Abstract
Objectives
Carbon monoxide (CO) can confer anti-inflammatory protection in rodent models of ventilator-induced lung injury (VILI). Caveolin-1 exerts a critical role in cellular responses to mechanical stress, and has been shown to mediate cytoprotective effects of CO in vitro. We sought to determine the role of caveolin-1 in lung susceptibility to VILI in mice. Furthermore, we assessed the role of caveolin-1 in the tissue protective effects of CO in the VILI model.
Design
Prospective experimental study
Setting
University laboratory
Subjects
Wild type (wt) and caveolin-1 deficient (cav-−/−) mice
Interventions
Mice were subjected to tracheostomy and arterial cannulation. Wt and cav-1−/− mice were ventilated with a tidal volume of 12 ml/kg body weight and a frequency of 80/min for 5 min as control, or for 8h with air in the absence or presence of CO (250 parts per million). Bronchoalveolar lavage (BAL) and histology were used to determine lung injury. Lung sections or homogenates were analyzed for caveolin-1 expression by immunohistochemical staining or Western Blotting, respectively.
Measurements and Main Results
Ventilation led to an increase in BAL protein concentration, cell count, neutrophil recruitment, and edema formation that was prevented in the presence of CO. While ventilation alone slightly induced caveolin-1 expression in epithelial cells, the application of CO during the ventilation significantly increased the expression of caveolin-1. In comparison to wt mice, mechanical ventilation of cav-1−/− mice led to a significantly higher degree of lung injury as compared to wt mice. In contrast to its effectiveness in wt mice, CO-administration failed to reduce lung injury markers in cav-1−/− mice.
Conclusions
Caveolin-1 null mice are more susceptible to VILI. Carbon monoxide executes lung protective effects during mechanical ventilation that are dependent in part, on caveolin-1 expression.
Keywords: ventilator induced lung injury, mechanical ventilation, carbon monoxide, caveolin-1, mechanotransduction, acute lung injury
INTRODUCTION
Mechanical ventilation is commonly used in the clinical management of respiratory failure. Despite recent progress in reducing mechanical ventilation-associated mortality by employing lower tidal volumes in patients (1), ventilator induced lung injury (VILI) remains a major problem in the intensive care unit. Ventilator associated lung injury arises from cyclic stretch to the lung imposed during mechanical ventilation, which disrupts cellular membranes, increases oxidative stress, induces pro-inflammatory cascades, and may subsequently lead to multiple organ failure (2). Therefore, supplementary therapeutic options, other than modulation of the ventilator setting, need to be developed in order to minimize VILI.
Over the last decade, carbon monoxide (CO) has been explored as a potential therapeutic agent in many disease models. Despite its noxious properties at elevated concentration, application of low dose CO exerts anti-inflammatory, anti-proliferative, anti-apoptotic, and anti-oxidative effects in preclinical models of inflammation, sepsis, ischemia/reperfusion (I/R)-injury, transplantation, among others (reviewed in (3, 4)). The organ protective action of inhaled CO is particularly effective in animal models of lung injury and disease. For instance, hyperoxic lung injury or bleomycin-induced pulmonary fibrosis were diminished in response to CO treatment in rodents (5, 6). Our laboratory previously demonstrated potent anti-inflammatory effects of inhaled CO in VILI using the combination of high-tidal volume ventilation and bacterial lipopolysaccharide (LPS) treatment in a rat model (7). However, the mechanism(s) by which CO prevents VILI remain incompletely understood.
CO potentially acts by modulating intracellular signal transduction pathways depending on the injury model. For example, guanylate cyclase-dependent production of cyclic-guanosine monophosphate has been implicated in the vascular effects of CO, whereas mitogen activated protein kinases (MAPK), and peroxisome proliferator-activated receptor-γ, among others, have been implicated in the anti-inflammatory effects of this gas (8, 9). Recently, we found that the anti-proliferative effect of CO in smooth muscle cells was mediated by caveolin-1 (10).
Caveolin-1, a 22 kDa protein, is the major structural protein of caveolae, small 50–100 nm flask-shaped invaginations of the plasma membrane. Caveolae, which occur abundantly in lung endothelial cells, type I pneumocytes, murine alveolar macrophages, and fibroblasts, facilitate endocytosis (11, 12). This mechanism is of major importance for the uptake of proteins into cells and the first step of transporting proteins through cellular barriers (transcytosis). For instance, caveolae-mediated transcytosis is thought to be the primary mechanism of carrying albumin across continuous endothelial cell barriers (13). Impaired clearance of proteins plays a pivotal role in lung edema and lung injury, and worsens outcome (14). In addition to inducing the biogenesis of caveolae, caveolin-1 plays a pivotal role in mediating signaling pathways involved in inflammation (12, 15). Interestingly, caveolin-1 has also been shown to mediate cell stretch-related effects in endothelial cells (16). To date, this has not been studied in lung epithelial cells which are compromised by cyclic stretch during mechanical ventilation.
