Abstract
Context
Induction of protective anti-HIV immune responses is the goal of an HIV vaccine. However, this may cause a reactive result by routine HIV testing in the absence of HIV infection.
Objective
This study evaluated the frequency of vaccine-induced seropositivity/reactivity (VISP) in HIV vaccine trial participants.
Design, Setting, and Participants
A routine diagnostic HIV algorithm was used for participants who completed 25 phase 1 and two phase 2 vaccine trials from 2000–2010 conducted in the United States, South America, Thailand, and Africa.
Main Outcome Measures
Three common FDA-approved enzyme immunoassay (EIA) HIV antibody kits were used to determine VISP. VISP was defined as being reactive on ≥ 1 EIA test, HIV-1 negative by nucleic acid testing, and Western blot negative, indeterminate, or atypical positive (profile consistent with vaccine product).
Results
Among 2176 HIV uninfected participants who received a vaccine product, 908 (41.7%; 95% confidence interval [CI], 39.6–43.8%) had VISP, but the occurrence of VISP varied substantially across different HIV vaccine product types: 399 of 460 (86.7%; 95% CI, 83.3–89.7%) adenovirus 5 product recipients, 295 of 552 (53.4%; 95% CI, 49.2–57.7%) recipients of poxvirus alone or as a boost, and 35 of 555 (6.3%; 95% CI, 4.4–8.7%) of DNA alone product recipients developed VISP. Overall, the highest proportion of VISP (40.9%; 891 of 2176 tested) occurred with the HIV 1/2 (rDNA) EIA compared to the rLAV EIA (21.4%; 150 of 700 tested), HIV-1 Plus O Microelisa System (14.7%; 193 of 1309 tested), and HIV 1/2 Peptide and HIV 1/2 Plus O (8.8%; 189 of 2150 tested) kits. Only 17 (1.9%) of the 908 participants with VISP tested nonreactive using the HIV 1/2 (rDNA) kit. All recipients of a gp140 vaccine (n= 70) had VISP, with 94.3% tested reactive to all three EIA kits tested. Among 901 participants with VISP and a Western blot result, 92 (10.2%) had a positive Western blot (displaying an atypical pattern consistent with vaccine product) and 592 (65.7%) had an indeterminate result. Only 8 participants with VISP received a vaccine not containing an env insert.
Conclusions
The induction of VISP in HIV vaccine recipients is common especially with vaccines containing both the HIV-1 envelope and gag proteins. Development and detection of VISP appear to be associated with the immunogenicity of the vaccine and the EIA assay used.
Introduction
With an estimated 7500 incident HIV infections each day worldwide, there is an urgent need to develop an effective prophylactic HIV vaccine.1 Over the last 20 years many potential vaccine candidates have been developed and assessed in human clinical trials in over 30,000 participants. These candidate vaccines have utilized a variety of approaches, including protein,2–5 DNA,6, 7 HIV peptides,8–10 and viral vectored strategies.11–15 In addition, a variety of HIV targets or inserts have been studied, including gag, pol, nef and env in a variety of combinations.16 Several of these candidate vaccines have advanced to large field trials.3, 4, 17–19
HIV-1 vaccines have the potential of confounding interpretation of HIV tests due to the antibody induced by vaccination.20–22 Depending on the HIV associated sequences used in the candidate vaccine, not only may the screening enzyme immunoassay (EIA) be reactive but the Western blot may also be difficult to interpret.23 Participants in early phase clinical trials, who are typically at low risk of HIV infection, may encounter difficulties with obtaining medical or disability/life insurance, donating blood or organs (which is based on a reactive EIA regardless of any confirmatory Western blot or RNA assay result), employment, and immigration due to a false positive HIV test. In volunteers who participate in later stage efficacy trials, this becomes an even more complex issue, as these individuals are at higher risk for contracting HIV and the candidate vaccines are generally known to be immunogenic.3, 4, 17, 19, 24, 25 In the US, the adoption of an “opt-out” HIV testing strategy recommended by the CDC facilitates identification of those infected with HIV and thus allows earlier access to care.26 However, this approach also has the potential to increase confusion around false positive HIV testing due to vaccine induced antibodies. In this report we assess the occurrence of vaccine induced seropositivity/reactivity (VISP) associated with different vaccine delivery systems and HIV inserts studied by the National Institutes of Health-sponsored HIV Vaccine Trials Network (HVTN).
METHODS
Participants
Data were combined from all phase 1 (n=25) and 2a (n=2) HIV-1 vaccine trials that had completed study follow-up visits (except for one phase 1 vaccine manufacturer managed trial), conducted by HVTN clinical trial sites located in nine different countries (Botswana, Brazil, Haiti, Jamaica, Peru, South Africa, Thailand, Trinidad and Tobago) and the US between December 14, 2000 and January 15, 2010. Clinical trial locations and participant demographics are shown in Table 1. Except for two phase 1 open-label trials, the trials were placebo-controlled, multicenter, double-blind, randomized trials. Participants were healthy, HIV-seronegative adults, aged 18–60 years. At the end of each trial, volunteers were tested using the end of study (EOS) HIV testing algorithm described below and in Figure 1, which established whether the participant had VISP. Each trial was approved by the institutional review board/ethics committees of the participating institutions and participants provided written informed consent for study participation.
Table 1.
