Abstract
The microsporidia are a group of obligate intracellular parasitic protists that have been implicated as both human and veterinary pathogens. The infectious process of these organisms is believed to be dependent upon the rapid influx of water into spores, presumably via aquaporins (AQPs), transmembrane channels that facilitate osmosis. An AQP-like sequence of the microsporidium Encephalitozoon cuniculi (EcAQP), when cloned and expressed in oocytes of Xenopus laevis, rendered these oocytes highly permeable to water. No permeability to the solutes glycerol or urea was observed. Pre-treatment of EcAQP-expressing oocytes with HgCl2 failed to inhibit their osmotic permeability, as predicted from EcAQP's lack of mercury-sensitive cysteine residues near the NPA motifs which line the AQP aqueous pore. EcAQP exhibits sequence identity to AQP A of Dictyostelium discoideum (26%) and human AQP 2 (24%). Further study of AQPs in microsporidia and their potential inhibitors may yield novel therapeutic agents for microsporidian infections.
Keywords: Microsporidia, Aquaporin, Germination, Water permeability, Encephalitozoon, Xenopus oocytes
1. Introduction
The phylum Microsporidia, a group of obligate intracellular parasitic protists, contains over 1200 species representing nearly 150 genera (Weiss, 2001; Garcia, 2002). Since the mid-1980s, these organisms have been increasingly implicated as agents of human disease, especially in their capacity as opportunistic parasites of AIDS patients (Cali, 1991; Weiss, 2001) and other immunosuppressed individuals (Franzen and Muller, 2001). The most common clinical manifestation is chronic diarrhea and wasting due to enteric infection, but the spectrum of disease is wide and includes most organ systems (Cali, 1991; Franzen and Muller, 2001). Albendazole and other benzimidazoles that bind tubulin, as well as fumagillin and its derivatives that inhibit methionine aminopeptidase type 2, have been identified as being useful agents for the treatment of microsporidiosis; however, effective and safe treatments do not yet exist for some of the microsporidian species that infect humans (Costa and Weiss, 2000).
Microsporidia produce an infectious, environmentally resistant spore, which is capable of extruding its internal polar filament and thereby inoculating its contents into a nearby host cell. The mechanism of spore germination has long been of interest to microsporidian researchers, and naturally it has been suggested that inhibition of this process would constitute an effective anti-microsporidian therapy (Keohane and Weiss, 1999). Germination stimuli are variable (Undeen and Epsky, 1990) but are postulated (reviewed in Keohane and Weiss, 1999) to result in cleavage of the disaccharide trehalose into glucose and its metabolites, producing an increase in intrasporal pressure and osmotic swelling that leads to extrusion of the polar filament. Because water flux across the lipid bilayer is limited, it has been suggested that microsporidia may possess aquaporins (AQPs) (Frixione et al., 1997), integral membrane channel proteins that facilitate osmosis (Verkman and Mitra, 2000; Agre and Kozono, 2003). This suspicion was bolstered by the observation that germination of Brachiola (Nosema) algerae spores is inhibitable by mercury salts (Frixione et al., 1997), which inhibit AQP function (Yang, 2000; Agre and Kozono, 2003). Further evidence suggesting the presence of AQPs in microsporidia surfaced with the identification of a single AQP-like sequence within the genome of Encephalitozoon cuniculi, a human-pathogenic species (Katinka et al., 2001).
The present study was undertaken to functionally characterise the putative E. cuniculi AQP (EcAQP) protein. Aquaporin function is typically assayed in RNA-injected Xenopus oocytes, where swelling occurs under osmotic stress due to expression of the exogenous AQP (Verkman and Mitra, 2000; Agre and Kozono, 2003). Utilizing this assay, the osmotic permeability and solute conductivity of EcAQP-injected oocytes were investigated. In addition, the EcAQP amino acid sequence was compared to those of human, plant, fungal, and other protist parasite AQPs.