To explore the significance of caveolin-1 in the lung during mechanical ventilation and to define its role in the protective effects of CO on VILI, we have employed a mouse model of VILI at physiologically relevant tidal volumes. In this study we demonstrate that CO reduces lung injury after mechanical ventilation, and that this protective effect depended on induction of caveolin-1. Furthermore, we demonstrate that caveolin-1 null mice are more susceptible to VILI. Our results implicate that caveolae represent essential cellular structures that limit stretch-induced injury.
MATERIALS and METHODS
Animals
All animals were housed in accordance with guidelines from the American Association for Laboratory Animal Care. The Animal Care and Use Committee of the University of Pittsburgh approved the protocols. Male C57/BL6 mice (wild type, wt) (Jackson Laboratories, Bar Harbor, ME) or caveolin-1 deficient (cav-1−/−) mice (Taconic, Hudson, NY) were anesthetized with ketamine (80 mg/kg, i.p.) and acepromazine (1 mg/kg, i.p.) and placed on a heating pad. A polyethylene catheter was inserted into the left carotid artery for direct blood pressure monitoring as well as for blood gas sampling, and a tracheostomy was established using a 20 G catheter. A rodent ventilator (Voltekenterprises, Toronto, Canada) was set to a tidal volume of 12 ml/kg body weight, frequency of 80/min, positive end-expiratory pressure (PEEP) of 2 cm H2O and connected to the tracheal canula. Muscular relaxation was achieved by applying pancuronium 2 mg/kg i.p. A 0.7 ml saline bolus was injected i.p. to compensate for evaporation during ventilation. Animals were randomized to receive ventilation with room air, or room air supplemented with carbon monoxide at a concentration of 250 parts per million (ppm) for 8h. Control animals were sham-operated and ventilated shortly for 5 minutes. Blood samples were withdrawn from control animals after 5 minutes and from ventilated animals at 1 h after onset of mechanical ventilation and blood gases were measured using an automated blood gas analyzer (ABL5, Radiometer, Bronshoi, Denmark). Blood gases revealed a mean pH 7.31+/−0.004, pO2 82+/−1.5 mmHg, and 34 +/−0.4 mmHg without differences between groups. After 8h of mechanical ventilation, the animals were sacrificed. Tissue and blood samples were snap frozen for subsequent analysis. To investigate the time course of cav-1 expression, additional animals (n=2/group) were ventilated with or without CO for 1 or 4 hours.
Bronchoalveolar lavage
At the end of each experiment, a bronchoalveolar lavage (BAL) was performed using 0.8 ml phosphate buffered saline (PBS). The recovered volume was centrifuged and the supernatant analyzed for protein concentration (Biorad Assay, Biorad, Hercules, CA). Furthermore, the pellet was re-dissolved in PBS and subsequently the number of total cells as well as the fraction of neutrophils were determined.
Polymerase chain reaction (PCR)
The right lower lung lobe was homogenized in TRIZOL in order to extract total RNA. Caveolin-1 mRNA was determined with a forward primer 5'-TTCAGGGAGGGGTGTCA-3' and a reverse primer 5'-AAAGTAGGTAGCAGGTTGGTAAAG-3'. GAPDH was used as the standard gene with a forward primer 5'-ACCACAGTCCATGCCATCAC-3' and a reverse primer 5'-TCCACCCTGTTGCTGTA-3'. In order to analyze the linear range of amplification, 23 cycles were used. PCR samples were run on a 1% agarose gel containing ethidium bromide and photographed. Densitometric analyses were performed using ImageJ software (National Institute of Health, www.rsb.info.nih.gov).
Immunoblotting
The right upper lung lobe was homogenized in 30 mM Tris Base including complete protease inhibitors (Roche Diagnostics, Mannheim, Germany). After determination of the protein content, equal amounts were loaded onto a 4–12% SDS page (Invitrogen, Carlsbad, CA, USA) and blotted onto a nitrocellulose membrane. The membranes were incubated with caveolin-1 (1:2000, sc-894, Santa Cruz, Santa Cruz, CA) overnight at 4°. After several washing steps, membranes were incubated for 1 h with the appropriate secondary antibody, detected (Supersignal, Peirce, Rockford, IL) and exposed to radiographic films. Re-blotting the membranes with β-actin (Sigma, Saint Louis, MO, USA) served to control for equal loading and transfer.