Baseline Characteristics of Non-HIV Infected Participants with End of Study HIV Test Results by Regiona
| Characteristics | US (n=1790) | Africa (n=176) |
South America/Caribbea n (n=200) |
Thailand (n=10) |
Total (n=2176) |
|
|---|---|---|---|---|---|---|
| No. of vaccine trials | 24 Phase I 2 Phase II |
3 Phase I 1 Phase II |
4 Phase I 1 Phase II |
1 Phase I | 25 Phase I 2 Phase II |
|
| Vaccine product category | ||||||
| DNA only | 491 (27.4) | 24 (13.6) | 30 (15.0) | 10 (100.0) | 555 (25.5) | |
| Peptide/protein alone or as a boostb | 284 (15.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 284 (13.1) | |
| Poxvirus alone or as a boostb | 463 (25.9) | 0 (0.0) | 89 (44.5) | 0 (0.0) | 552 (25.4) | |
| Adenovirus 5 alone or as a boostb | 330 (18.4) | 103 (58.5) | 27 (13.5) | 0 (0.0) | 460 (21.1) | |
| Adenovirus 35 | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (<0.1) | |
| Alphavirus | 55 (3.1) | 49 (27.8) | 0 (0.0) | 0 (0.0) | 104 (4.8) | |
| Canarypox + protein or peptide boost | 166 (9.3) | 0 (0.0) | 54 (27.0) | 0 (0.0) | 220 (10.1) | |
| Men | 1019 (56.9) | 86 (48.9) | 114 (57.0) | 7 (70.0) | 1226 (56.3) | |
| Age (in years) | ||||||
| 18–23 | 414 (23.1) | 64 (36.4) | 65 (32.5) | 0 (0.0) | 543 (25.0) | |
| 24–28 | 482 (26.9) | 68 (38.6) | 54 (27.0) | 4 (40.0) | 608 (27.9) | |
| 29–38 | 406 (22.7) | 30 (17.0) | 52 (26.0) | 4 (40.0) | 492 (22.6) | |
| 39–60 | 488 (27.3) | 14 (8.0) | 29 (14.5) | 2 (20.0) | 533 (24.5) | |
| Median (minimum, maximum) | 29 (18,60) | 25 (18, 49) | 27 (18, 58) | 33 (24, 42) | 29 (18, 60) | |
| Race/ethnicityc | ||||||
| White, not Hispanic | 1252 (69.9) | 1 (0.6) | 22 (11.0) | 0 (0.0) | 1275 (58.6) | |
| Black, not Hispanic | 319 (17.8) | 174 (98.9) | 73 (36.5) | 0 (0.0) | 566 (26.0) | |
| Hispanic | 106 (5.9) | 0 (0.0) | 65 (32.5) | 0 (0.0) | 171 (7.9) | |
| Other | 113 (6.3) | 1 (0.6) | 40 (20.0) | 10 (100.0) | 164 (7.5) | |
Abbreviation: US, United States
Data are presented as No. (%) unless otherwise indicated.
The products given as a boost were either to a product within the product category or as a boost to a DNA prime.
Race/ethnicity is based on participant self-report.
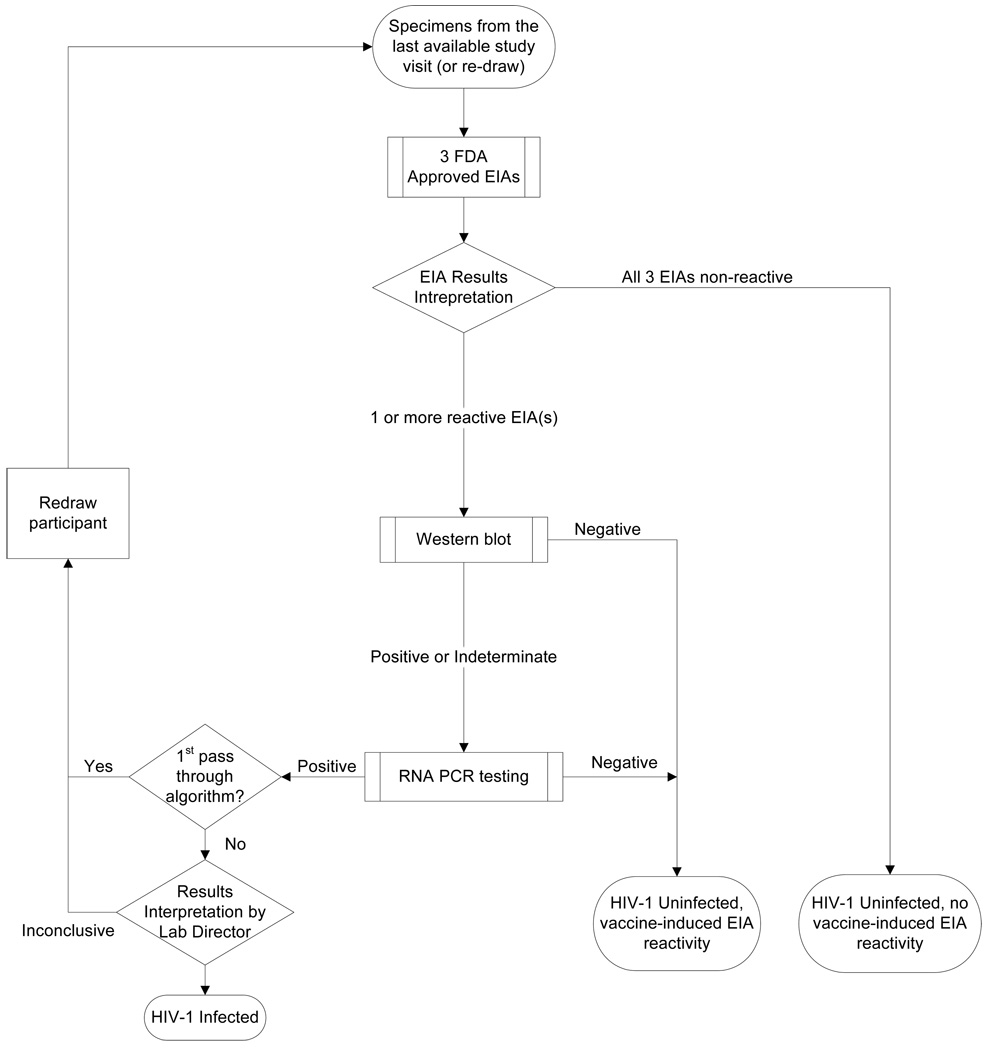
Figure 1.
Algorithm for HIV infection and VISP testing
Specimens from a participant’s last available study visit were tested for VISP using an algorithm that started with 3 different EIA tests. The algorithm proceeded directly to the Western blot if all three EIA tests were reactive. If 1 or 2 of the EIA tests were initially reactive then the EIAs were repeated in duplicate per manufacturer’s instructions to confirm reactivity before proceeding to the Western blot. Western blots were interpreted according to CDC criteria. Additionally, evaluation of the Western blot required consideration of whether the bands were consistent with a vaccine-induced response or an actual HIV infection. Specimens that were Western blot positive or indeterminate had quantitative RNA PCR testing. Additional HIV nucleic acid tests were performed to aid in a clear interpretation of infection status. If HIV infection was suspected, specimens from participant were re-drawn and the algorithm repeated until a clear interpretation was made.
The phase 1 trials typically involved dose escalation. Except for three phase 1 trials that administered vaccines by the subcutaneous route and one phase 1 trial that compared different routes of administration for boosting, vaccines were administered intramuscularly. Because of the potential for participant unblinding, HIV testing was typically performed at 6 month intervals during the trials by the HVTN Laboratory Program using an algorithm that was able to distinguish true infection from VISP. Participants were informed about the risk of developing VISP and potential negative consequences thereof before signing the consent document. This information was also discussed with participants at study visits. As required by NIH-sponsored trials, participants self-reported their ethnicity and race, using standardized categories (including “other”). Most studies were 12–18 months in length and end of study testing typically occurred 6–12 months after the last vaccination.
