Abstract
Introduction
Quantitation of the expression levels of proteins involved in drug transport and disposition is needed to overcome limitations of film-based detection of chemiluminescent immunoblots.
Purpose
The purpose was to describe and validate a quantitative immunofluorescent blotting method for detection of ATP-Binding Cassette Transporter Isoform C2/Multidrug Resistance-associated Protein 2 (ABCC2/MRP2).
Methods
Western blotting was performed by electrophoresis of membrane vesicle protein isolated from Sf9 cells overexpressing MRP2 subsequently blotting with infrared labeled secondary antibody. The bound complex was detected using the Odyssey Infrared Imaging System (Li-Cor; Lincoln, NE). The images were analyzed using the Odyssey Application Software to obtain the integrated intensities, followed by linear regression of the intensity data.
Results
The limits of quantitation for the time-insensitive technique described here were from 0.001μg to 0.5μg of total membrane protein, the coefficient of variation of the slope was 8.9%; r2 values were 0.986 ± 0.012. The utility and sensitivity of this technique was demonstrated in quantitating expression of MRP2 in human placental tissue samples, in which MRP2 was present in low abundance.
Discussion
The immunofluorescent blotting technique described provides sensitive, reproducible, and quantitative determinations of large, integral membrane proteins such as MRP2, all with potential long-term cost savings.
Keywords: ATP-binding cassette transporters, Blotting, Human, Immunofluorescence, Methods, MRP2/ABCC2, Placenta, Protein detection, Protein quantitation, Western blotting
Introduction
Immunoblotting is a standard technique for the detection of numerous proteins. Most commonly, the technique requires polyacrylamide gel electrophoresis, transfer to a nitrocellulose membrane, and blotting with a primary antibody followed by a secondary antibody providing a means of detection such as horseradish peroxidase-mediated chemiluminescence, and finally exposure to film. Film is then commonly analyzed by densitometry using widely available programs such as ImageJ or NIH Image. However, these widely used methods are not conducive to quantitative comparisons due to time-sensitivity of the chemiluminescence and the narrow linear range of film. Additionally, it can be subjective in terms of film exposure times, and can be difficult to reproduce.
More recently, other methods avoiding the use of film have arisen. There are high-density imagers capable of directly capturing the chemiluminescence with a CCD camera. While these imagers have sophisticated capabilities for image analysis, they still require chemiluminescence. Additionally, makers of chemiluminescence kits have also improved their products to provide a more constant, longer-lasting signal. However, such improvements result in recurring expenses which can be significant.
In the past several years, a new approach has been marketed in which the secondary antibody is tagged with a fluorophore rather than conjugated with an enzyme. This approach frees the user from the time-sensitivity of chemiluminescence, while reducing long-term costs. Additionally, this approach can enable quantitative rather than semi-quantitative comparisons over a wide dynamic range. However, to date there are no available reports validating the quantitation of immunofluorescent blotting of ATP-Binding Cassette (ABC) transporters.
Furthermore, the current literature is deficient in papers applying and validating this technique for many commonly studied proteins. The time-insensitive technique described here has a 500-fold linear range of quantitation, with absolute intra-gel and inter-gel variabilities below 15%. Although this technique has been applied to several soluble proteins (for example, estrogen receptor α (Long and Nephew, 2006) and the chemokine receptor CXCR2 (Baugher and Richmond, 2008)), it has rarely been applied to integral membrane proteins such as ABC transporters. Finally, we applied this technique to the quantitation of human Multidrug Resistance-associated Protein 2 (MRP2) in human placental villous tissue samples, where MRP2 is variably expressed.
Materials and Methods
Sf9 cells were grown in SFII-900 SFM (Invitrogen) in suspension culture (27°C, 140rpm) to ~3 × 106 cells/ml at >97% viability (as determined using trypan blue exclusion) and diluted to 1 × 106cells/ml before infection. Then 5 × 108 cells were infected in the presence of 5% fetal bovine serum using a high-titer recombinant baculovirus stock containing the full coding region of the human MRP2/ABCC2 (NM_000392) at a multiplicity of infection of six; 48 hours later, sucrose-fractionated plasma membrane vesicles were isolated and stored as described (Ito et al., 2001; Gerk et al., 2004). Protein concentrations were determined as described (Lowry et al., 1951; Gerk et al., 2004).