Immunohistochemistry
Immunostaining of lung tissue was performed in the left lung lobe that was infused with OCT (Sakura, Torrance, CA) and stored at –80°C. Cryosections were subjected to hematoxylin & eosin staining (H&E). Caveolin-1 staining and confocal single cell microscopy were conducted using the above mentioned antibody (1:50). Secondary antibody and immunofluorescence staining were conducted according to the Center of Biological Imaging protocol (CBI, Pittsburgh, PA, www.cbi.pitt.edu). The color green indicates caveolin-1, red indicates f-actin / phalloidin, and blue (dapi) indicates nuclei.
Cytokine/Chemokine measurements
BAL aliquots were analyzed using interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Statistical analysis
Data represent means +/− standard error of means (SEM) for n=6/group. Statistical analyses were performed using the analysis of variances (ANOVA) for multiple group comparison and the Student-Newman-Keuls posthoc test (Sigmastat statistical software, Systat Inc., Erkrath, Germany). For two group comparison, the Student-t-test was used to compare parametric, and the Mann-Whitney Rank Sum test to compare non-parametric data. p<0.05 was considered significant.
RESULTS
Effect of ventilation and carbon monoxide on lung injury
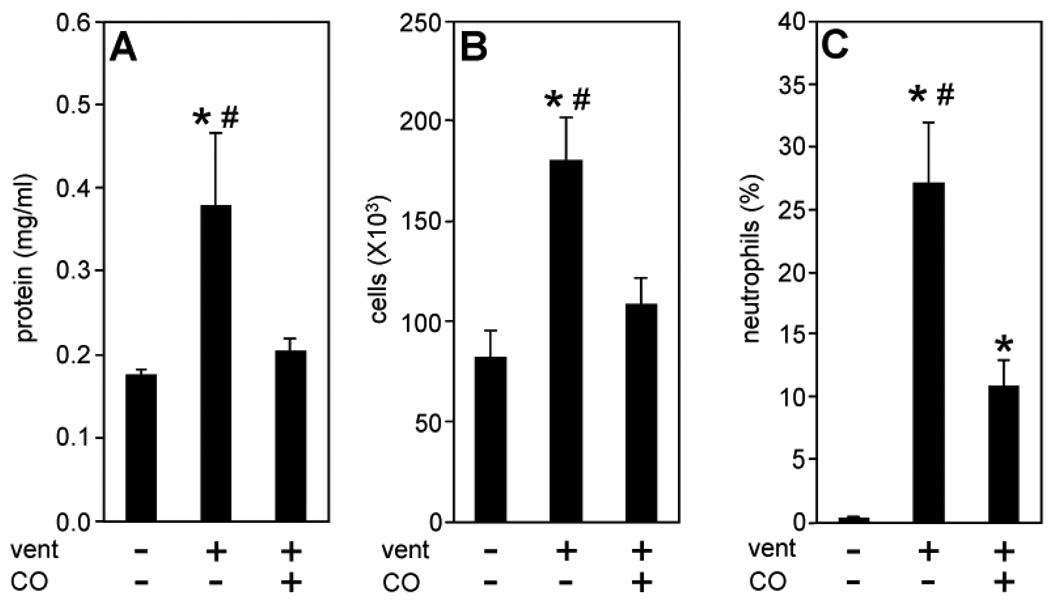
We used a total of 62 male C57/BL6 mice for the current study. Ventilation of wild-type (wt) mice with 12 ml/kg led to a significant increase in BAL protein concentrations after 8h, relative to control animals (Fig. 1a). Moreover, BAL cell count and the percentage of neutrophils were notably elevated as a result of ventilation (Fig. 1b and c). In sharp contrast, the presence of carbon monoxide (CO, 250 ppm) during ventilation substantially diminished these injury markers (Fig. 1a–c) indicating that CO protected the lung from VILI.
Figure 1. Effect of ventilation and carbon monoxide on lung injury.
Wild type mice were ventilated with 12 ml/kg tidal volume for 5 min (control), 8h with air (vent), or for 8h with air+carbon monoxide 250 ppm (vent+CO). Bronchoalveolar lavage (BAL) was recovered at the end of each experiment. (A) BAL protein concentration, (B) BAL total cell count, (C) BAL neutrophil fraction. Data represent means + SEM for n=6/group. ANOVA, *p<0.05 vs control, #p<0.05 vs vent+CO.