Vaccine constructs and study regimens
Table 2 lists the 25 different vaccine products tested in these trials, which were given alone or in combination as noted. The number of vaccinations ranged from 1 to 5. Products tested in the largest number of participants were the canarypox vector vaccine ALVAC HIV vCP1452 (Aventis Pasteur), the two DNA Vaccine Research Center (VRC)-HIVDNA009-00VP (4 plasmid) and VRC-HIVDNA016-00-VP (6 plasmid) vaccines, and the recombinant adenovirus serotype 5 (Ad5) vector vaccine VRC-HIVADV014-00-VP.
Table 2.
Product and Study Descriptions
| Vaccine Type and Product Description |
HIV Insert(s) | HVTN Protocol |
N | Product Notes |
|---|---|---|---|---|
| DNA only | ||||
| EP HIV-1090 (Epimmune) | gag, pol, vpr, nef, rev, env | 048, 064 | 54 | |
| EP-1233 (Pharmexa-Epimmune) | gag, pol, vpr, nef, rev, env | 067 | 2 | |
| pGA2/JS2 (GeoVax) | gag, pol, env, tat, vpu, rev | 045 | 24 | |
| pGA2/JS7 (GeoVax) | gag, pol, env, tat, rev, vpu | 065 | 5 | |
| VRC-HIVDNA009-00-VP (4 plasmid) (VRC) | gag, pol, nef, env | 044, 052, 068, 069 | 132 | |
| VRC-HIVDNA-016-00-VP (6 plasmid) (VRC) | gag, pol, nef, env | 204 | 22 | |
| VRC-HIVDNA044-00-VP (VRC) | env | 072 | 10 | |
| gag DNA (Wyeth) | gag | 060, 063 | 217 | |
| PENNVAX-B (University of Pennsylvania) | env, gag, pol | 070 | 89 | |
| Peptide or protein only or as boost | ||||
| LIPO-5 (Aventis-Pasteur) | gag, pol, nef | 042 | 23 | |
| Gp140 (Chiron) | env | 049 | 70 | Used alone or with Chiron DNA/PLG (gag, env, tat, vpu, rev) |
| EP-1043 (Epimmune) | pol, vpu, env, gag | 064 | 47 | Used alone or with EP HIV-1090 |
| NefTat (GSK) only | nef, tat | 041 | 10 | |
| NefTat (GSK) + gp120W61D (GSK) | nef, tat + env | 041 | 59 | Products administered together |
| HIV CTL MEP (Wyeth) | env, gag, nef | 056 | 75 | |
| Poxvirus only or as boost | ||||
| ALVAC HIV vCP1452 (Aventis Pasteur) | env, gag, pol, nef | 026, 039, 203, 042 | 305 | |
| rFPV (TBC-F357+TBC-F349) (Therion) only | env, gag, tat, rev, nef | 055 | 35 | |
| rMVA (TBC-M358+TBC-M335) (Therion) only | env, gag, tat, rev, nef | 055 | 40 | |
| rMVA (TBC-M358+TBC-M335)+rFPV (TBC-F357+TBC-F349) boost (Therion) | env, gag, tat, rev, nef | 055 | 50 | |
| MVA/HIV62 (GeoVax) | gag, pol, env | 065 | 94 | Used alone or with GeoVax DNA vaccine pGA2/JS7 (gag, pol, env, tat, rev, vpu, pol) |
| MVA-mBN32 (Pharmexa-Epimmune, Bavarian Nordic) | gag, pol, vpr, nef, rev, env | 067 | 28 | Used with Pharmexa-Epimmune DNA vaccine EP-1233 (gag, pol, vpr, nef, rev, env) |
| Adenovirus 5 only or as boost | ||||
| HIVADV038-00-VP (VRC) | env | 072 | 2 | Used in combination with VRC-HIVDNA 044-00-VP |
| VRC-HIVADV014-00-VP (VRC) | gag, pol, env | 054, 057, 068, 069, 204 | 423 | Used alone or in combination with VRC-HIVADVO14-00-VP |
| MRKAd5 HIV-1 gag/pol/nef (Merck) | gag, pol, nef | 071 | 35 | |
| Adenovirus 35 | ||||
| HIVADV027-00-VP (VRC) | env | 072 | 1 | |
| Alphavirus | ||||
| AVX101 (AlphaVax) | gag | 040, 059 | 104 | |
| Other combination regimens | ||||
| ALVAC HIV vCP1452 (Aventis Pasteur) + protein or peptide boost | env, gag, pol, nef | 026, 042, 203 | 220 | Used with HIV-1 MN rgp120 (VaxGen), LIPO-5 or AIDSVAX™ B/B(gp120) (VaxGen) |
Abbreviations: VRC, Vaccine Research Center; GSK, GlaxoSmithKline Biologicals
References and/or ClinicalTrial.gov number: HVTN 026, reference 15 and NCT00011037; HVTN 039, reference 12 and NCT00027261; HVTN 040, NCT00063778; HVTN 041, reference 5 and NCT00027365; HVTN 042, NCT00076063; HVTN 044, NCT00069030; HVTN 045, reference 7 and NCT00043511; HVTN 048, reference 10 and NCT00054860; HVTN 049, NCT00073216; HVTN 052, NCT00071851; HVTN 054, NCT00119873; HVTN 055, NCT00083603; HVTN 056, reference 8 and NCT00076037; HVTN 057, NCT00091416; HVTN 059, NCT00097838; HVTN 060, NCT00111605; HVTN 063, NCT00115960; HVTN 064, reference 9, NCT00141024; HVTN 065, NCT00301184; HVTN 067, NCT00428337; HVTN 068, NCT00270218; HVTN 069, NCT00384787; HVTN 070, NCT 00528489; HVTN 071, NCT00486408; HVTN 072, NCT00472719; HVTN 203, reference 11 and NCT00007332; HVTN 204, NCT00125970
Laboratory testing
The EOS HIV testing algorithm consisted of three US Food and Drug Administration (FDA)-licensed EIA tests administered equally in all trials (except for 182 participants on the earliest trials who had only an Abbott test [n=11] or an Abbott and one other EIA kit run [n=171]): 1) Abbott HIVAB HIV 1/2 (rDNA); 2) Bio-Rad Genetic Systems HIV 1/2 Peptide, which was replaced in March 2006 with the next generation Bio-Rad Genetic Systems HIV 1/2 Plus O EIA; and 3) bioMerieux Vironostika HIV-1 Plus O Microelisa System, which due to product discontinuation was replaced in October 2007 with the Bio-Rad Genetic Systems rLAV. Kits were used according to manufacturer’s instructions. Participant specimens testing repeatedly reactive on any one or more of the three different EIA kits were further tested by Western blot. For early trials, a validated Western blot kit developed by the testing lab from commercially available antigens was used (California Department of Health Services Viral and Rickettsial Disease Laboratory, Richmond, CA), which was replaced in June 2005 with the Bio-Rad Genetic Systems HIV-1 Western Blot kit. Blots were interpreted according to CDC criteria and the manufacturer’s product insert, requiring the presence of any 2 of the bands gp41, gp120/160, or p24 for positivity.27 Specimens testing positive or indeterminate had quantitative RNA PCR performed to rule out true infection. During the time span of the study, the quantitative RNA PCR kits included Amplicor HIV-1 Monitor (Roche Diagnostics), Abbott Realtime HIV-1 (Abbott Molecular), and a validated real-time HIV-1 RNA assay developed at the Department of Laboratory Medicine, University of Washington (Seattle, Washington). Anyone with a positive RNA PCR had a repeat sample drawn to confirm true infection.
