Abstract
Background
Previous studies have reported that the adoption of a single-platform flow cytometry cell counting method resulted in lower interlaboratory variation in absolute T cell counts as compared to predicate dual-platform flow cytometry methods which incorporate independent automated lymphocyte counts (Schnizlein-Bick et al., Clin Diagn Lab Immunol 2000;7:336–343; Reimann et al., Clin Diagn Lab Immunol 2000;7:344–351). In the present study, we asked whether use of a single-platform method could reduce variation in absolute cell counts across the laboratories in the Multicenter AIDS Cohort Study (MACS) (n = 4), as suggested by the studies cited.
Methods
Identical study samples were shipped overnight to the MACS laboratories either by the National Institute of Allergy and Infectious Diseases, Division of AIDS Immunology Quality Assessment (NIAID-IQA) proficiency-testing program (n = 14), or by the Los Angeles site of the MACS (n = 10). For each sample, two tubes of blood were received; one was used for an automated complete blood count and differential, and the other for flow cytometry. The latter was performed using both our current dual-platform method (three-color CD45 gating and automated hematology) and the single-platform method (with TruCOUNT® beads to generate the absolute counts).
Results
The median percent coefficients of variation (%CVs) for the dual-platform and single-platform methods were 6.6 and 9.9, respectively, for CD4 T cell counts, and 5.9 and 8.5, respectively, for CD8 T cell counts (n = 24). These differences were not statistically significant. The differences in absolute T-cell counts between the MACS sites and the median of all laboratories participating in the NIAID-IQA were smaller for the dual-platform than for single-platform absolute count method.
Conclusion
In contrast to previous reports, we did not observe lower interlaboratory variation across the MACS sites for single-platform absolute lymphocyte subset counting relative to dual-platform methods. This result may be at least partly explained by the lower interlaboratory variation with the optimized dual-platform method in this study relative to the previous reports.
Key terms: HIV, absolute CD4 counts, flow cytometry, single platform, dual platform, interlaboratory variation
INTRODUCTION
The absolute CD4+ T-cell count is an important prognostic marker of HIV-1 disease progression and death and a key component of all guidelines on (i) when to initiate antiretroviral therapy (ART) and (ii) when to change therapy due to immunologic failure (1). For example, people who commenced ART with a CD4+ T-cell count <200 cells/mm3 were reported to be at higher risk of disease progression and death in the long term than those with higher baseline CD4+ T-cell counts (2,3).
A variety of flow cytometry-based methods for determining absolute cell counts exist. The dual-platform counting method multiplies the flow cytometry-derived CD4+ T-cell percentage by the absolute lymphocyte count derived from a hematology analyzer to calculate the CD4 absolute count. The single-platform method uses flow cytometry alone to calculate the absolute cell count from the ratio of stained cells to a standardized number of beads added to a known volume of blood. The beads, or fluorospheres, are either pre-positioned in the staining tube [e.g., TruCOUNT® beads (BD Biosciences, San Jose, CA)], or are added to the stained sample prior to analysis [e.g., Flow-Count beads (Beckman Coulter, Fullerton, CA.)]. In previous studies, use of the bead-based single-platform counting methods significantly reduced interlaboratory variation in absolute cell counts compared to predicate dual-platform methods (4,5). However, these studies used older methods based on lymphocyte light scatter gating (4,5) and either lyse-wash staining (4) or lyse-no wash staining (5), rather than lyse no-wash staining and CD45 gating, which we and others have shown to reduce interlaboratory variation in T-cell subset percents and absolute counts (4,6,7).
The purpose of the present study, therefore, was to determine whether the bead-based single-platform method could reduce the interlaboratory variation in absolute cell counts across the four laboratories of the Multicenter AIDS Cohort Study (MACS) relative to the optimized dual-platform method (CD45-gating and lyse no-wash staining), as currently used in the MACS laboratories. To facilitate this analysis, the present study utilized the data from a single tube prepared with the CD45-gated, lyse-no-wash method to generate both the dual- and single-platform absolute counts for each sample. The TruCOUNT® bead method was selected for this comparison because it uses the same three-color lyse-no wash reagents as our current dual-platform method.