Effect of ventilation and carbon monoxide on caveolin-1 expression
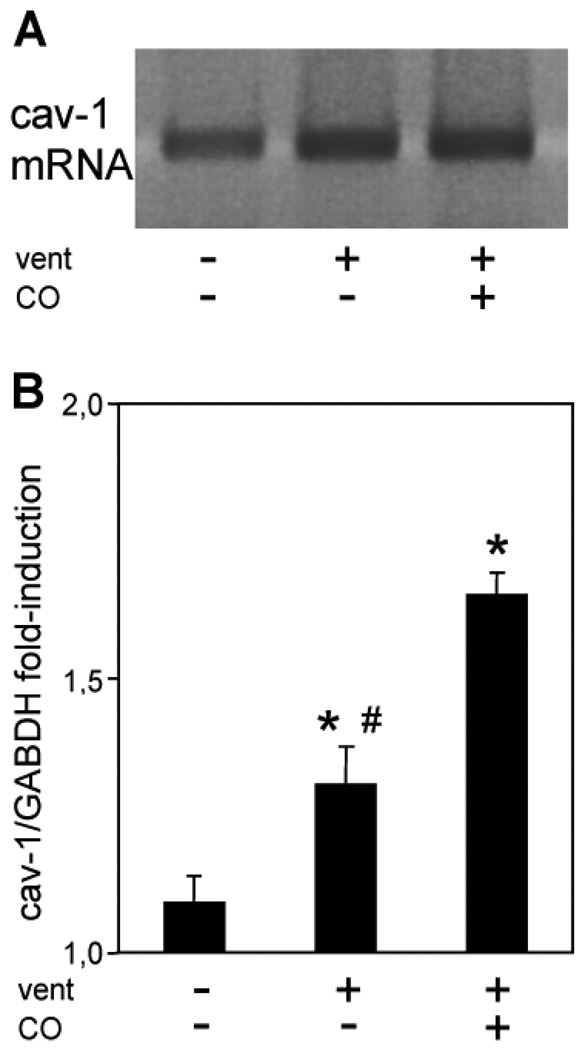
Next, we investigated the underlying mechanism(s) that mediate the protective effects of CO application during mechanical ventilation. Recent in vitro experiments by our group have demonstrated that certain protective effects of CO are transduced by caveolin-1 (10). Therefore, we analyzed the effect of ventilation in the absence and presence of CO on the regulation of caveolin-1. To quantify the modulation of caveolin-1 by ventilation or CO administration, PCR as well as immunoblots for caveolin-1 were performed using lung tissue homogenates. Figure 2 demonstrates increased accumulation of caveolin-1 mRNA in response to mechanical ventilation that was even further upregulated in CO treated animals (Fig. 2a–b). The transcriptional upregulation of cav-1 was paralleled by increased protein synthesis that was detected by Western Blot analysis (Fig. 3a–b). To investigate the time course of cav-1 expression by ventilation and CO-treatment, additional animals were ventilated for 1 and 4 hours. Our results demonstrated an increased cav-1 protein synthesis as early as 4 hours after the onset of CO inhalation (Fig. 3c).
Figure 2. Effect of ventilation and carbon monoxide on caveolin-1 transcriptional regulation.
Wild type mice were ventilated with 12 ml/kg tidal volume for 5 min (control), 8h with air (vent), or for 8h with air+carbon monoxide 250 ppm (vent+CO). RNA was extracted from lung tissue at the end of the experiments and subjected to polymerase chain reaction. (A) Representative PCR for caveolin-1. (B) Densitometric analysis of caveolin-1 / GAPDH. Data represent means + SEM for n=6/group. ANOVA, *p<0.05 vs control, #p<0.05 vs vent+CO.
Figure 3. Effect of ventilation and carbon monoxide on caveolin-1 protein expression.
Wild type mice were ventilated with 12 ml/kg tidal volume for 5 min (control), 8h with air (vent), or for 8h with air+carbon monoxide 250 ppm (vent+CO). Lung tissue was homogenized at the end of the experiments and subjected to Western Blot analysis. (A) Representative Western blot of total caveolin-1 protein accumulation (upper panel). β-actin detection of the same membrane for normalization (lower panel). (B) Densitometric analysis of caveolin-1 / β-actin. Data represent means + SEM for n=6/group. ANOVA, *p<0.05 vs control , #p<0.05 vs vent+CO. (C) Time course of caveolin-1 regulation. Representative Western Blot of caveolin-1 expression at indicated time points.
As shown in Figure 4 with immunofluorescence microscopy and confocal single cell microscopy, caveolin-1 (green immunofluorescence) was abundantly expressed in epithelial cells of untreated animals (Fig. 4a,d, arrow). Ventilation of these animals with 12 ml/kg for 8h further increased caveolin-1 staining (Fig. 4b,e). Most interestingly, the administration of CO clearly upregulated caveolin-1 in lung parenchymal cells (Fig. 4c,f).
Figure 4. Effect of ventilation and carbon monoxide on the localisation of caveolin-1 .
