious behavior and neurodevelopmental disorders. Brain, Beh, Imm. 2009b;23:365–370. doi: 10.1016/j.bbi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, Bienenstock J. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J. Immunol. 1999;163:2410–2415. [PubMed] [Google Scholar]
- Thompson T, Hackenberg T, Cerutti D, Baker D, Axtell S. Opioid antagonist effects on self-injury in adults with mental retardation: Response form and location as determinants of medication effects. Am J Ment Retard. 1994;99:85–102. [PubMed] [Google Scholar]
- Thompson T, Schroeder SR, editors. Ment Retard Dev Dis Res Rev. Vol. 1 1995. Self-injury in developmental disabilities: Neurobiological and environmental mechanisms. [Google Scholar]
- Vanderah TW, et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–7709. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Grisel JE, Silbert LH, Maier SF. Parallel activation of multiple spinal opiate systems appears to mediate 'non-opiate' stress-induced analgesias. Brain Res. 1992;594:99–108. doi: 10.1016/0006-8993(92)91033-b. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1101. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses, and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Wall PD, Melzack R, editors. Textbook of pain. 3rd. Ed. New York: Churchill Livingstone; 1994. [Google Scholar]
- Woolf CJ. The pathophysiology of peripheral neuropathic pain – abnormal peripheral input and abnormal central processing. Act Neurochir Suppl. (Wien) 1993;58:125–130. doi: 10.1007/978-3-7091-9297-9_29. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: Implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin scratching in mice. J Dermatol Sci. 2007;46:41–51. doi: 10.1016/j.jdermsci.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: A key role of mast cells. Pain. 2003;105:467–479. doi: 10.1016/S0304-3959(03)00261-6. [DOI] [PubMed] [Google Scholar]