2. Materials and methods
2.1. Parasites
Encephalitozoon cuniculi was cultured in RK13 cells (rabbit kidney cells CCL37; American Type Culture Collection, Rockville, Md.) at 37 8C and 5% CO2. Infected RK13 cells were maintained in continuous culture in minimum essential medium supplemented with 7% heat-inactivated FCS, 1% penicillin–streptomycin and 1% amphotericin B (Fungizone; Invitrogen, Carlsbad, CA) and subpassaged every week by trypsin-EDTA treatment (Invitrogen, Carlsbad, CA). Spores were harvested from culture medium twice weekly.
2.2. Cloning and expression of EcAQP
Genomic DNA was isolated from disrupted spores of E. cuniculi by SDS and proteinase K treatment and homogenization, followed by phenol–chloroform extraction, as previously described (Keohane et al., 1998). Recognition sites for the restriction enzymes Xma I and Xba I were engineered onto the N- and C-termini, respectively, of EcAQP (GenBank accession no. NP_586002), by PCR-amplification of genomic DNA. PCR was performed using Pfx DNA polymerase and 15 μM of each primer (primers, restriction sites are italicised: 5′GGACCTCCCGGGATGACCAGAGAGACATTGAAG3′ (forward), 5′GACCCTCTAGACTAAAAGCTGAGCTTGT ACAG3′ (reverse)); DNA was amplified for 35 cycles (45 s denaturation at 94 °C, 45 s annealing at 40 °C and 60 s extension at 60 °C). The amplicon was cloned into the Xma I–Xba I multiple cloning site of the pGEMHE Xenopus expression vector (Liman et al., 1992) by Xma I–Xba I digestion and mutual ligation of the amplicon and vector, yielding pGEMHE-EcAQP. Escherichia coli strain DH5α was subsequently transformed by pGEMHE-EcAQP; large-scale plasmid purification from ampicillin-screened colonies was accomplished by the HiSpeed Kit (Qiagen, Valencia, CA). Identity of pGEMHE-EcAQP was confirmed by restriction digestion analysis and by sequencing. pGEMHE-EcAQP was linearised downstream of the 3′ untranslated region (UTR) by digestion with the restriction enzymes Sph I or Nhe I. cRNA was generated in vitro by the mMessage mMachine kit (Ambion, Austin, TX) as per manufacturer's instructions using T7 RNA polymerase, nucleotide phosphate (NTP), 7-methyl-guanosine cap analog, and RNase-inhibitor. Xenopus laevis maintenance and surgical oocyte removal were performed as previously described (Mak and Foskett, 1994). Defolliculated stages V and VI oocytes were injected with 55 ng in 37 nL of pGEMHE-EcAQP mRNA or 37 nL water (controls). Oocytes were incubated in isoosmotic ND96 buffer/pyruvate (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 2.5 mM pyruvate, pH 7.6) at 16 °C for 4–6 days, adding fresh buffer after 3 days.
2.3. Oocyte swelling assays
Swelling assays were conducted at room temperature in plastic 96-well culture plates. For measurement of water permeability, oocytes were transferred to hypotonic source of ND96/pyruvate (1:3 diluted ND96 buffer/0.83 mM pyruvate) or, if indicated, pre-incubated for 5 min in isoosmotic ND96/pyruvate +0.1, 1, 10, 200 μM, or 1 mM HgCl2 and then transferred to hypotonic ND96/pyruvate+HgCl2. For measurement of solute conductivity, oocytes were transferred to ND96/pyruvate where 65 mM NaCl had been replaced with 130 mM glycerol or urea. Swelling was video-monitored every 3 s with a Zeiss SV11 dissecting microscope (Zeiss, Göttingen, Germany) at 66×magnification, and a Retiga 1300 digital camera and IPLab software (Scanalytics, Fairfax, VA). Image analysis was accomplished with ImageJ software (National Institutes of Health, US; URL: http://rsb.info.nih.gov/ij/) by converting images to binary and treating the oocyte as a growing sphere whose volume could be inferred from its cross-sectional area. Water permeability (Pf, cm/s) was calculated based on the first 60 s of the assay and according to the following equation: Pf={[V0][d(V/V0/dt]}/[(S)(Vw)(osmin−osmout)]; V0 and S are the initial volume and surface area of each individual oocyte, respectively; d(V/V0)/dt, the relative volume increase per unit time; Vw, the molecular volume of water (18 cm3/mol); and osmin−out, the osmotic gradient between the inside and outside of the oocyte (140×10−6 mol/cm3). Solute conductivity was inferred from d(V/V0)/dt. Water permeabilities and swelling rates were statistically compared with the Student's t-test (two-tailed).