Wild type mice were ventilated with 12 ml/kg tidal volume for 5 min (A, control), 8h with air (B, vent), or for 8h with air+carbon monoxide 250 ppm (C, vent+CO). Lung sections were taken at the end of each experiment and representative slides were immunofluorescence stained for caveolin-1 protein. (A–C) 200 X magnification, (D–F) confocal single cell microscopy, 2000 X magnification. Green color: caveolin-1 (arrow), red color: f-actin / phalloidin, blue color: dapi (nuclei, n).
Effect of caveolin-1 deficiency on lung injury
First, we confirmed cav-1 gene depletion in the knock out animals (Fig. 5a). Next, we determined the degree of lung injury in cav-1−/− mice as a result of 8h mechanical ventilation (Fig. 5b–c). Under control conditions, a slight increase in BAL protein concentrations was present in cav-1−/− animals. However, in these mice neither protein concentration nor total cell count were statistically different from wt mice. Theses results on lung injury parameters exclude the possibility that cav-1−/− mice might present severe pre-existing lung tissue damage (17). After mechanical ventilation, caveolin-1 deficiency led to a substantial increase of BAL protein and cell counts as compared to wt animals. While CO exerted protective effects in wt mice and decreased the protein leakage and cellular infiltration, this protection was absent in cav-1−/− mice (Fig. 5b–c). The protective effects of CO inhalation in wild type and the deleterious consequences of cav-1 deficiency were evident by H&E staining of lung sections (Fig. 6). In wt, H&E staining of control lungs showed no obvious signs of lung injury (Fig. 6a). However, ventilation led to significant lung damage reflected by thickening of alveolar septae and infiltration of pro-inflammatory cells (Fig. 6b). In the presence of applied CO, both edema formation as well as the cellular infiltration were absent despite ventilation (Fig. 6c). Interestingly, under control conditions, the lung histology of cav-1−/− mice (Fig. 6d) was comparable to that of wt mice (Fig. 6a). Mechanical ventilation led to massive lung injury in cav-1−/− mice (Fig. 5e) that was more pronounced than in wt mice (Fig. 6b). While CO exerted protective effects in wt mice (Fig. 6c), these effects were absent in cav-1−/− mice (Fig. 6f).
Figure 5. Effects of ventilation and carbon monoxide on lung injury in caveolin-1 deficiency.
Wild type (wt, black bars) and caveolin-1−/− mice (cav-1−/−, white bars) were ventilated with 12 ml/kg tidal volume for 5 min (control), 8h with air (vent), or for 8h with air+carbon monoxide 250 ppm (vent+CO). (A) Representative Western Blot for caveolin-1 (upper panels) and β-actin (lower panels) in wt and cav-1−/− mice. (B) BAL protein concentration, (C) BAL total cell count. Data represent means + SEM for n=6/group. ANOVA for comparison within wild type or caveolin-1−/− groups, *p<0.05 vs control, #p<0.05 vs vent+CO. p-value for comparison between wild type and caveolin-1−/− groups as indicated.
Figure 6. Effect of ventilation and carbon monoxide on lung histology in wild type and caveolin-1 deficient mice.
Wild type (A-C, wt) and caveolin-1−/− (D-F, cav-1−/−) mice were ventilated with 12 ml/kg tidal volume for 5 min (A, D, control), 8h with air (B, E, vent), or for 8h with air+carbon monoxide 250 ppm (C, F, vent+CO). Representative lung sections were taken at the end of each experiment and stained with H&E (400 X magnification).
Effect of caveolin-1 deficiency on neutrophils and cytokine release
Most interestingly and contrary to our expectations, mechanical ventilation did not further recruit neutrophils in cav-1−/− mice as compared to wt mice (Fig. 7a). However, CO significantly reduced neutrophil influx in ventilated wt mice, but did not impact neutrophil influx in cav-1−/− mice. To validate this observation, BAL cytokines and chemokines were measured (Fig. 7b–c). Interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) were elevated in ventilated wt mice as compared to control animals. The presence of CO during ventilation diminished both IL-6 as well as MIP-2 levels in wt mice. In cav-1−/− mice, IL-6 levels were further increased in response to mechanical ventilation, relative to wt mice. CO application significantly reduced ventilation-induced IL-6 levels in both wt and cav-1−/− mice. In contrast, MIP-2 levels in response to ventilation were comparable between wt and cav-1−/− mice, and were not significantly reduced by CO in cav-1−/− mice.
Figure 7. Effect of ventilation and carbon monoxide on neutrophil recruitment, interleukin-6 and macrophage inflammatory protein-2 in wild type and caveolin-1−/− mice.