MATERIALS AND METHODS
Blood Specimens
Samples of whole blood were collected in ethylenediamine tetra-acetic acid (EDTA) by the NIAID-IQA (n = 14) and shipped overnight to the laboratories of the MACS, located in Baltimore, Chicago, Los Angeles and Pittsburgh. To increase the sample size for more reliable estimation of the interlaboratory CVs, additional samples (n = 10) were sent similarly by the Los Angeles site of the MACS. Samples did not include a stabilizing reagent. All samples shipped from the IQA were HIV-1 infected, as were five of the 10 samples shipped from the Los Angeles site. Each of the 24 blood samples was analyzed in all laboratories to permit interlaboratory comparison. The samples were received approximately 24 h after phlebotomy, two tubes per sample. Flow cytometry was performed immediately on one tube, and the other was immediately sent to the commercial laboratory (Quest Diagnostics) for complete blood count and differential. Thus, hematology analyses were performed approximately 30–36 h after blood draw.
In a confirmatory study to further increase the number of samples analyzed for interlaboratory variability using the dual-platform method, especially for specimens with <250 CD4+ T-cells/mm3, an additional 28 unique samples shipped by the IQA were analyzed for absolute T-cell counts and lymphocyte percentages. For IQA shipments which included replicate samples, only the data from the first replicate was included in this analysis.
IRB approval was in place for collection and analysis of peripheral blood samples at the IQA and the MACS laboratories.
Instrumentation
Flow cytometry was performed with a FACSCalibur and Cellquest software (BDIS, San Jose, CA) at MACS sites 1 and 2, and with an EPICS XL flow cytometer using System 2 software (Beckman Coulter, Hialeah, FL) at sites 3 and 4.
Hematologic analyses were performed with a Coulter STKS at sites 1, 2, and 3 and a Coulter Gen-S at site 4.
Three-Color Flow Cytometry
All specimens were stained with Tritest reagents (BD Biosciences, San Jose, CA) in TruCOUNT® tubes using a lyse no-wash sample preparation method and CD45 lymphocyte gating. Specifically, 50 μl of whole blood was stained with monoclonal antibodies, followed by lysis of red blood cells and no washing, according to the manufacturer’s instructions. The monoclonal antibodies were conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or peridinin chlorophyll protein (PerCP). The CD4+ and CD8+ T-cell subset percentages were obtained from samples stained with the following two-tube antibody combinations: CD3-FITC, CD4-PE, and CD45 PerCP, and CD3-FITC, CD8-PE, and CD45 PerCP, respectively. Samples were run on the flow cytometer after the staining. For analysis of lymphocytes, appropriate low right angle light scatter and CD45bright events were gated. The lymphocyte events were subsequently analyzed on plots of lymphocytes expressing CD3 versus CD4 and CD3 versus CD8 to determine the percentages of CD3+ CD4+ and CD3+ CD8+ lymphocytes, the event counts of which were used for calculation of the absolute CD4+ and CD8+ T-cell counts, respectively. An ungated dot plot of the FL1 parameter (FITC) versus the FL2 parameter (PE) was used to capture the total number of acquired TruCOUNT® bead events. For the single-platform analysis, absolute counts were calculated as:
For the calculation of absolute lymphocyte subset counts per μl of whole blood by the dual-platform method, the percentage of CD4+ or CD8+ T-cells derived by flow cytometry was multiplied by the absolute lymphocyte count derived from the automated hematology analyzer (calculated as the product of the white blood cell count (WBC) and the lymphocyte percentage). The dual-platform confirmatory study utilized the same methods as described above except that the specimens were stained with Tritest reagents in tubes that did not contain TRUCount® beads.
Flow Cytometry Quality Assurance
All four MACS laboratories have participated in the NIAID IQA proficiency testing program since its inception and have remained certified during this time. All are certified for the three-color CD45 gated, lyse-no-wash methodology. From 2006 through 2008, for CD4+ and CD8+ T-cell percentage measurements the laboratories had zero “bad” values out of 144 possible replicate values for intralaboratory variabilitiy, and only one “bad” interlaboratory value out of 432 possible values, according to IQA acceptability standards of ≤3% variation across replicates (for intralaboratory performance) and <5% difference from the consensus median value (for interlaboratory performance). For absolute count performance there were of 13 of 144 “bad” intralaboratory values and four of 432 “bad” interlaboratory values during this same time period. Acceptable intralaboratory absolute count ranges are within 75 cells/mm3 for CD4 and within 150 cells/mm3 for CD8 (maximum − minimum). Acceptable interlaboratory absolute counts are within 100 or 200 cells/mm3 for CD4 and CD8, respectively, from the IQA median absolute counts.