Statistical analysis
To summarize the risk of developing VISP for these diverse HIV-1 vaccine trials, vaccine regimens were grouped into general strategy (product) categories based on vaccine insert (DNA, peptide or protein), viral vector (poxvirus, adenovirus 5/35, or alphavirus), or canarypox prime with peptide or gp120 boost. These general categories were further subdivided when VISP rates within a category substantially varied by product type. Participants who did not complete their vaccine regimen were included in the analysis based on the products they received. Since the specific HIV-1 gene inserts effect seropositivity/reactivity, we also present data by HIV-1 gene insert groupings.
For all categories, the VISP rate and exact 95% binomial confidence intervals (CIs) were calculated. The VISP rate was calculated as the number of participants testing reactive on one of the three different EIA kits tested divided by the number of participants tested. Because the HIV 1/2 (rDNA) kit nearly always detected VISP, we also included in the rate calculations those participants who had been tested with this kit but were missing data from one or both of the other kits (n=182). Additionally, positivity rates for the different EIA kits are provided based on all participants with a result for the kit. Gender, age (below vs above the median), and racial (white vs black) differences were evaluated with Fisher exact tests. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Carey, North Carolina). P values <0.05 were considered statistically significant. No adjustment for multiple testing was performed.
RESULTS
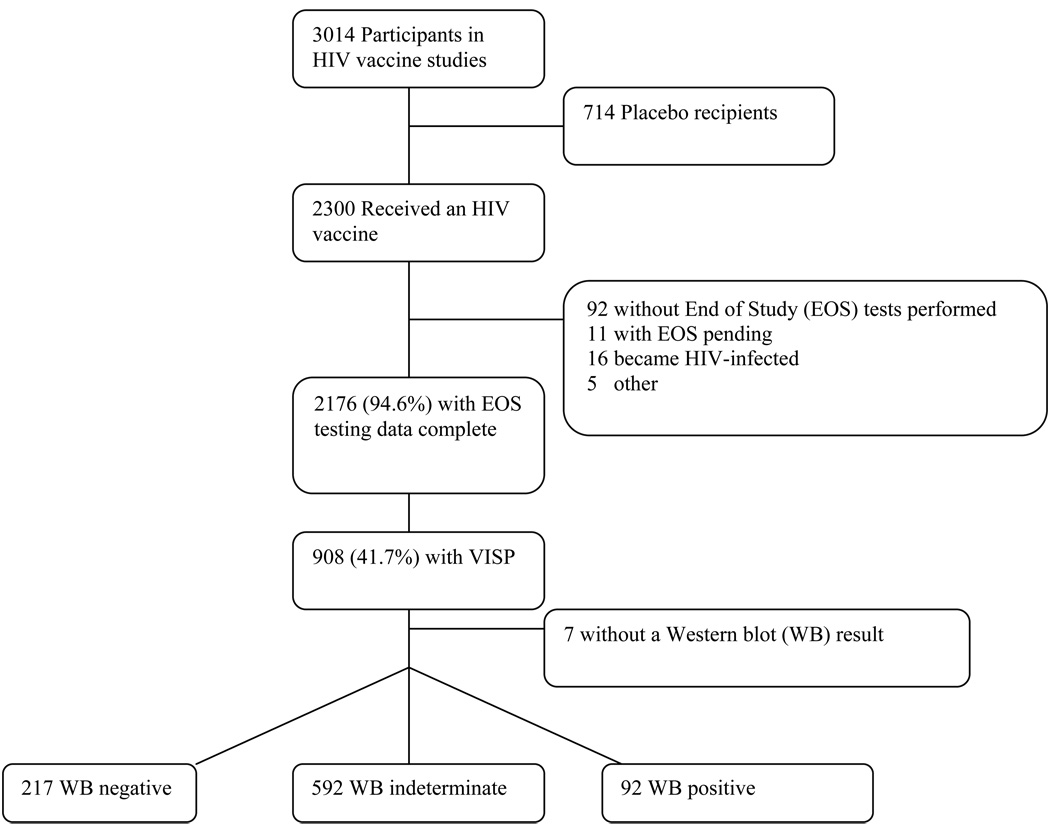
A total of 3014 participants were enrolled in the trials, of whom 2300 (76.3%) were vaccinees (and 714 [23.7%] placebo recipients), with 2176 (94.6%) of vaccinees having EOS testing data available (11 [0.5%] had EOS testing pending and 92 [4.0%] did not have EOS testing done, the majority due to loss to follow-up), with 16 excluded from the analysis due to becoming HIV-1 infected and 5 for vaccine administration issues (eg, wrong product administered, product viability uncertain) (Figure 2).
Figure 2.
Flow of Subjects
There were 2176 non-HIV infected vaccine recipients with HIV test results available from the EOS testing algorithm at the time of data analysis. Although the algorithm specified that three different EIA kits be run, 182 (8.4%) participants from the earliest trials were missing kit results other than HIV 1/2 (rDNA) results from one (n=171) or two (n=11) kits.
The majority of participants (82%) in the analysis were from the US (Table 1). Among US participants, 57% were men, 70% were white, and the median age was 29 years. African participants had a slightly younger median age of 25, and 49% were male. Participants from South America and the Caribbean were drawn from ethnic/racial groups representative of their respective countries and had a median age of 27, with 57% being male.