Wild type (black bars) and caveolin-1−/− mice (white bars) were ventilated with 12 ml/kg tidal volume for 5 min (control), 8h with air (vent), or for 8h with air+carbon monoxide 250 ppm (vent+CO). Bronchoalveolar lavage (BAL) was obtained at the end of each experiment. (A) BAL neutrophil fraction. (B) ELISA from BAL Interleukin-6 concentration. (C) ELISA from BAL macrophage inflammatory protein-2 concentration. Data represent means + SEM for n=6/group. ANOVA for comparison within wild type or caveolin-1−/− groups, *p<0.05 vs control, #p<0.05 vs vent+CO. p-value for comparison between wild type and caveolin-1−/− groups as indicated.
DISCUSSION
In the current study, we have demonstrated that mechanical ventilation with 12 ml/kg for 8h induced severe lung injury as reflected by histological evaluation of lung sections, as well as increased protein concentrations in the BAL, and infiltration of macrophages and neutrophils. CO administration at low concentration (250 ppm) significantly reduced all lung injury parameters.
The effectiveness of applying low dose CO in order to employ its organ protective effects has been established in many in vitro and in vivo studies over the last decade (reviewed in (3, 4)). Presently, several ongoing clinical studies address the effectiveness of CO-treatment in humans (NIH, www.clinicaltrials.gov). In animal experiments, CO confers tissue protection through anti-proliferative, anti-oxidative, vasodilatory, anti-inflammatory, and membrane stabilizing characteristics (18, 19). The latter two are most important regarding the development of VILI. For instance, our laboratory has recently demonstrated the beneficial effects of CO-administration against LPS-induced lung injury in overventilated rats (7). The data in the present study shows that in a mouse model using moderate tidal volumes (12 ml/kg), mechanical ventilation led to a significant degree of lung injury that was abolished in the presence of CO. Given these protective properties of CO during mechanical ventilation, we attempted to identify the underlying mechanism. Signaling of CO might be transduced by several pathways including cGMP (8), MAPK (18), or caveolin-1, as recently described in vitro (10).
Our results demonstrate that mechanical ventilation induced the expression of caveolin-1 mRNA and protein in the lung in vivo, which was dramatically further upregulated by the application of CO during ventilation. Caveolin-1 orchestrates membrane trafficking, endocytosis, regulation of cholesterol, and signal transduction in cellular growth and apoptosis (reviewed in (20)). Interestingly, in endothelial cells, caveolin-1 appears to play an important role in mechanotransduction (16). Previous in vitro data on caveolin-1 expression upon flow mediated shear stress in lung endothelial cells increased caveolin-1 density at the luminal plasma membrane (21). Lung caveolin-1 expression is not restricted to endothelial cells but includes type I epithelial cells (11). Here we show for the first time that mechanical stress by the means of mechanical ventilation induced caveolin-1 in lung epithelial cells. Immunohistochemistry and confocal microscopy of epithelial cells demonstrated basal expression of caveolin-1 in the alveolar luminal surface that was further enhanced by ventilation. These cells cover more than 95% of the internal surface area, which is consistent with our finding that increased expression of caveolin-1 at the luminal surface is paralleled by upregulation of caveolin-1 protein in whole lung homogenates.
We hypothesized that caveolin-1 upregulation by ventilation alone might reflect an adaptive mechanism in order to reduce stretch-induced lung injury. Therefore, we employed the mouse VILI model in caveolin-1 deficient mice. Our results show clearly that caveolin-1 deficiency aggravated lung injury in response to mechanical ventilation, most pronounced with respect to protein leakage, edema formation, and macrophage infiltration. It has been described that cav-1−/− mice exert increased microvascular permeability (22) and pre-existing lung injury (17, 22–24) which may contribute to the susceptibility to VILI in these mice. However, our results did not reveal significant alterations in lung morphology under control conditions between wild type and cav-1−/−, with the exception of a trend in elevated BAL protein concentrations in cav-1−/− controls without ventilation. The latter is most likely based on impaired caveolae-mediated transcytosis that is thought to be the primary transport path for albumin at physiological concentration (13). Thus, the clearance of alveolar proteins in the intact lung depends on caveolae (14). Most interestingly, caveolae mediated transcytosis at high protein concentrations, seems to play a minor role (14). In this respect, an increased nitric oxide dependent paracellular protein transport may compensate for the lack of caveolae in cav-1−/− animals (22).
The precise mechanism responsible for the higher susceptibility to mechanical ventilation in cav-1−/− mice remains unclear. Cav-1 serves as a major signaling protein, which interacts with and modulates the activation state of several major cellular signaling pathways, e.g. mitogen activated protein kinases, integrins, and nitric oxide synthases (NOS). For instance, it has been reported that cav-1 deficiency leads to activation of the endothelial isoform of NOS thus overproducing nitric oxide (15). As a possible consequence, increased NO levels may have aggravated lung injury in response to ventilation in our model. However, whether NO promotes or inhibits the development of VILI is still controversial (25–27). Studies which systematically investigate the influence of NO in a long term model of VILI with moderate tidal volumes, as we applied, are not available.