Western blotting was performed by loading various amounts (0.001–1μg) of membrane protein on 8% tris-glycine denaturing 10- or 15-well gels (Novex) and separating proteins by at electrophoresis, then transferring to nitrocellulose. After blocking nonspecific binding with the Odyssey blocking buffer (Li-Cor Biosciences), mouse anti-MRP2 (M2-III6, Alexis) subsequently, infrared labeled secondary antibodies goat anti-mouse IRDye 800 (Li-Cor Biosciences) was added to bind to the primary antibody. The bound complex was detected using the Odyssey Infrared Imaging System (Li-Cor; Lincoln, NE). The images were analyzed using the Odyssey Application Software, version 1.2 (Li-Cor) to obtain the integrated intensities. Numerical data were analyzed using GraphPad Prism 4.0, using unweighted non-linear regression (y=a+bx) to compare the best-fit model (y=a+bx vs. y=bx) by an F-test.
Application
Reports of the expression of human MRP2 range from very low or undetectable in the human placenta (Pascolo et al., 2003; Evseenko et al., 2006) to comparable to hepatic expression in term placentae (Meyer Zu Schwabedissen et al., 2005), while other reports do not provide quite enough information to make this comparison (St-Pierre et al., 2000; Azzaroli et al., 2007). Some reports demonstrate high interpatient variability in MRP2 expression (Meyer Zu Schwabedissen et al., 2005; Vaidya et al., 2009). Therefore, the above methods were applied to detect and quantitate MRP2 protein in normal human placental homogenate. Human placentas from normal pregnancies were obtained under approval from the VCU Institutional Review Board; subjects gave written informed consent prior to enrollment. After removing the fetal and maternal plates, the soft villous tissue was excised, cleared of major blood vessels, finely minced, and thoroughly rinsed with normal saline. The tissue (200–300mg wet tissue) was homogenized with a Polytron homogenizer at 16,000rpm for 30 seconds. The samples were then centrifuged at 500 rcf at 4°C for 10 minutes, and the supernatant was aliquoted and stored at -80°C. After determining protein concentrations as described above, samples (25ug protein) were analyzed by immunoblotting as above. Sample intensities were compared to a calibration curve on the same blot to determine the equivalent amount of MRP2 protein using MRP2-expressing membranes as a standard.
Protocol
1. Reagents and Solutions
-
1.1
Tris-glycine SDS sample buffer (2X):
2ml glycerol (#0854-1L; Amresco, Solon, OH)
2.5ml Trizma base 0.5M (T-6066; Sigma-Aldrich, St. Louis, MO)
4ml 10% sodium dodecyl sulfate (SDS) (L-4509; Sigma-Aldrich)
0.5ml of 0.1% bromphenol blue (BP-114-25; Fisher, Pittsburgh, PA)
1N hydrochloric acid (HX0603-75; EMD, Gibbstown, NJ); to adjust pH to 6.8
18M.Ω H2O to adjust total volume to 10ml
-
1.2
Running buffer: prepared 10X (dilute to 1X before use)
30.54 g Trizma base
144.13 g Glycine (G-8898; Sigma-Aldrich)
10 g SDS
1N HCl to adjust pH to 8.3
18M.Ω H2O to adjust total volume to 1000ml
-
1.3
Transfer buffer: (pre-chilled to 4°C)
1.45g Trizma base
7.2g glycine, add both to ~750ml dH2O
200ml HPLC grade methanol (JT9093-3; VWR Scientific, West Chester, PA)
18M.Ω H2O to adjust total volume to 1000ml, let stir ~5–10min for thorough mixing
-
1.4
Blocking Buffer: Odyssey Blocking buffer (927-40000; Li-Cor Biosciences, Lincoln, NE)
-
1.5
TBS Binding buffer: (prepared 10X)
24.2g Trizma base
80g sodium chloride (S-7653; Sigma-Aldrich)
add both of the above to approximately 800ml 18M.Ω water
adjust pH using 5N HCl to pH 7.6 (about 20ml)
18M.Ω H2O to adjust total volume to 1000ml
-
1.6
Other reagents:
Tween 20 (20605-500ml; USB Corp., Cleveland, OH)
10% w/v SDS solution (dissolve 1g SDS in 8–9ml 18M.Ω H2O, adjust volume to 10ml)
-
1.7
Antibodies:
Primary antibody: mouse anti-MRP2 (M2-III6; Alexis Biochemicals, San Diego, CA)
Secondary antibody: goat anti-mouse Alexa Fluor 680 (827-06901; Li-Cor Biosciences)
2. Consumables
Novex 8% Tris-Glycine pre-cast 10-well gels (EC6015BOX; Invitrogen, Carlsbad, CA)
Benchmark prestained protein ladder (10748010; Invitrogen)
Whatman qualitative filter paper grade 1 (1001-917; GE Healthcare, Piscataway, NJ)
Nitrocellulose 0.2μm Protram BA85 (10-402-594; Schleicher&Schuell, Keene, NH)
3. Apparatus
Pipette roller: break a 10ml disposable polystyrene pipette to a length corresponding to 4ml markings (ie, break off pieces below 2ml and above 6ml; retain piece between 2ml and 6ml)
For running the gel: X-Cell Sure Lock Mini Cell (EI905; Invitrogen)
For transfer: Biorad transfer apparatus (Mini Trans-Blot Cell; Bio-Rad, Hercules, CA)
DC Power Supply (PS500X; Hoefer Scientific Instruments, San Francisco, CA)
4. Procedure
-
4.1
Prepare samples appropriately, in 1X sample buffer, to deliver an appropriate amount of protein per well, in a maximum of 20μl. Routinely, it is better to plan on adding 20μl to each well. Mix well, briefly centrifuge.
