Our laboratory has been interested in the patterns of synaptic plasticity after cortical stroke in animal models.1 These protein expression studies suggest that regions both ipsilateral and contralateral to the stroke are involved during the recovery period. An emerging issue of interest is whether the recovered function is indeed normal or reflects some compensatory function.2 One approach to resolving these questions is to assess different patterns of functional recovery in patients who do and do not recover function with and without therapy and compare them to normal patterns.
Investigation of the molecular mechanisms of brain plasticity after injury suggests that functional activity stimulates this recovery and guides the development of functional pathways.3,4 Several investigators postulate that recovery from stroke resembles “use-dependent” learning.5 For this report, we review pertinent literature related to this phenomenon, assess how recovery of language resembles normal language function, and provide examples from our own research on functional imaging that assess patterns of activation related to recovery. The primary treatment modality that we review is constraint-induced language therapy (CILT), in which stroke patients are restricted to using verbal communication.6
CILT is based on principles of constraint-induced therapy for other conditions.6 CILT is performed in chronic stroke patients with moderate aphasia by a visual physical barrier between participants and provided tasks they must communicate. Control subjects are allowed to use all forms of communication. In a recent report, the ability to communicate improved in both CILT and “control” aphasic subjects, but CILT-trained subjects used words more often whereas “control” subjects used more gestures and fewer words.7 This finding is particularly interesting because gestures are an important part of normal language development. Gestures enhance the use of words and facilitate communication, postulated to occur by a mechanism termed reduction in “cognitive load.”8,9 It is therefore interesting that in the case of the recovering stroke patient, gestures inhibit the use of words. This difference contrasts in an important way from earlier language development.
The evidence that the recovered language function reflects “use-dependent” learning is quite strong. One reflection of this phenomenon is whether training subjects to name objects or other types of word training is generalizable, meaning does the stroke patient learn only what they are taught or are they able to learn a wider communication scheme. Raymer et al show that intensive training for either nouns or verbs increased the use of words that were taught, but there was little if any spill over to untrained words.10 The frequent observation of lack of generalizability11 is also consistent with the concept that recovering language function differs from normal language development where exposure to conversation is an essential part of learning that generalizes to overall communication.
These features of communication after aphasic stroke led us to hypothesize that recovery of language function resembles learning a new language, where the rules, phonemes (meaningful sounds), syntax, and grammar need to be taught along with vocabulary. Of interest, there is a growing literature on the functional and structural substrates of learning a foreign language. There is evidence that the volume of left hemisphere Heschl gyrus, a subset of the primary auditory cortex, is positively related to ability to learn a new language.12 Functional MRI (fMRI) studies demonstrated that subjects who successfully learned a new language showed increased activation in the left posterior superior temporal region (plus right hemisphere activation) after training, whereas subjects performing at a lower level demonstrated increased activation in the right superior temporal region and right inferior frontal gyrus.13
We have been exploring patterns of brain activation using magnetoencephalography (MEG) in aphasic stroke patients undergoing CILT.14 MEG is a noninvasive functional imaging method which allows for the construction of brain activation maps of extremely high resolution on the basis of measurement of the magnetic fields generated during sensory, motor and cognitive tasks, and recorded by magnetic flux sensors on the surface of the head.15 Our initial experience with CILT in a cohort of 16 subjects demonstrated that half responded to CILT based on improvement in a verbal task. MEG was performed during a word recognition task for spoken works where the subject was taught a series of target words and asked to recognize these words from a larger group of distractors. Two blocks of trials were averaged and the event related fields time-locked to the words recorded using a whole head neuromagnetometer (4D 3600, 4D NeuroImaging). Source locations were coregistered on T1-weighted MRI obtained from the subject. The Figure demonstrates activation surrounding the injured left cortex time locked to 5 epochs of a 1000-ms recording. Comparing the subjects who improved in language ability after CILT to those who did not, the responders were characterized by a right hemisphere laterality of activation pre-CILT which then became bilaterally represented. This is in contrast to bilateral activation in both pre- and post-CILT in the nonresponders without subsequent additional left hemisphere activation after training.
Figure.