Other three-color quality assurance procedures used by the MACS laboratories include, but are not limited to, verifying (i) lymphocyte recoveries of ≥95% by backgating (8,9), (ii) lymphocyte purity of ≥98% by inclusion of ≤2.0% CD3-CD4dim monocytes, (iii) 3% or less variation in CD3% across tubes measuring this phenotype, (iv) difference of 5% or less between the CD3% and sum of CD3+CD4+% and CD3+CD8+% (the check-sum difference) (8), and (v) confirmation of any CD3% result <55%. Specimens yielding unusual results are restained to confirm the original result, or stained with additional antibodies to determine the cause for the unusual result, e.g., analysis of proportions of CD3+CD4−CD8− or CD3+CD4+CD8+ lymphocytes if the check-sum difference is elevated, or enumerating B and NK cell percentages if the CD3% is <55%. In addition, the total of the T-cell, B-cell, and NK-cell percentages should be ≥95% (8). Other atypical staining patterns are repeated or further analyzed, such as identification of elevated levels of dim CD45+ lymphocytes (typically CD19+ B-cells) or prewashing the whole blood to remove serum factors that cause artifactual staining (10).
Statistical Analysis
Interlaboratory Variation, Dual- vs. Single-Platform
For assessment of the variations in dual- and single-platform absolute counts across sites, we report the median percent coefficient of variation (median %CV). The CV was calculated for each sample across sites and the median % CV for all samples was derived. The Wilcoxon Sign Rank test (SYSTAT Software, Richmond, CA) was used to test significance of differences in median %CV between dual- and single-platform variation across sites. Data from the 14 unique samples shipped by the IQA and the 10 unique samples shipped by the Los Angeles MACS site were used for this analysis.
Analysis of Contributors to Dual-Platform Variation, T-cell Percentage vs. Lymphocyte Count
We computed the median %CV across sites for the percentage of CD4+ and CD8+ T-cells and absolute lymphocyte counts, to determine their relative contribution to the overall variation in absolute CD4+ and CD8+ T-cell counts.
Analysis of Differences Between Single- and Dual-Platform Absolute Counts, MACS Laboratories vs. IQA and Within MACS Laboratories
For each of the 14 IQA samples, we calculated the difference between the absolute counts obtained by each method at each of the MACS laboratories and the median of the results reported to the IQA program from all laboratories participating in the IQA for each specimen. Differences were calculated as the MACS value minus the IQA median and plotted in Figure 1. Statistical difference between the T cell counts for each MACS site and the IQA median were assessed by the Wilcoxon Sign Rank Test. Single- and dual-platform median absolute counts and interquartile ranges were calculated for each MACS site and presented in Table 3 (n = 24). The Wilcoxon Sign Rank Test was used to determine whether the single-platform counts were statistically different from the dual-platform counts within each MACS site, using all 24 unique shipped samples.