VISP occurred in 908 of the 2176 participants (41.7%; 95% confidence interval [CI], 39.6%–43.8%, Table 3). VISP rates varied greatly by type of HIV vaccine administered. For viral vectored vaccines, alphavirus replicon products containing the gag antigen alone were found to have the lowest VISP (1.0%; [95% CI, 0.0%–5.2%]). In contrast, Ad5 products containing envelope antigens given alone or as a boost to a DNA prime were found to have the highest VISP (92.7%; [95% CI, 89.8%–95.0%]). Within the DNA only product category VISP occurred in 18.9% (95% CI, 13.2%–25.7%) of participants receiving a VRC DNA product, whereas it occurred in only 1% (95% CI, 0.3%–2.6%) of participants receiving other DNA vaccines. For poxvirus products given alone or as a boost to a DNA or poxvirus prime, 53.4% (95% CI, 49.2%–57.7%) of recipients had VISP. This was similar to the 48.6% (95% CI, 41.9%–55.4%) occurrence for canarypox given in combination with a protein or peptide product. All 70 recipients of a gp140 vaccine had VISP, whereas recipients of other protein and peptide products given alone or as a DNA boost had a VISP rate of 0.5% (95% CI, 0.0%–2.6%).
Table 3.
Vaccine Induced Seropositivity/Reactivity Rates by Type of HIV-1 Vaccine and EIA Kit
| Overalla | EIA Kit Ratesb | |||||
|---|---|---|---|---|---|---|
| Product Category | Rate | Exact 95% CI | HIV 1/2 (rDNA) |
HIV 1/2 Peptide, HIV 1/2 Plus Oc |
HIV-1 Plus O Microelisa Systemd |
rLAV |
| All products combinede | 908/2176 = 41.7% | (39.6%,43.8%) | 891/2176 = 40.9% | 189/2150 = 8.8% | 193/1309 = 14.7% | 150/700 = 21.4% |
| DNA only | 35/555 = 6.3% | (4.4%,8.7%) | 33/555 = 5.9% | 1/555 = 0.2% | 3/251 = 1.2% | 0/304 = 0.0% |
| Non-VRC DNA VTN | 4/391 = 1.0% | (0.3%,2.6%) | 3/391 = 0.8% | 1/391 = 0.3% | 0/141 = 0.0% | 0/250 = 0.0% |
| VRC DNA | 31/164 = 18.9% | (13.2%,25.7%) | 30/164 = 18.3% | 0/164 = 0.0% | 3/110 = 2.7% | 0/ 54 = 0.0% |
| Peptide or Protein only or as boostf | 71/284 = 25.0% | (20.1%,30.5%) | 70/284 = 24.6% | 70/284 = 24.6% | 67/198 = 33.8% | 0/17 = 0.0% |
| gp140 only | 70/70 =100.0% | (94.9%,100.0%) | 70/70 =100.0% | 70/70 =100.0% | 66/ 70 = 94.3% | not used |
| Non-gp140 products | 1/214 = 0.5% | (0.0%,2.6%) | 0/214 = 0.0% | 0/214 = 0.0% | 1/128 = 0.8% | 0/17 = 0.0% |
| Poxvirus only or as boostf | 295/552 = 53.4% | (49.2%,57.7%) | 288/552 = 52.2% | 78/538 = 14.5% | 20/310 = 6.5% | 38/149 = 25.5% |
| Fowlpox only | 2/35 = 5.7% | (0.7%,19.2%) | 2/35 = 5.7% | 0/35 = 0.0% | 0/22 = 0.0% | 0/13 = 0.0% |
| Canarypox only | 151/305 = 49.5% | (43.8%,55.3%) | 150/305 = 49.2% | 3/291 = 1.0% | 7/212 = 3.3% | not used |
| MVA +/− fowlpox boost | 89/119 = 74.8% | (66.0%,82.3%) | 85/119 = 71.4% | 49/119 = 41.2% | 5/64 = 7.8% | 16/55 = 29.1% |
| MVA boost to DNA prime (HVTN 065) | 53/65 = 81.5% | (70.0%,90.1%) | 51/65 = 78.5% | 26/65 = 40.0% | 8/12 = 66.7% | 22/53 = 41.5% |
| MVA boost to DNA prime (HVTN 067) | 0/28 = 0.0% | (0.0%,12.3%) | 0/28 = 0.0% | 0/28 = 0.0% | not used | 0/28 = 0.0% |
| Adenovirus 5 only or as boostf, | 399/460 = 86.7% | (83.3%,89.7%) | 395/460 = 85.9% | 33/460 = 7.2% | 102/231 = 44.2% | 112/229 = 48.9% |
| Ad5 only (containing env antigens) | 63/71 = 88.7% | (79.0%,95.0%) | 63/71 = 88.7% | 13/71 = 18.3% | 27/69 = 39.1% | 0/2 = 0.0% |
| Ad5 as boost to DNA prime (containing env antigens) | 331/354 = 93.5% | (90.4%,95.8%) | 327/354 = 92.4% | 20/354 = 5.6% | 75/162 = 46.3% | 112/192 = 58.3% |
| Ad5 only (not containing env antigens) | 5/35 = 14.3% | (4.8%,30.3%) | 5/35 = 14.3% | 0/35 = 0.0% | not used | 0/35 = 0.0% |
| Alphavirus only | 1/104 = 1.0% | (0.0%,5.2%) | 0/104 = 0.0% | 1/104 = 1.0% | 0/104 = 0.0% | not used |
| Other combination regimens | ||||||
| Canarypox + protein or peptide boost | 107/220 = 48.6% | (41.9%,55.4%) | 105/220 = 47.7% | 6/208 = 2.9% | 1/215 = 0.5% | not used |
Abbreviation: EIA, enzyme immunoassay; VRC, Vaccine Research Center; MVA, modified vaccinia Ankara; Ad5, adenovirus 5
The overall rate is determined by reactivity on 1 or more EIA kits.
The reactivity to specific EIA kits was calculated by the number of participants with specimens reactive to a given kit divided by the total number of participants whose specimens were tested using the kit.
26 participants are missing results from these kits, 14 for canarypox alone products and 12 for canarypox + protein or peptide boost regimens.
167 participants are missing results for the Vironostika kit; 69 for the non-gp140 peptide/protein products, 93 for canarypox only products, and 5 for canarypox + protein or peptide boost regimens.