2.4. Sequence analysis
The BLAST program (Altschul et al., 1997) was used to search the databases for proteins similar to EcAQP. The Biology Workbench (URL: http://workbench.sdsc.edu/) was employed for Kyle–Doolittle hydropathy analysis (Kyle and Doolittle, 1982; Pearson and Lipman, 1988; Pearson, 1990), transmembrane segment prediction (Persson and Argos, 1994), CTREE phylogenetic construction (algorithm and program: The Biology Workbench, David J. States), and multiple protein sequence alignment (Felsenstein, 1989; Higgins et al., 1992; Thompson et al., 1994).
3. Results
3.1. Functional characterization of EcAQP
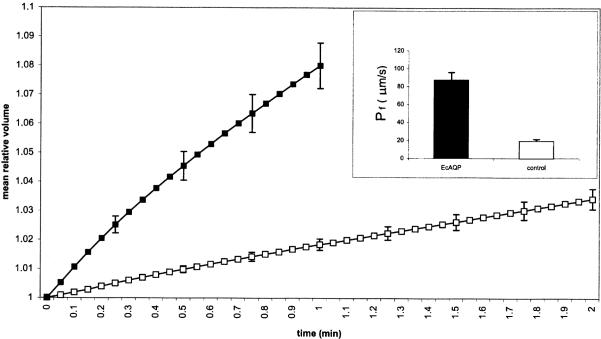
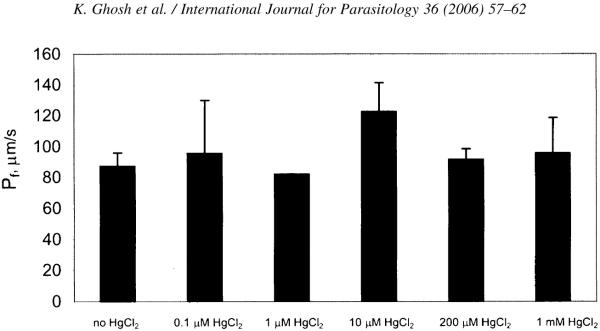
Mean swelling rates of EcAQP- and water-injected oocytes in hypotonic medium are plotted in Fig. 1; only 1 min of EcAQP swelling is plotted due to bursting of some oocytes between 1 and 2 min (data not shown). At 1 min, EcAQP oocytes swelled on average 8.0±0.8%, compared with 1.8±0.2% for water-injected control oocytes; at 2 min, control oocytes had only swelled 3.4±0.35%. Endogenously expressed AQP 3 (Schreiber et al., 2000) as well as osmosis across the lipid bilayer may account for swelling of control oocytes. The osmotic permeability, Pf, of EcAQP oocytes, 87 μm/s, differed significantly (P⪡0.001) from that of controls (19 μm/s) (Fig. 1, inset). Osmotic permeability of HgCl2-treated and -untreated EcAQP oocytes did not differ significantly for any of the tested HgCl2 concentrations (Fig. 2). Conductivities of EcAQP oocytes to glycerol (1×10−4±6×10−5 d(V/V0)·s−1) and urea (3×10−4±6×10−5 d(V/V0)·s−1) did not differ significantly from those of control oocytes.
Fig. 1.
EcAQP-injected Xenopus laevis oocyte swelling assay. After 1 min, EcAQP-injected oocytes had swelled an average of 8.0 vs. 1.8% water-injected controls. Inset: Altered water permeability (P⪡0.001) of EcAQP-injected oocytes (87.3±8.6 μm/s; n=12) vs. water-injected (19.3±2.0 μm/s; n=15). Legend: closed marker/bar, EcAQP-injected; open marker/bar, water-injected. (Mean±S.E.M; for clarity, error bars are only displayed every 15 s.).