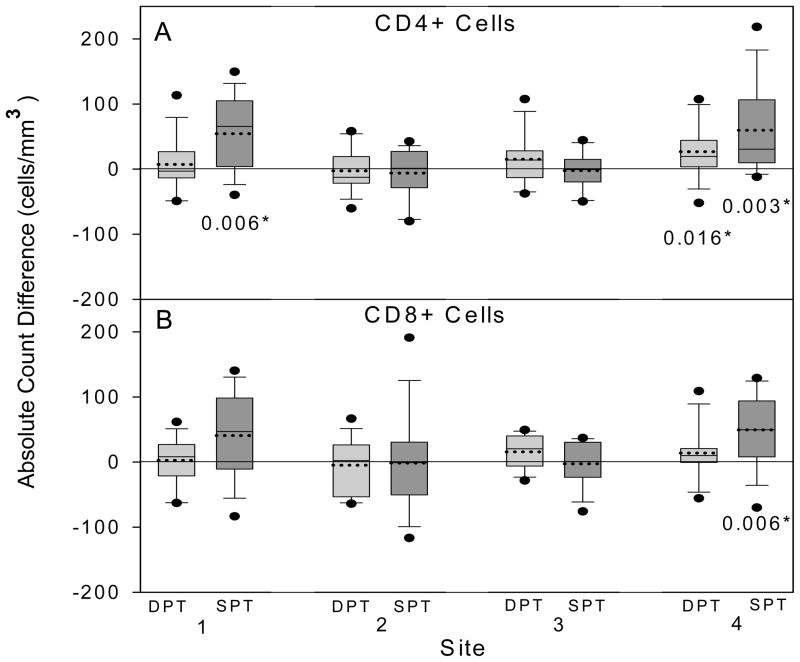
Fig. 1.
Box-plots of differences between the absolute cell counts obtained at the four MACS sites and the median absolute cell counts obtained by the IQA program (calculated as MACS result minus IQA median). For each MACS site, data were obtained by the dual-platform (DPT) (light shading) and single-platform (SPT) (dark shading) methods, and are shown for CD4+ (A) and CD8+ (B) cells. The lower and upper bounds of each box represent the 25th and 75th percentiles of the data for 14 unique samples, respectively. The horizontal lines within each box represent the mean (dashed line) and the median (solid line) of the distribution; lower and upper whiskers represent the 10th and 90th percentiles, and circles represent data points outside the 10th and 90th percentiles. P values for significant (P < 0.05) differences between a site’s absolute counts and the median absolute counts obtained by the IQA are shown on the figure (Wilcoxon sign rank test).
Table 3.
Comparison of Absolute T Cell Counts (cells/mm3) Determined by Dual- and Single-Platform Methods, Across MACS Sites (n= 24)
| Site | CD4 subset |
Pa | CD8 subset |
Pa | ||
|---|---|---|---|---|---|---|
| Dual-platform | Single-platform | Dual-platform | Single-platform | |||
| 1 | 485 (235–809)b | 513 (244–852)b | 0.022 | 634 (445–863)b | 653 (444–904)b | 0.092 |
| 2 | 506 (224–743) | 505 (247–786) | 0.648 | 623 (420–807) | 581 (396–850) | 0.607 |
| 3 | 490 (250–768) | 480 (219–770) | 0.022 | 595 (452–849) | 573 (426–824) | 0.010 |
| 4 | 522 (240–760) | 547 (255–925) | 0.007 | 657 (402–815) | 700 (500–939) | 0.004 |
Wilcoxon sign rank test.
Median (interquartile range).
Confirmatory Study of Dual-Platform Variation
In order to evaluate the consistency of interlaboratory variability using the dual-platform method, an additional 28 unique IQA samples shipped to each of the MACS laboratories were analyzed as described above. CVs and median % CVs were determined as described above and presented in Table 4.
Table 4.
Interlabortory Variation in Absolute T Cell Counts Measured by Dual-Platform Methods Across the Four MACS Sites (Confirmatory Study)
| Subset | CD4 stratum (cells/mm3) | n | Cell count (cells/mm3) | %CV dual-platform |
|---|---|---|---|---|
| CD4 | All | 28 | 237 (181–467)a | 6.3 (5.0–8.7)a |
| <250 | 14 | 181 (176–205) | 7.6 (5.2–9.6) | |
| <400 | 18 | 187 (178–225) | 6.7 (5.1–9.6) | |
| >400 | 10 | 471 (466–565) | 6.3 (5.0–6.6) | |
| CD8 | All | 28 | 707 (561–911) | 5.3 (3.7–6.9) |
| <250 | 14 | 645 (522–709) | 6.0 (4.0–6.9) | |
| <400 | 18 | 665 (425–718) | 5.8 (4.0–6.9) | |
| >400 | 10 | 921 (933–1020) | 4.2 (1.7–7.0) |
Median (interquartile range).