The “all products combined” category includes 1 participant who received HIVADV027-00-VP (rAd35). This person is not included in a specific product category.
The products given as a boost were either to a product within the product category or as a boost to a DNA prime.
The HIV 1/2 (rDNA) kit was selected for inclusion in the EOS testing algorithm since it is a commonly used diagnostic kit in the US and has been noted to be particularly sensitive to detecting vaccine induced HIV antibodies. Only 17 (1.9%) of 908 participants with VISP did not test reactive using the HIV 1/2 (rDNA) kit (Table 3). Within product categories VISP occurred more frequently with the HIV 1/2 (rDNA) kit than the other kits (except for alphavirus replicon category, wherein a single participant was found to have VISP detected with only the HIV 1/2 Peptide kit. The gp140 product was unique in that 94.3% of recipients were reactive on all three kits. The other kits varied on their rates of reactivity, especially for viral-vectored vaccines (Table 3). Although kit data are summarized for all participants with a kit result, data are similar when limiting comparisons to participants tested with the same three kits.
Almost all of VISP was associated with vaccines that contain env, either alone or in combination with other HIV-1 antigens (Table 4). Only 8 participants with VISP received a vaccine not containing env: 5 received Ad5 (gag, pol, nef inserts) and tested reactive using the HIV 1/2 (rDNA) kit; 2 received gag DNA and tested reactive using the HIV 1/2 (rDNA) kit; and 1 received alphavirus replicon (gag insert) and tested reactive using a HIV 1/2 Peptide kit.
Table 4.
Vaccine Induced Seropositivity/Reactivity Rates and Western Blot Resultsa by Vaccine Insert HIV-1 Genesb
| Western Blot Resultc | ||||||
|---|---|---|---|---|---|---|
| Positive | Indeterminate | Negative | ||||
| Gene Insert |
Other HIV-1 antigens included |
VISP Rate (95% CI) |
No. with VISP and WB Data |
No. (%) | No. (%) | No. (%) |
| Env | None or other antigens | 900/1787 = 50.4% (48.0%, 52.7%) | 893 | 92 (10.3) | 586 (65.6) | 215 (24.1) |
| None | 29/42 = 69.0% (52.9%, 82.4%) | 29 | 1 (3.4) | 24 (82.8) | 4 (13.8) | |
| Gag | None or other antigens | 879/2065 = 42.6% (40.4%, 44.7%) | 872 | 91 (10.4) | 568 (65.1) | 213 (24.4) |
| Other antigens, but no env | 5/58 = 8.6% (2.9%, 19.0%) | 5 | 0 (0.0) | 5 (100.0) | 0 (0.0) | |
| None | 3/321 = 0.9% (0.2%, 2.7%) | 3 | 0 (0.0) | 1 (33.3) | 2 (66.7) | |
Abbreviation: WB, Western blot; VISP, vaccine induced seropositivity/reactivity
A Western blot was run if VISP was detected. WB data are missing for 7 participants with VISP.
Vaccines may include only part of the indicated gene.
Within each gene insert category, the percentage for the specific WB result is the number with the WB result divided by the number in the category developing VISP and having a WB result.
Within the vaccine sub-product categories, no gender or age differences in VISP were observed within geographical regions or for regions combined. One racial difference was observed for US participants receiving VRC Ad5 alone (93.2% white vs 50.0% black; P = .01; no participants in Africa received this product), although this is based on only 6 black individuals and was not observed for VRC DNA prime and Ad5 boost. This analysis is not adjusted for multiple testing.
For those testing reactive on one or more EIA kits, all except 7 had a Western blot result (Table 5, Figure 2). Overall, 10.2% (n=92) had a positive Western blot, 65.7% (n=592) tested indeterminate, and 24.1% (n=217) tested negative; however, the distribution of results varied by product. For DNA and fowlpox vaccines, ≥ 50.0% had negative Western blots. The env-containing Ad5 vaccine given alone or as a DNA vaccine boost had the highest proportion of positive results (25.4% and 13.6%, respectively). Poxvirus regimens had higher percentages of indeterminate results (50.0%–97.3%) and positive results <10%.
Table 5.
Western Blot Results for Participants with Vaccine Induced Seropositivity/Reactivity by Vaccine Product Category
| Western Blot Resulta | ||||||
|---|---|---|---|---|---|---|
| Positive | Indeterminate | Negative | ||||
| Product Category | No. with VISP |
No. with WB resultsb |
No. (%) | No. (%) | No. (%) | |
| All products combined | 908 | 901 | 92 (10.2) | 592 (65.7) | 217 (24.1) | |
| DNA only | 35 | 34 | 1 (2.9) | 11 (32.4) | 22 (64.7) | |
| Non-VRC DNA | 4 | 3 | 0 (0.0) | 0 (0.0) | 3 (100.0) | |
| VRC DNA | 31 | 31 | 1 (3.2) | 11 (35.5) | 19 (61.3) | |
| Peptide or Protein only or as boostc | 71 | 71 | 6 (8.4) | 57 (80.2) | 8 (11.3) | |
| gp140 only | 70 | 70 | 6 (8.6) | 56 (80.0) | 8 (11.4) | |
| Non-gp140 products | 1 | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| Poxvirus only or as boostc | 295 | 292 | 14 (4.8) | 234 (80.1) | 44 (15.1) | |
| Fowlpox only | 2 | 2 | 0 (0.0) | 1 (50.0) | 1 (50.0) | |
| Canarypox only | 151 | 150 | 1 (0.7) | 146 (97.3) | 3 (2.0) | |
| MVA+/− fowlpox boost | 89 | 88 | 8 (9.1) | 56 (63.6) | 24 (27.3) | |
| MVA boost to DNA prime (HVTN 065) | 53 | 52 | 5 (9.6) | 31 (59.6) | 16 (30.8) | |
| Adenovirus 5 only or as boostc | 399 | 398 | 61 (15.3) | 195 (49.0) | 142 (35.7) | |
| Ad5 only (containing env antigens) | 63 | 63 | 16 (25.4) | 22 (34.9) | 25 (39.7) | |
| Ad5 as boost to DNA prime (containing env antigens) | 331 | 330 | 45 (13.6) | 168 (50.9) | 117 (35.5) | |
| Ad5 only (not containing env antigens) | 5 | 5 | 0 (0.0) | 5 (100.0) | 0 (0.0) | |
| Alphavirus | 1 | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| Canarypox + protein or peptide boost | 107 | 105 | 10 (9.5) | 94 (89.5) | 1 (1.0) | |
Abbreviations: VISP, vaccine induced seropositivity/reactivity; WB, Western blot; VRC, Vaccine Research Center; MVA, modified vaccinia Ankara; Ad5, adenovirus 5
Within each product category, the percentage for the specific WB result is the number with the WB result divided by the number in the category developing VISP and having a WB result.