Fig. 2.
Swelling of EcAQP oocytes is not inhibited by HgCl2. Permeability differences between HgCl2-treated and -untreated oocytes were not significant (where n>1) for all tested concentrations (0.1 μM, n=2; 1 μM, n=1; 10 μM, n=5; 200 μM, n=4; 1 mM, n=2; untreated, n=12).
3.2. Sequence analysis and phylogenetic construction
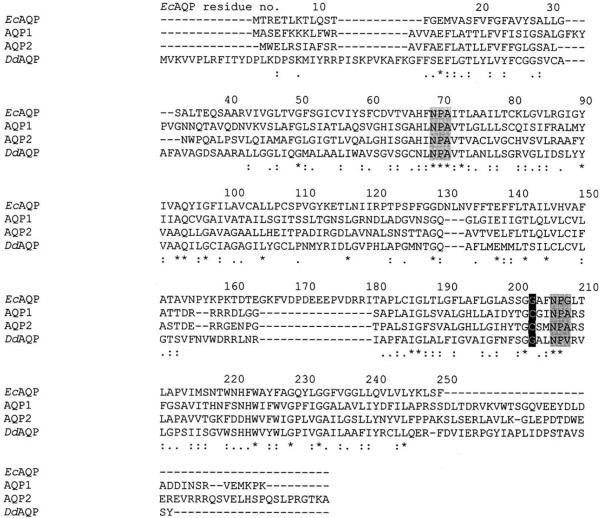
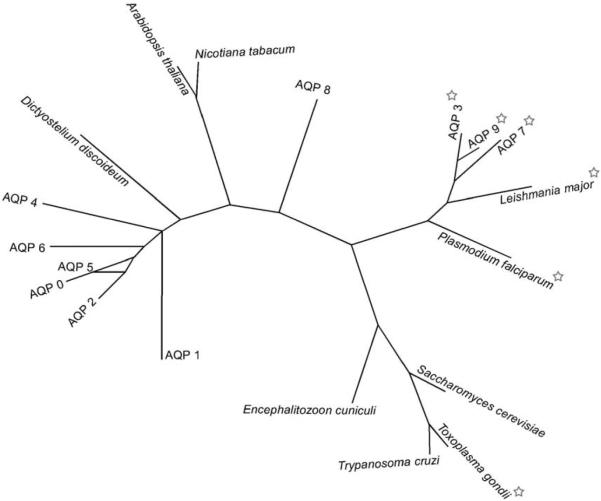
Kyle–Doolittle hydropathy analysis reveals EcAQP to be a highly hydrophobic protein, as would be expected for an aquaporin; six transmembrane segments are predicted (residues 13–37, 42–68, 79–107, 131–157, 180–208 and 222–250). Comparison of EcAQP with existing proteins in the databases using BLAST revealed AQP A of Dictyostelium discoideum to be the most similar (26% identity) among named, characterised proteins; among human aquaporins, AQP2 was the closest match (24% identity). In an unrooted phylogenetic construction of EcAQP, human AQPs 0–9, and AQPs from several parasitic protists, two plants, and a yeast (Fig. 4), EcAQP clusters neither with the orthodox AQPs, which conduct only water (AQPs 0, 1, 2, 4–6), nor with the water- and solute-conducting aquaglyceroporins (AQPs 3, 7 and 9). Instead it branches closely with two other protist AQPs, one of which is an orthodox AQP of Trypanosoma cruzi (Montalvetti et al., 2004) while the other is an aquaglyceroporin of Toxoplasma gondii (Pavlovic-Djuranovic et al., 2003), and with an orthodox AQP from the yeast Saccharomyces cerevisiae (Laize et al., 2000). Alignment of EcAQP with human AQP 1 (Fig. 4) demonstrates the presence within EcAQP of the two NPA motifs believed to line the AQP aqueous pore (Jung et al., 1994), and the absence of the cysteine residue aligned with C189 of AQP 1, which is believed to confer mercury-sensitivity to AQP 1 (Preston et al., 1993).