RESULTS
Interlaboratory Variation, Dual- vs. Single-Platform
Table 1 summarizes the comparison of interlaboratory variation in absolute CD4+ and CD8+ T-cell counts using the single- and dual-platform methods across the four MACS sites. The CVs for samples with <400 and > 400 CD4+ T-cells/mm3 were both lower for the dual-platform method than for the single-platform method. For samples with CD4+ T-cell counts below 250/mm3, the single-platform CVs were slightly lower for both CD4+ T-cell counts and for CD8+ T-cell counts. Variation was always lower for CD8+ T-cell counts than for CD4+ T-cell counts. Differences in CVs between the two platforms were not statistically significant.
Table 1.
Interlaboratory Variation in Absolute T Cell Counts Measured by Dual- and Single-Platform Methods Across the Four MACS Sites
| Subset | CD4 Stratum (cells/mm3) | n | Cell count (cells/mm3) | %CV |
Pa | |
|---|---|---|---|---|---|---|
| Dual-Platform | Single-platform | |||||
| CD4 | All | 24 | 499 (234–767)b | 6.6 (4.8–10.9)b | 9.9 (8.1–11.4)b | 0.424 |
| <250 | 6 | 164 (143–204) | 9.7 (4.8–11.3) | 9.0 (6.3–12.4) | 0.753 | |
| <400 | 9 | 204 (158–282) | 8.9 (4.4–12.4) | 9.2 (7.7–11.0) | 0.594 | |
| >400 | 15 | 763 (505–816) | 6.3 (5.1–9.3) | 10.6 (8.2–11.5) | 0.363 | |
| CD8 | All | 24 | 622 (430–834) | 5.9 (3.3–10.8) | 8.5 (6.5–10.1) | 0.230 |
| <250 | 6 | 534 (410–930) | 6.8 (3.7–9.3) | 6.7 (5.4–9.3) | 0.753 | |
| <400 | 9 | 583 (392–948) | 6.3 (3.3–11.7) | 6.7 (4.8–9.3) | 0.953 | |
| >400 | 15 | 662 (473–814) | 5.9 (3.3–11.0) | 9.1 (7.4–12.8) | 0.078 | |
Wilcoxon sign rank test.
Median (interquartile range).
Relative Contributions of T-Cell Percentage and Lymphocyte Count to Dual-Platform Interlaboratory Variation
To assess the relative contribution of percentage of CD4 or CD8 T-cells and the hematology-derived absolute lymphocyte count to the overall dual-platform variation, we calculated the interlaboratory variations for each of these variables. As shown in Table 2, the median %CVs for the CD4 and CD8 percentages for all samples were 2.8 and 2.0%, respectively, while that for the absolute lymphocyte count was 4.9%. Thus, the largest contributor to variation in absolute lymphocyte subset counts was the automated hematology instrumentation (i.e., the absolute lymphocyte count). Variation in CD4 percentage and in absolute lymphocyte count was slightly higher for samples with < 400 CD4+ cells/mm3 compared to those with >400 CD4+ cells/mm3.
Table 2.
Interlaboratory Variation in Dual-Platform Absolute Lymphocyte Count, Percent CD4+ T-Cells, and Percent CD8+ T-Cells Across MACS Sites
| CD4 stratuma | n | Absolute lymphocyte counta | %CV |
||
|---|---|---|---|---|---|
| Absolute Lymphocyte Count | CD4 percent | CD8 percent | |||
| All | 24 | 1577 (1239–2014)b | 4.9 (3.9–10.6)b | 2.8 (1.9–3.7)b | 2.0 (1.6–3.1)b |
| <400 | 9 | 1196 (901–1573) | 5.5 (4.3–10.4) | 3.7 (3.4–6.6) | 1.8 (1.1–4.0) |
| >400 | 15 | 1839 (1342–2144) | 4.7 (3.8–10.9) | 2.4 (1.5–2.9) | 2.0 (1.9–3.2) |
cells/mm3.
Median (interquartile range).