We chose to measure neutrophil fraction, IL-6, and MIP-2 as indicators of a potentially altered immunoresponse in cav-1 deficiency. Neutrophil infiltration is thought to play an essential role in the development of lung injury after mechanical ventilation (28). It is important to note that in this study, neutrophils were measured as a fraction of the sum of macrophages and neutrophils in the BAL. As mentioned earlier in this paper and as reported previously by our laboratory, neutrophils are not detectable in control lungs and migrate into the lungs upon mechanical ventilation (29). Intriguingly, neutrophil recruitment into the lung was not further increased in cav-1−/− mice, and comparable to wt animals. These immune cells migrate upon stimulation from the endovascular to the alveolar space passing the endothelial and the epithelial cell barrier. It has been demonstrated by others that cav-1 deficiency inhibits the endothelial transmigration of neutrophils upon pro-inflammatory stimuli (15, 30). In this regard, neutrophil migration depends at least in part on transcytosis that is facilitated by a number of mediators (i.e. vascular endothelial growth factor-receptor, platelet endothelial adhesion molecule-1, or intercellular adhesion molecule-1). These molecules are either located within or translocate to caveolae upon pro-inflammatory stimuli (31–33). Furthermore, it has been demonstrated, that endo- and transcytosis processes are impaired in cav-1 deficiency (22). Also, neutrophils express caveolin-1 (34). The attachment of neutrophils in cav-1−/− mice is severely inhibited and their ability to transmigrate over cellular barriers impaired (34). Therefore, inhibition of neutrophil migration would most likely explain the lack of a further neutrophil infiltration in cav-1−/− mice in our model.
The cytokine IL-6 and the small chemokine MIP-2 are known to be upregulated in response to mechanical ventilation with high tidal volumes and represent major regulators of neutrophil recruitment into the lung. In pilot experiments, we observed these cytokines and chemokines to be present at 8 h of mechanical ventilation (data not shown). Most interestingly, the release of the neutrophil chemoattractant MIP-2 upon mechanical ventilation in cav-1−/− animals was not further increased as compared to wt and paralleled the migration pattern of neutrophils. These results are surprising, since MIP-2 is produced by macrophages which are outnumbered in cav-1 deficiency as compared to wt after ventilation. Nevertheless, cytokines and chemokines seem to be differentially regulated in cav-1 deficiency upon pro-inflammatory stimuli (35). Regarding the influence of caveolin-1 on MIP-2 release, we provide evidence that MIP-2 regulation is not dependent on cav-1 expression and furthermore, an increase in MIP-2 production that would represent aggravated lung injury seems to be prevented in cav-1 deficiency. Due to the lack of data on MIP-2 in cav-1 deficiency, we can only speculate on possible explanations for this observation. The release of IL-8, a functional human homologue of mouse MIP-2, in response to proinflammatory stimuli has been shown to be inhibited in vascular endothelial and renal epithelial cell lines with disrupted caveolae (36–38). Here, altered pathways such as the MAPK, activation of sphingomyelinase, or toll like receptors might play a role. However, the role of caveolin-1 in the regulation of IL-8 is far from understood and its impact on MIP-2 in the lung in response to mechanical stress literally unknown.
In contrast to neutrophils and MIP-2, IL-6 production was substantially upregulated in ventilated cav-1−/− mice as compared to wt. IL-6 is secreted by macrophages and might on one hand reflect the tremendous increase of macrophages in the BAL. Even though total cell count in the BAL includes all cells containing nuclei, macrophages represent the majority of these cells. On the other hand, previous reports from us and others demonstrated elevated IL-6 levels in cav-1−/− macrophage cell lines (12, 35). As reported earlier, LPS challenge of a macrophage cell line resulted in significant IL-6 production that was even further increased when cav-1 was silenced (12). Conversely, overexpression of cav-1 in these cells led to a significant decline of IL-6 protein providing evidence for anti-inflammatory role of cav-1. This assumption from in vitro data is in agreement with our in vivo results showing the first time that IL-6 release after mechanical ventilation is pronounced in the absence of caveolin-1.
Since CO further upregulated caveolin-1 which correlated with increased protection from VILI, we speculated that CO-protection requires caveolin-1. In fact, while CO exerted major protective effects in wild type mice, this was no longer the case in caveolin-1 deficiency. Protein leakage, edema formation as well as macrophage and neutrophil infiltration, which were prevented in wt mice by CO-administration, were not reduced by CO treatment in cav-1−/− animals. However, cytokine upregulation (i.e., IL-6) was partially inhibited by CO in both strains with comparable effectiveness. Thus, the inhibitory effect of CO on IL-6 production appears to be independent of caveolin-1 in this model.