Fig. 4.
Alignment of EcAQP, human AQPs 1 (accession no. NP_932766), 2 (NP_000477), and AQP A of Dictyostelium discoideum (BAA85158). Highlighted in black is the residue position at which the presence of a cysteine confers mercury-sensitivity to AQP 1; gray highlights are the NPA motifs thought to line the water-conducting pore of each AQP monomer. Asterisks indicate fully conserved residues; two dots, conservation of strong groups; one dot, conservation of weak groups.
4. Discussion
Hundreds of AQPs or putative AQPs have been identified, from each of the three domains of life. Aquaporins are thought to exist natively as a homotetramer, with each 26–34 kDa monomer forming its own pore (Verkman and Mitra, 2000). The expected molecular weight of the EcAQP protein is approximately 26.8 kDa; thus it is within the range for an AQP monomer. An `hourglass model' has been posited for the shape of the monomer (Jung et al., 1994), in which six transmembrane domains surround the pore, formed in part by two NPA motifs. These characteristics are also predicted for EcAQP (Fig. 4). In addition, the BLAST similarity searches strongly suggest that EcAQP is a member of the AQP protein family.
The significantly increased permeability of EcAQP-injected Xenopus oocytes as compared to controls (Fig. 1, inset) provides further evidence that the AQP-like gene within the E. cuniculi genome (Katinka et al., 2001) is indeed an aquaporin. Aquaporins of selected other protistan parasites have measured Pf s of 32 (T. cruzi; (Montalvetti et al., 2004), 40 (T. gondii;(Pavlovic-Djuranovic et al., 2003), and 276 μm/s (Plasmodium falciparum;(Hansen et al., 2002). The EcAQP Pf (87 μm/s) is similar to those of human AQPs 3, 2, and 5 (80, 100 and 100 μm/s, respectively, (Yang and Verkman, 1997), which are considered to be in the high range for human aquaporins (King et al., 2004). However, because of differing levels of expression attributable to, among other factors, different amounts of cRNA injected by each investigator, and their varying translational and post-translational processing efficiencies, these comparisons are not strictly quantitative.
Aquaporins are divided into two phylogenetically and functionally distinct groups (reviewed in Heymann and Engel, 1999): the classic, or orthodox AQPs, which are permeable to water, and the aquaglyceroporins, which are also permeable to glycerol and other small solutes. Neither the presence nor absence of solute conductivity by EcAQP can be predicted based on the phylogeny in Fig. 3, as it clusters with neither group among the human AQPs, and branches closely with non-human AQPs of both types. However, its lack of solute conductivity, 3×10−4 d(V/V0)·s−1, not significant relative to water-injected controls, and approximately an order of magnitude lower than the reported swelling rates of ~2×10−3 d(V/V0)·s−1 for the T. gondii (Pavlovic-Djuranovic et al., 2003) and P. falciparum aquaglyceroporins (Hansen et al., 2002), is perhaps not surprising in light of the fact that EcAQP shares highest identity (24%) among the human AQPs with orthodox AQP 2. EcAQP also branches closely with a yeast AQP (S. cerevisiae AQP 2; (Laize et al., 2000) (Fig. 3), which is consistent with recent data on the fungal origins of the phylum Microsporidia (Thomarat et al., 2004).
Fig. 3.
An unrooted phylogenetic tree of aquaporins (AQP) including EcAQP. This is based on CTREE alignment of protein sequences of EcAQP (GenBank accession no. NP_586002), closest BLAST match AQP A of Dictyostelium discoideum (BAA85158), human AQPs 0-9 (NP_036196, NP_932766, NP_000477, NP_004916, P55087, NP_001642, Q13520, NP_001161, O94778, NP_066190, respectively), plant aquaporins of Arabidopsis thaliana (P25818) and Nicotiana tabacum (CAA69353), parasitic protist aquaporins of Leishmania major (AAS73184), Plasmodium falciparum (CAC88373), Toxoplasma gondii (CAE46485), Trypanosoma cruzi (AAM76680), and AQP 2 of the yeast Saccharomyces cerevisiae (AAD10058). Stars indicate aquaglyceroporins.