Differences Between Single- and Dual-Platform Absolute Counts, MACS vs. IQA
To assess the accuracy of the absolute cell counts obtained by the single- and dual-platform methods at the MACS sites, we calculated differences between the counts obtained at each MACS site and the median absolute cell counts of the laboratories that analyzed the same specimens in the IQA (36–75 laboratories). Figure 1 summarizes the distributions of these differences, for both CD4+ and CD8+ T-cell subsets. Overall, the absolute counts derived by the dual-platform methods were closer to the IQA median counts than those derived by the single-platform. CD4+ cell count differences from IQA medians were lower for dual-platform than single-platform at sites 1 and 4, and low with both methods at sites 2 and 3; differences were significant at sites 1 and 4 for the single-platform method and at site 4 for the dual-platform method. For CD8+ T-cell counts, the pattern of differences was similar, and the only one that was significant was for the single-platform counts at Site 4.
Comparison of Absolute Counts at each MACS Site, Dual- vs. Single-Platform
Figure 1 also shows the within-laboratory bias in absolute counts between the methods. Sites 1 and 4 showed higher CD4+ and CD8+ cell counts for single-platform than for dual-platform, a finding reported in other studies (11,12).
To better define the bias between the dual- and single-platform methods for the individual laboratories, we also analyzed data from all 24 samples. Table 3 shows that CD4+ T-cell counts were slightly higher for single-platform than for dual-platform at sites 1 and 4, and slightly lower at site 3. A similar trend was seen for CD8+ T-cells, with a large difference at site 4. At site 2, there were no significant differences between single- and dual-platform results for either CD4+ or CD8+ T-cells.
Confirmatory Study of Dual-Platform Interlaboratory Variation
The consistency of the dual-platform variation across the four MACS sites was assessed by measuring the median %CV on 28 additional unique IQA shipped samples. The results are shown in Table 4. They compare very favorably with those derived from the initial 24 samples analyzed, as shown in Table 1, thus demonstrating the stability over time of the low interlaboratory variability with the dual-platform method.
DISCUSSION
The present study differs from previous reports (4,5) in that enumeration of absolute T-cell subset counts by the dual-platform method yielded interlaboratory variation similar to, if not better than, that observed with the single-platform method. In addition, our dual-platform absolute counts were closer than the single-platform absolute counts to the median absolute counts of the IQA laboratories. The IQA median count represented a consensus of 36–75 reporting laboratories and provides a good reference value for measuring individual laboratory performance on shared, shipped samples. On the basis of the data obtained, adopting the single-platform methods in the MACS would result in a slight shift in CD4+ T-cell absolute counts at three of the four sites with no significant improvement in interlaboratory variability.
The different conclusion in the present study is primarily due to the fact that the inter-laboratory variability with the dual-platform method was substantially smaller in the present study than in the previous studies that compared dual- and single-platform methods. Specifically, in the studies by Schnizlein-Bick et al (4) and Reimann et al. (5), dual-platform variation ranged from 16 to 18.2 median %CV for CD4+ T-cell counts and 11 to 16.9 median %CV for CD8+ T-cell counts, while the corresponding values in the present study were 6.6% for CD4+ T-cells and 5.9% for CD8+ T-cells. Because dual-platform variation depends on the variation in both the T-cell subset percentages and the absolute lymphocyte count, we considered these variables in detail. The median interlaboratory variation in CD4 percent in the present study was 2.8 %CV, much lower than the 9 %CV reported for the dual-platform method by Schnizlein-Bick et al.
This discrepancy may be explained by several factors. The most important was probably the preparation of samples by the newer lyse-no wash, CD45-gating method, as opposed to the older lyse-wash, light scatter-gating method used in whole or in part in the previous comparative studies (4,5). The MACS laboratories previously observed a similar reduction in median interlaboratory CV for both CD4 and CD8 T-cell percentages when they switched to the lyse-no wash, CD45 gating method, with the median interlaboratory %CV for CD4+ T-cell percent decreasing from 7.0 to 3.9 and that for CD8+ T-cell percent from 4.6 to 2.6. The lyse-no wash, CD45 gating method yields improved results for a variety of technical reasons. It avoids centrifugation, which causes not only cell loss but also the formation of cellular aggregates which “escape” from the lymphocyte scatter gate because of their increased light scatter (13). Reducing aggregate formation results in higher lymphocyte recoveries compared to older methods. Our data are consistent with a previous report in which laboratories that used the lyse-no-wash dual-platform method had lower interlaboratory variation on a single shared sample than laboratories using lyse-wash dual-platform methods (14). In addition, the CD45 gate was optimized to attain lymphocyte purities of ≥98%, as opposed to the 85% required by the 1993 NIAID immunophenotyping guidelines for light scatter gating (15). This improvement in gate purity is a result of exclusion of red blood cell contamination of the lymphocyte gate. The lymphocyte gate recovery for CD45 gating was ≥95%, while the guidelines for light scatter gating required only 90%. Lymphocyte recovery is also improved because the CD45-side scatter gate leads to formation of a more distinct lymphocyte cluster that is resolved completely from the contaminating red blood cell debris compared to scatter gating and thus leads to a more inclusive and objective lymphocyte gate.