7 participants with VISP are missing WB results.
The products given as a boost were either to a product within the product category or as a boost to a DNA prime.
For those with a positive Western blot result, all but 1 had a positive gp160 band (11 had missing data). For gag bands, all but 1 was positive for p24, and 44 (53.7%) were positive for p55 (30 received an Ad5 product and 14 a poxvirus vectored vaccine; 10 had missing data). For pol bands, p51 was positive for only 6 participants (4 received an Ad5 vaccine and 2 received poxvirus; 10 had missing data) and no one had a positive p31 band (11 had missing data). For those with an indeterminate Western blot, p24 was positive for 91.1% who received a poxvirus vectored vaccine regimen, and 17.8% who received other vaccines (3 had missing data). Data on other bands were not consistently available for participants with an indeterminate Western blot who received a poxvirus vectored vaccine. For other products, only the gp160 band had a substantial number of positives, particularly for a gp140 vaccine, 83.9% of which were positive.
COMMENT
These data demonstrate that VISP is a common but highly variable outcome of preventive HIV vaccine trials. The variability of the occurrence of VISP is dependent on the immunogenicity of the vaccine product, the HIV gene inserts, and the HIV testing kit used. This analysis did not specifically examine the vaccine dose, number of doses, administration schedule, and use of adjuvants as contributing factors to the development of VISP as it would be difficult to assign relative importance to these factors in this cross-study analysis. By product category, rates ranged from 1% for an alphavirus replicon construct containing only a gag insert up to 100% for a gp140 vaccine. VISP occurred most frequently with the HIV 1/2 (rDNA) kit (98% of those with VISP tested positive by this kit). An awareness of the potential occurrence of VISP allows for appropriate counseling and education to be provided to study subjects.
Among participants with a reactive EIA, Western blot distinguished only 24% as HIV negative, with most (66%) having an indeterminate Western blot result with positive bands that were consistent with the genes included in the vaccine insert. A limitation of the HVTN data are that testing for VISP with multiple EIA kits is performed only at the end of the study so that it is not possible to distinguish effects of multiple inoculations with a given vaccine or the contribution of a single product when given as series of inoculations in combination with other products. Given the likely specificity for an insert (especially env based inserts) to cross react with a given detection system (EIA assay) it is difficult to predict the potential rate of VISP associated with future vaccines as delivery systems, inserts, and potentially EIA detection assays are modified.
This analysis significantly expands on previous studies of VISP, which were limited in scope to a relatively small number of candidate vaccines.20, 23, 28 In contrast, our analysis includes data on 25 vaccine products given alone or in combination. The most extensive previous assessment of VISP focused on gp120, vaccinia constructs, and ALVAC canarypox HIV-1 vaccines with or without a protein boost. In the analysis by Ackers et al, none of 118 samples from studies of gp120 constructs were reactive with the HIV 1/2 (rDNA) or HIV-1 Plus O Microelisa System kits and 13 (11%) were reactive using the rLAV EIA kit, which was not used for the HVTN protein/peptide trials.28 The results with the HIV 1/2 (rDNA) and HIV-1 Plus O Microelisa System kits were similar to our results of 1 positive among 214 subjects receiving a non-gp140 protein or peptide constructs. For trials of various generations of canarypox-vectored vaccines boosted with gp120, Ackers et al observed low proportions of VISP detected by the HIV-1 Plus O Microelisa System kit, which was confirmed in our analysis. For these regimens, they observed VISP proportions of 3% to 31%, depending on the trial, using the rLAV EIA kit (not used in our analysis for these regimens). They did not use the HIV 1/2 (rDNA) kit, whereas for this kit we observed that 48% of those receiving a canarypox vaccine given with a protein or peptide boost developed VISP.
The occurrence of VISP is dependent on the immune response to the vaccine product and the sensitivity of the kit to detect reactivity to the HIV antigens it contains. In these HVTN trials, VISP was rare for vaccines not containing an env insert. This is in contrast to a 41% rate observed among participants in some trials who received either an Ad5-vectored clade B HIV-1 monovalent gag or trivalent gag/pol/nef vaccine.23 Although the difference may in part be due to the immunogenicity of different types of vaccines (HVTN non-env studies were primarily of alphavirus replicon or DNA vaccines), the difference may also be due to the EIA kits used. To increase the likelihood of detecting VISP, the HVTN EOS testing algorithm uses commercial HIV testing kits that each have a different set of HIV antigens. An example of the importance of the match (or mis-match) of vaccine insert to kit antigens is that in a study by Quirk et al, 2 of 432 non-env containing Ad5 recipients were reactive to env-only containing rapid EIA kits.23 Development of a rapid, accurate HIV test which is unlikely to cross react with vaccine construct would be a useful approach for improved serologic tests. One promising approach utilizes sequences which are universally found in natural infections but if omitted from the vaccine insert would reliably differentiate vaccination from natural infection by serological testing.29 Until such an approach is available we recommend the inclusion of an HIV RNA assay to differentiate vaccination from infection (see Figure 1). As participants with VISP may subsequently become infected with HIV, it is imperative that appropriate follow-up testing be conducted, including HIV RNA testing, to minimize potential misinterpretation of HIV test results.
HVTN trial data are limited in that testing for VISP only occurs at the end of the trial (typically 6–12 months following the final vaccination). Although participants are offered long-term HIV testing services through the HVTN if they have VISP, the HIV testing data from longer time points has not been systematically collected to date. Through requests for HIV testing services, the HVTN is aware that some participants continue to have VISP for many years (sometimes greater than 15 years) following their study participation. An observational study of VISP post-trial termination is currently in development in the HVTN.
Testing for VISP at the end of the study and providing participants with their VISP status is critically important to prevent social harms, incorrect HIV diagnosis, and inaccurate reporting to health agencies. A misinterpretation of VISP can be minimized by clinicians obtaining a complete patient history (eg, participation in an HIV vaccine trial) and interpretation of the Western blot and HIV RNA. However, given the added time and cost associated with obtaining this information, clinicians may overlook or not pursue this information. During the course of HIV vaccine study participation, the detection of VISP might influence a study participant’s perception of the vaccine product received and may influence their behavior.30 To date, social harms related to HIV testing outside the context of the study sites have been relatively rare. However, with the recommendations by the CDC for routine HIV screening and “opt out” HIV testing practices, the education of participants and health care providers will be important to keep social harms related to HIV testing low for HIV vaccine trial participants.