Mercury-inhibition of osmotic permeability is a hallmark of many aquaporins (Yang, 2000) and the observation that germination of spores of the microsporidian B. algerae was inhibited by treatment with mercury salts (Frixione et al., 1997) was interpreted as circumstantial evidence for microsporidian AQPs. It is also possible, however, that the inhibitory effect of mercury on germination observed by Frixione et al. (1997) is attributable to modification of other cysteine-containing micro-sporidian proteins. For example, Hayman et al. (2001) identified two Encephalitozoon intestinalis spore proteins with N-terminal cysteine-rich motifs, whose functions are as yet unknown. General cytotoxic effects of mercury may also be partially or wholly responsible for the observed inhibition of germination.
Pre-treatment of EcAQP-expressing oocytes with HgCl2 did not inhibit swelling. Nonetheless, this does not preclude the classification of EcAQP as an AQP, as several AQPs have documented mercury-insensitivity, e.g. the prototypical mercurial-insensitive AQP 4 (Yang et al., 1995). Cysteine residue 189 (C189), which is close in primary sequence to the second of the NPA motifs which have been postulated to line the aqueous pore (Jung et al., 1994), has been shown by site-directed mutagenesis to be the mercury-sensitive residue of AQP 1 (Preston et al., 1993) and site-directed mutagenesis to cysteine of any of four amino acids near the first NPA motif or one amino acid near the second NPA was found to confer mercury-sensitivity to AQP4 of Rattus norvegicus (Shi and Verkman, 1996). Alignment of EcAQP and AQP 1 amino acid sequences (Fig. 4) demonstrates that the EcAQP amino acid near the second NPA corresponding to the C189 of AQP 1 is glycine (i.e. EcAQP G203), as well as a lack of any cysteine residues in the immediate vicinity of the first NPA motif. The absence of these cysteines may explain the apparent mercury-insensitivity of EcAQP. Among human AQPs, the mercurial-insensitive AQP 4 is the second-closest match (22% identity) to EcAQP according to BLAST analysis. In the future, it may be interesting to examine whether mercury-sensitivity could be conferred upon EcAQP by site-directed mutagenesis of G203 to a cysteine residue.
In conclusion, we believe that the functional swelling assay in Xenopus oocytes and amino acid sequence analysis provide evidence that the putative AQP-like sequence identified in the E. cuniculi genome (Katinka et al., 2001) may indeed be considered an AQP. As the germination of microsporidian spores is believed to depend on the rapid influx of water (reviewed in Keohane and Weiss, 1999), it is hoped that further study of microsporidian AQPs and potential inhibitors of these proteins (e.g. gold and silver salts (Niemietz and Tyerman, 2002)) may yield novel therapeutic agents for human infections with these opportunistic pathogens.
Acknowledgements
This work was supported by grants from the National Institutes of Health (AI31788) and the Department of Defense (DAMD 17-02-1-0209).