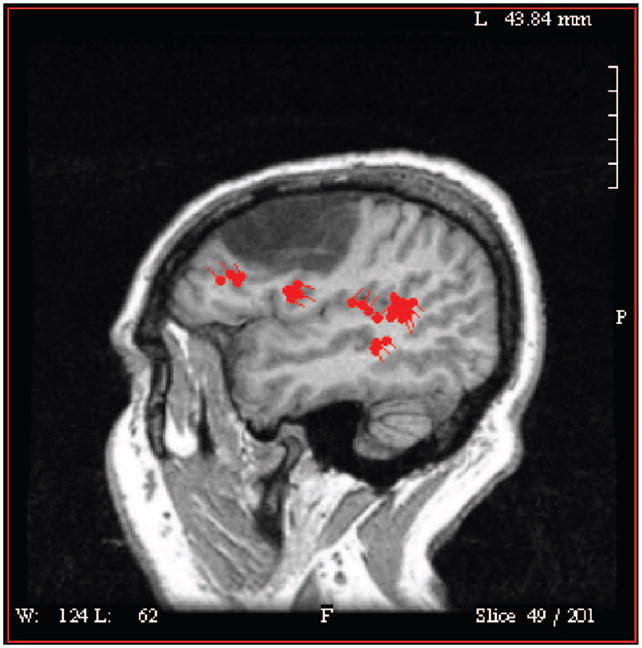
This figure represents the regions of activation (red areas) during a word recognition test using MEG localization in a subject who suffered a left hemisphere stroke (hypodense region) and resulting aphasia. Both the primary auditory cortex and regions anterior and medial to the infarction show activation demonstrating plasticity of the brain response.
We conclude that use-dependent learning is a strong feature of language recovery after intensive training with characteristics that resemble the process of learning a new language. Functional imaging indicates that in increase in engagement of left hemisphere was important in recovery, similar to those that successfully learned a new language in fMRI studies in healthy controls. There are both mechanistic and treatment implications of this research. For example, it is possible that an important feature of learning both in aphasia and in a foreign language should involve emphasis on the phenomic structure of the language of interest. Preliminary results in a small cohort of aphasics given a phoneme-based treatment paradigm found suggestions of beneficial treatment effect, generalization and persistence.11 It is our intention to pursue these findings using both functional and structural imaging.
Acknowledgments
Sources of Funding
This work was supported by NIH/NINDS grant #P51-NS046588 to A.C. Papanicolaou.
Footnotes
Disclosures
None.
References
- 1.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 2.Metz GA, Antonow-Schlorke I, Otto WW. Motor improvements after focal cortical ischemia in adult rats are mediated by compensatory mechanisms. Behav Brain Res. 2005;162:71–82. doi: 10.1016/j.bbr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 4.Ivanco TL, Greenough WT. Physiological consequences of morphologically detectable synaptic plasticity: potential uses for examining recovery following damage. Neuropharmacology. 2000;39:765–767. doi: 10.1016/s0028-3908(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 5.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulvermüller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub T. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 7.Maher LM, Kendall D, Swearengin JA, Rodrigues A, Leon SA, Pingel K, Holland A, Rothi LJ. A pilot study of use-dependent learning in the context of Constraint Induced Language Therapy. J Int Neuropsychol Soc. 2006;12:843–852. doi: 10.1017/S1355617706061029. [DOI] [PubMed] [Google Scholar]
- 8.Roth WM. Gestures: Their role in teaching and learning. Rev Ed Res. 2001;71:365–392. [Google Scholar]
- 9.Moresella E, Krauss RM. The role of gestures in spatial working memory and speech. Am J Psychol. 2004;117:411–424. [PubMed] [Google Scholar]
- 10.Raymer AM, Ciampitti M, Holliway B, Singletary F, Blonder LX, Ketterson T, Anderson S, Lehnen J, Heilman KM, Rothi LJG. Semantic-phonologic treatment for noun and verb retrieval impairments in aphasia. Neuropsycholog Rehab. 2007;17:244–270. doi: 10.1080/09602010600814661. [DOI] [PubMed] [Google Scholar]
- 11.Kendall DL, Rosenbek JC, Heilman KM, Conway T, Klenberg K, Rothi LJG, Nadeau SE. Phoneme-based rehabilitation of anomia in aphasia. Brain Language. 2008;105:1–17. doi: 10.1016/j.bandl.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Wong PCM, Warrier CM, Penhune VB, Roy AK, Sadehh A, Parrish TB, Zatorre RJ. Volume of left Heschl’s gyrus and linguistic pitch. Learning Cerebral Cortex. 2008;18:828–836. doi: 10.1093/cercor/bhm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong PCM, Perrachione TK, Parrish TB. Neural characteristics of successful and less successful speech and word learning in adults. Human Brain Mapping. 2007;28:995–1006. doi: 10.1002/hbm.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breier J, Maher LM, Schmadeke S, Hasan KM, Papanicolaou AC. Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: a case study. Neurocase. 2007;13:169–177. doi: 10.1080/13554790701448200. [DOI] [PubMed] [Google Scholar]
- 15.Papanicolaou AC, Pazo-Alvarez P, Castillo EM, Billingsley-Marshall RL, Breier JI, Swank PR, Buchanan S, McManis M, Clear T, Passaro AD. Functional neuroimaging with MEG: Normative language profiles. Neuroimage. 2006;33:326 –342. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]