Acknowledgments
Funding/Support: This work was funded by NIAID grants U01 AI046747, AI068614, AI46725, AI068618, AI046703, AI068635, AI069412, and the University of Washington Center for AIDS Research (CFAR) AI27757.
Role of the Sponsor: The funder had no role in the design, conduct or analysis of these data.
Footnotes
Author Contributions: Drs Cooper, Metch, and Baden all had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosures: No potential conflicts of interest are reported.
NIAID HIV Vaccine Trials Network (HVTN) Vaccine-Induced Seropositivity (VISP) Task Force:
Mary Allen, Sarah Alexander, Constance Ducar, Alan Fix, Renee Holt, Shelly Karuna, Genevieve Meyer, Miko Robertson, Kyle Rybczyk, Richard Shikiar, Steve Wakefield, Margaret Wecker.
Additional Contributions: We would like to thank Haynes “Chip” Sheppard, PhD, at the California Department of Health Services Viral and Rickettsial Disease Laboratory for the pre-2006 HIV testing for the End of Study algorithms and HIV testing data. Dr Sheppard was previously under contract with the NIAID HIV Vaccine Trials Network.
Previous Presentation: Presented in part at AIDS Vaccine 2008; October 14, 2008; Cape Town, South Africa.
REFERENCES
- 1.Global Summary of the AIDS epidemic. [Accessed March 15, 2010];2007 December; Available at < http://www.who.int/hiv/data/en/index.html.
- 2.Pialoux G, Hocini H, Perusat S, et al. Phase I study of a candidate vaccine based on recombinant HIV-1 gp160 (MN/LAI) administered by the mucosal route to HIV-seronegative volunteers: the ANRS VAC14 study. Vaccine. 2008;26:2657–2666. doi: 10.1016/j.vaccine.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Pitisuttithum P, Nitayaphan S, Thongcharoen P, et al. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. The Journal of infectious diseases. 2003;188:219–227. doi: 10.1086/376506. [DOI] [PubMed] [Google Scholar]
- 4.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 5.Goepfert PA, Tomaras GD, Horton H, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25:510–518. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Graham BS, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. The Journal of infectious diseases. 2006;194:1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan MJ, Russell ND, Celum C, et al. Excellent safety and tolerability of the human immunodeficiency virus type 1 pGA2/JS2 plasmid DNA priming vector vaccine in HIV type 1 uninfected adults. AIDS research and human retroviruses. 2006;22:678–683. doi: 10.1089/aid.2006.22.678. [DOI] [PubMed] [Google Scholar]
- 8.Spearman P, Kalams S, Elizaga M, et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine. 2009;27:243–249. doi: 10.1016/j.vaccine.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X, Newman MJ, De-Rosa S, et al. A novel HIV T helper epitope-based vaccine elicits cytokine-secreting HIV-specific CD4+ T cells in a Phase I clinical trial in HIV-uninfected adults. Vaccine. 2009;27:7080–7086. doi: 10.1016/j.vaccine.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorse GJ, Baden LR, Wecker M, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Russell ND, Graham BS, Keefer MC, et al. Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: negative results fail to trigger a phase 3 correlates trial. Journal of acquired immune deficiency syndromes (1999) 2007;44:203–212. doi: 10.1097/01.qai.0000248356.48501.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goepfert PA, Horton H, McElrath MJ, et al. High-dose recombinant Canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. The Journal of infectious diseases. 2005;192:1249–1259. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 13.McCormack S, Stohr W, Barber T, et al. EV02: a Phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine. 2008;26:3162–3174. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 14.Priddy FH, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 15.Cleghorn F, Pape JW, Schechter M, et al. Lessons from a multisite international trial in the Caribbean and South America of an HIV-1 Canarypox vaccine (ALVAC-HIV vCP1452) with or without boosting with MN rgp120. Journal of acquired immune deficiency syndromes (1999) 2007;46:222–230. doi: 10.1097/QAI.0b013e318149297d. [DOI] [PubMed] [Google Scholar]
- 16.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 19.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DH, Mazumdar A, Winston S, Harkonen S. Utility of various commercially available human immunodeficiency virus (HIV) antibody diagnostic kits for use in conjunction with efficacy trials of HIV-1 vaccines. Clinical and diagnostic laboratory immunology. 1995;2:268–271. doi: 10.1128/cdli.2.3.268-271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silbermann B, Tod M, Desaint C, et al. Short communication: Long-term persistence of vaccine-induced HIV seropositivity among healthy volunteers. AIDS research and human retroviruses. 2008;24:1445–1448. doi: 10.1089/aid.2008.0107. [DOI] [PubMed] [Google Scholar]
- 22.Simonsen L, Buffington J, Shapiro CN, et al. Multiple false reactions in viral antibody screening assays after influenza vaccination. American journal of epidemiology. 1995;141:1089–1096. doi: 10.1093/oxfordjournals.aje.a117374. [DOI] [PubMed] [Google Scholar]
- 23.Quirk EK, Mogg R, Brown DD, et al. HIV seroconversion without infection after receipt of adenovirus-vectored HIV type 1 vaccine. Clin Infect Dis. 2008;47:1593–1599. doi: 10.1086/593313. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert PB, Peterson ML, Follmann D, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. The Journal of infectious diseases. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 25.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. Jama. 2008;300:945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 27.CDC. Interpretation and use of the Western blot assay for sero-diagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep. 1989;38 suppl 7:1–7. [Google Scholar]
- 28.Ackers ML, Parekh B, Evans TG, et al. Human immunodeficiency virus (HIV) seropositivity among uninfected HIV vaccine recipients. The Journal of infectious diseases. 2003;187:879–886. doi: 10.1086/368169. [DOI] [PubMed] [Google Scholar]
- 29.Khurana S, Needham J, Park S, et al. Novel approach for differential diagnosis of HIV infections in the face of vaccine-generated antibodies: utility for detection of diverse HIV-1 subtypes. Journal of acquired immune deficiency syndromes (1999) 2006;43:304–312. doi: 10.1097/01.qai.0000242465.50947.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gust DA, Wiegand RE, Para M, Chen RT, Bartholow BN. HIV testing outside of the study among men who have sex with men participating in an HIV vaccine efficacy trial. Journal of acquired immune deficiency syndromes (1999) 2009;52:294–298. doi: 10.1097/QAI.0b013e3181ab6e93. [DOI] [PubMed] [Google Scholar]