It is also possible that the extensive flow cytometry experience and long history of certification by the IQA program of the MACS laboratories may have contributed to our different results in undefined ways, but these are not factors that can be compared to the previous studies.
With respect to the hematology-derived absolute lymphocyte count, the four MACS laboratories in the present study used similar hematology instrumentation to derive this measurement. This may have reduced interlaboratory variation in absolute lymphocyte counts, because it is possible that different hematology analyzers may have bias toward higher or lower lymphocyte counts (4). Data are not available to compare our variation in absolute lymphocyte counts with those of the previous comparisons of single- and dual-platforms.
Although we did not study the effect of sample degradation on variation in the absolute count, one would expect that if sample degradation affected the dual-platform method more than the single-platform method, higher variation would have been observed with the dual-platform method, which is the opposite of what we observed. Thus, it is unlikely that the present results were biased by effects of the shipping process on sample integrity. Similarly, although we did not evaluate the relative effects of single- and dual-platform methods on intralaboratory variation, lower within-lab variation for single-platform compared to dual-platform method could not account for the findings of the present study because lower intralaboratory variation for the single-platform method would lead to higher interlaboratory variation for the dual platform method, which we did not find.
A potential alternative explanation for the improved performance of the dual-platform method relative to the single-platform method in this study might be a lack of experience with the single-platform method. For example, variation in pipetting could have spuriously increased the single-platform variation across sites. However, this explanation is unlikely, because the median %CVs of MACS laboratories using the single-platform method, namely 9.9% for the absolute CD4+ T-cell cell count and 8.5% for the absolute CD8+ T-cell count, were almost identical to those in the two previous comparative studies: 9–10.8% and 7–10.1%, respectively (4,5).
The agreement with the IQA median absolute cell counts was better with the dual-platform method than with the single-platform method. This could reflect better performance of the dual-platform method, or it could be at least partially explained by the fact that the majority (~70%) of the IQA laboratories used the dual-platform method to measure absolute counts. Data to distinguish between these two possibilities are not available.
The results of this study, including the dual-platform confirmatory study performed on 28 additional IQA samples, suggest that if hematology laboratories achieve comparable results and flow cytometry is well performed, then the dual-platform and single-platform methods can achieve comparable interlaboratory variations, even for samples with CD4 T cell counts below 250/mm3. However, the present report should not be taken as a recommendation that laboratories or studies using single-platform methods switch to the dual-platform method used in this study. It should also be mentioned that participation in a proficiency testing program such as the NIAID IQA program, not only makes it easier to monitor whether good immunophenotyping is being performed throughout a study, but also permits comparison of the absolute counts from the individual laboratories of a multicenter study with those of the proficiency testing program and thus tests whether the individual labs are performing similarly.
Acknowledgments
Grant sponsors: National Institutes of Health (IQA Program), NIAID, MACS, National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), Office of AIDS Research, HIV Prevention Trials Network; Grant sponsor: DAIDS; Grant number: N01-AI 95356; Grant sponsor: National Cancer Institute; the National Heart, Lung and Blood Institute; Grant numbers: UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041; Grant sponsor: DHHS; Grant number: U01-AI-068613.
The authors thank Patricia Hultin, Natysha Tucker, Edwin Molina, Carlos Nahas, Tricia Nilles, and Kim Stojka for their contributions in the MACS flow cytometry laboratories; and Mr. Raul Louzao for management of the IQA project and for data review and technical assistance to MACS flow cytometry units.