References
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali A. General microsporidian features and recent findings on AIDS isolates. J. Protozool. 1991;38:625–630. [PubMed] [Google Scholar]
- Costa SF, Weiss LM. Drug treatment of microsporidiosis. Drug Resist. Updat. 2000;3:384–399. doi: 10.1054/drup.2000.0174. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Franzen C, Muller A. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 2001;3:389–400. doi: 10.1016/s1286-4579(01)01395-8. [DOI] [PubMed] [Google Scholar]
- Frixione E, Ruiz L, Cerbon J, Undeen AH. Germination of Nosema algerae (Microspora) spores-conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J. Eukaryot. Microbiol. 1997;44:109–116. doi: 10.1111/j.1550-7408.1997.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Garcia L. Laboratory identification of microsporidia. J. Clin. Microbiol. 2002;40:1892–1901. doi: 10.1128/JCM.40.6.1892-1901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kun JFJ, Schultz JE, Beitz E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. J. Biol. Chem. 2002;277:4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- Hayman JR, Hayes SF, Amon J, Nash TE. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect. Immun. 2001;69:7057–7066. doi: 10.1128/IAI.69.11.7057-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Engel A. Aquaporins: phylogeny, structure and physiology of water channels. News Physiol. Sci. 1999;14:187–194. doi: 10.1152/physiologyonline.1999.14.5.187. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Bleasby AJ, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput. Appl. Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Jung J, Preston G, Smith B, Guggino W, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- Katinka MD, Duprat S, Cornillot E, Metenier G, Thomarat F, Prensier G, Barbe V, Peyretaillade E, Brottier P, Wincker P, Delbac F, Alaoui HE, Peyret P, Saurin W, Gouy M, Weissenbach J, Vivares CP. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature. 2001;414:450–453. doi: 10.1038/35106579. [DOI] [PubMed] [Google Scholar]
- Keohane EM, Weiss LM. The structure, function, and composition of the microsporidian polar tube. In: Wittner M, editor. The Microsporidia and Microsporidiosis. ASM Press; Washington, DC: 1999. pp. 196–224. [Google Scholar]
- Keohane EM, Orr GA, Zhang HS, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol. Biochem. Parasitol. 1998;94:227–236. doi: 10.1016/s0166-6851(98)00071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Kyle J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laize V, Tacnet F, Ripoche P, Hohmann S. Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast. 2000;16:897–903. doi: 10.1002/1097-0061(200007)16:10<897::AID-YEA583>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian KC channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Mak D, Foskett J. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J. Biol. Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- Pavlovic-Djuranovic S, Schultz JE, Beitz E. A single aquaporin gene encodes a water/glycerol/urea facilitator in Toxoplasma gondii with similarity to plant tonoplast intrinsic proteins. FEBS Lett. 2003;555:500–504. doi: 10.1016/s0014-5793(03)01313-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. Rapid and sensitive sequence comparison with FASTP and FASTA. Meth. Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B, Argos P. Prediction of transmembrane segments in proteins utilising multiple sequence alignments. J. Mol. Biol. 1994;237:182–192. doi: 10.1006/jmbi.1994.1220. [DOI] [PubMed] [Google Scholar]
- Preston G, Jung J, Guggino W, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- Schreiber R, Pavenstadt H, Greger R, Kunzelmann K. Aquaporin 3 cloned from Xenopus laevis is regulated by the cystic fibrosis transmembrane conductance regulator. FEBS Lett. 2000;475:291–295. doi: 10.1016/s0014-5793(00)01689-6. [DOI] [PubMed] [Google Scholar]
- Shi LB, Verkman AS. Selected cysteine point mutations confer mercurial sensitivity to the mercurial-insensitive water channel MIWC/AQP-4. Biochemistry. 1996;35:534–538. doi: 10.1021/bi9520038. [DOI] [PubMed] [Google Scholar]
- Thomarat F, Vivares CP, Gouy M. Phylogenetic analysis of the complete genome sequence of Encephalitozoon cuniculi supports the fungal origin of Microsporidia and reveals a high frequency of fast-evolving genes. J. Mol. Evol. 2004;59:780–791. doi: 10.1007/s00239-004-2673-0. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994:22. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undeen AH, Epsky ND. In vitro and vivo germination of Nosema locustae (Microspora: Nosematidae) spores. J. Invertebr. Pathol. 1990;56:371–379. [Google Scholar]
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop. 2001;78:89–102. doi: 10.1016/s0001-706x(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Yang B. The human aquaporin gene family. Curr. Genomics. 2000;1:91–102. [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Yang B, Ma T, Verkman AS. cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. Evidence for distinct transcriptional units. J. Biol. Chem. 1995;270:22907–22913. doi: 10.1074/jbc.270.39.22907. [DOI] [PubMed] [Google Scholar]