Previous studies from this laboratory have suggested a relationship between caveolin-1 and CO-mediated tissue protection. For example, CO application induced caveolin-1 in neointimal lesions of injured rat aorta coincident with a tissue protection (10). In addition, depletion of cav-1 using siRNA abolished the anti-proliferative effect of CO in pulmonary smooth muscle cells. In agreement with previous studies, the present investigation indicates that CO requires caveolin-1 to execute its protective effects during mechanical ventilation. Furthermore, these studies identify caveolin-1 as a novel therapeutic target for modulating the outcome of VILI.
CONCLUSIONS
In summary, mechanical ventilation with moderate tidal volume led to a substantial degree of lung injury that was prevented in the presence of carbon monoxide. Caveolin-1, the major structural protein of caveolae, plays a crucial role in limiting lung injury during mechanical ventilation and mediates the protective effects of carbon monoxide application.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to Alexander Hoetzel (DFG HO 2464/1-1); by awards from the American Heart Association to S. W. Ryter, (AHA #0335035N), and H. P. Kim (AHA #0525552U) and NIH grants R01-HL60234, R01-HL55330, R01-HL079904, and P01-HL70807 awarded to A. M. K. Choi.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 4.Hoetzel A, Dolinay T, Schmidt R, et al. Carbon Monoxide in Sepsis. Antioxid Redox Signal. 2007 doi: 10.1089/ars.2007.1762. [DOI] [PubMed] [Google Scholar]
- 5.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Song R, Fattman CL, et al. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol. 2005;166:27–37. doi: 10.1016/S0002-9440(10)62229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinay T, Szilasi M, Liu M, et al. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 8.Morse D, Sethi J, Choi AM. Carbon monoxide-dependent signaling. Crit Care Med. 2001;30:S12–S17. [PubMed] [Google Scholar]
- 9.Bilban M, Bach FH, Otterbein SL, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Kim HP, Wang X, Nakao A, et al. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci U S A. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlin K, Mager EM, Allen L, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 12.Wang XM, Kim HP, Song R, et al. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hastings RH, Folkesson HG, Matthay MA. Mechanisms of alveolar protein clearance in the intact lung. Am J Physiol. 2004;286:L679–L689. doi: 10.1152/ajplung.00205.2003. [DOI] [PubMed] [Google Scholar]
- 15.Garrean S, Gao XP, Brovkovych V, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Bergaya S, Murata T, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 18.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 19.Farrugia G, Lei S, Lin X, et al. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–8570. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AW, Hnasko R, Schubert W, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo V, Morton C, DePaola N, et al. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 22.Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-name, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 23.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 24.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broccard AF, Feihl F, Vannay C, et al. Effects of L-NAME and inhaled nitric oxide on ventilator-induced lung injury in isolated, perfused rabbit lungs. Crit Care Med. 2004;32:1872–1878. doi: 10.1097/01.ccm.0000139605.38527.1b. [DOI] [PubMed] [Google Scholar]
- 26.Choi WI, Quinn DA, Park KM, et al. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1627–1632. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- 27.Takenaka K, Nishimura Y, Nishiuma T, et al. Ventilator-induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am J Physiol. 2006;290:L1078–L1086. doi: 10.1152/ajplung.00239.2005. [DOI] [PubMed] [Google Scholar]
- 28.Karzai W, Cui X, Heinicke N, et al. Neutrophil stimulation with granulocyte colony-stimulating factor worsens ventilator-induced lung injury and mortality in rats. Anesthesiology. 2005;103:996–1005. doi: 10.1097/00000542-200511000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Hoetzel A, Dolinay T, Vallbracht S, et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejana E. The transcellular railway: insights into leukocyte diapedesis. Nat Cell Biol. 2006;8:105–107. doi: 10.1038/ncb0206-105. [DOI] [PubMed] [Google Scholar]
- 31.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 32.Mathew R, Huang J, Shah M, et al. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499–1506. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 33.Millan J, Hewlett L, Glyn M, et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 34.Hu G, Ye RD, Dinauer MC, et al. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol. 2008;294:L178–L186. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 35.Medina FA, de Almeida CJ, Dew E, et al. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:6665–6674. doi: 10.1128/IAI.00949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walton KA, Gugiu BG, Thomas M, et al. A role for neutral sphingomyelinase activation in the inhibition of LPS action by phospholipid oxidation products. J Lipid Res. 2006;47:1967–1974. doi: 10.1194/jlr.M600060-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Walton KA, Cole AL, Yeh M, et al. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Nord EP. Functional caveolae are a prerequisite for CD40 signaling in human renal proximal tubule cells. Am J Physiol. 2004;286:F711–F719. doi: 10.1152/ajprenal.00308.2003. [DOI] [PubMed] [Google Scholar]