LITERATURE CITED
- 1.O’Gorman MRG, Zijenah LS. CD4 T cell measurements in the management of antiretroviral therapy—A review with an emphasis on pediatric HIV-infected patients. Cytometry Part B. 2008;74B (Suppl 1):S19–S26. doi: 10.1002/cyto.b.20398. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D’Armino Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy; a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 3.Anastos K, Barrón Y, Miotti P, Weiser B, Young M, Hessol N, Greenblatt RM, Cohen M, Augenbraun M, Levine A, Munos A. Risk of progression to AIDS and death in women infected with HIV-1 initiating highly active antiretroviral treatment at different stages of disease. Arch Intern Med. 2002;162:1973–1980. doi: 10.1001/archinte.162.17.1973. [DOI] [PubMed] [Google Scholar]
- 4.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JKA, O’Gorman MRG Site Investigators, and The NIAID DAIDS New Technologies Evaluation Group. Evaluation of TruCOUNT absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus- positive adults. Clin Diagn Lab Immunol. 2000;7:336–343. doi: 10.1128/cdli.7.3.336-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimann KA, O’Gorman MRG, Spritzler J, Wilkening C, Sabath DE, Helm K, Campbell DE The NIAID DAIDS New Technologies Evaluation Group. Multisite comparison of CD4 and CD8 T-lymphocyte counting by single- versus multiple-platform methodologies: Evaluation of Beckman coulter flow-count fluorospheres and the tetraONE system. Clin Diagn Lab Immunol. 2000;7:344–351. doi: 10.1128/cdli.7.3.344-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultin LE, Menendez FA, Hultin PM, Jamieson BD, O’Gorman MRG, Borowski L, Matud JL, Denny TN, Margolick JB. Assessing immunophenotyping performance: Proficiency-validation for adopting improved flow cytometry methods. Cytometry Part B (Clinical Cytometry) 2007;72B:249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelman R, Wilkening C. Analyses of quality assessment studies using CD45 for gating lymphocytes for CD3+CD4+% Cytometry (Communications in Clinical Cytometry) 2000;42:1–4. doi: 10.1002/(sici)1097-0320(20000215)42:1<1::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Schenker EL, Hultin LE, Bauer KD, Ferbas J, Margolick JB, Giorgi JV. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14:307–317. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 9.Hultin LE, Hultin P. Flow cytometry based immunophenotyping method and applications. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. 7. Washington, DC: ASM Press; 2006. pp. 147–157. [Google Scholar]
- 10.Nicholson JKA, Rao PE, Calvelli T, Stetler-Stevenson M, Browning SW, Marti GE. Artifactual staining of monoclonal antibodies in two-color combinations is due to an immunoglogullin in serum and plasma. Cytometry. 1994;18:140–146. doi: 10.1002/cyto.990180305. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson JKA, Stein D, Mui T, Mack R, Hubbard M, Denny T. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin Diagn Lab Immunol. 1997;4:309–313. doi: 10.1128/cdli.4.3.309-313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohler T, Au von M, Klose N, Muller K, Coulibaly B, Nauwelaers F, Spengler HP, Kynast-Wolf G, Krausslich HG. Evaluation of a simplified dual-platform flow cytometric method for measurement of lymphocyte subsets and T-cell maturation phenotypes in the population of Nouna, Burkina Faso. Clin Vaccine Immunol. 2007;14:775–781. doi: 10.1128/CVI.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson A. Basic phenotyping of lymphocytes: Selection and testing of reagents and interpretation of data. Clin Immunol Newslett. 1990;10:43–55. [Google Scholar]
- 14.Gratama JW, Kraan J, Keeney M, Granger V, Barnett D. Reduction of variation in T-cell subset enumeration among 55 laboratories using single-platform, three or four-color flow cytometry based on CD45 and SSC-based gating of lymphocytes. Cytometry (Clinical Cytometry) 2002;50:92–101. doi: 10.1002/cyto.10084. [DOI] [PubMed] [Google Scholar]
- 15.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: A report from the National Institute of Allergy and Infectious Diseaes, Division of Aids. Cytometry. 1993;14:702–714. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]