-
4.2
Heat denature at 37°C for 30 minutes (note that high temperatures even for short times will destroy MRP2). Gently flick tubes, then briefly centrifuge again.
Set voltage to 125V to begin electrophoresis. Let the gel run about 45 minutes (1 gel) to an hour (2 gels), but typically not past the point at which the dye runs out the bottom of the gel.
-
4.3
Set up the BioRad transfer apparatus in an ice bucket, such that the transfer cell is covered by ice on all sides, place the BioRad cooling unit (ice-slab), and a stir bar.
-
4.4
Lift the gel from the paper towels, and while holding it over the glass pan, drizzle some transfer buffer over it from the corner of a sponge. Next, place one filter paper and a sponge on top of the gel.
-
4.5
Place the electrode cap on it, and plug in the probes, and set the voltage to 100V (irrespective of number of gels), and let it run about 1 hour 15 minutes in the cold room on ice, with stirring.
-
4.6
After the transfer is done, disassemble the apparatus, carefully observing if any of the colored protein ladder marker is remaining in the gel. It should all be transferred to the nitrocellulose; if not, transfer was not complete and results will not be good.
-
4.7
Place each nitrocellulose membrane into a square tray (Millipore), and rinse briefly with deionized water to remove the transfer buffer. Drain the water.
-
4.8
Add 4 ml of Odyssey Blocking buffer and place on the platform rocker at 4°C overnight or at room temperature for 1 hour.
-
4.9
After the blocking step, incubate on the rocking platform for 1 hour at room temperature after adding 4 μl Tween-20 to the same blocking buffer solution, and the appropriate primary antibody. We have used the following dilution for the primary antibody anti-MRP2 (M2-III6) 1:4000. For two-color detection primaries from different species can be used simultaneously on the same blot.
-
4.10
Rinse three times with TBS-T (~40ml each time), rocking 5 min each time.
-
4.11
Mix 4 ml of Odyssey blocking buffer, 4 μl Tween-20, 4 μl 10%w/v SDS and 0.4 μl of each of the appropriate secondary antibody solutions. Use the goat anti-mouse Alexa Fluor 680, goat anti-rabbit IRDye 800 (827-06905; Li-Cor) or donkey anti-goat IRDye 800 (827-06903; Li-Cor) to bind to the primary antibodies from the mouse, rabbit or goat respectively. For two-color detection when primaries from different species are used simultaneously on the same blot, use appropriate secondary antibodies that can be visualized in different fluorescence channels (700 and 800 nm). For example, for a western blot to detect both MRP2 and actin, use anti-MRP2 (mouse) and anti-β-actin (rabbit) (RB-9421-P0; NeoMarkers, Fremont, CA) as the primaries and goat anti-mouse Alexa Fluor 680 and goat anti-rabbit IRDye 800 as the secondary antibodies. Allow to rock for 1 hour at room temperature; protect from light for all further steps by placing paper towels over the tray.
-
4.12
Rinse three times with TBS-T (~40ml each time), rocking 5 min each time. Then rinse once with TBS (no Tween 20) for 30 min, then once with deionized water for 5min.
-
4.13
Dry the membrane by pressing between filter papers and wrap it in an Al foil envelope.
-
4.14
Begin the scan by selecting ‘Scan’ from the File menu or the blue arrow on the tool bar. Give an appropriate name for the analysis, set the resolution to 169, distance from the surface to 0, select the appropriate channel depending upon the secondary antibodies used, begin with a scanning intensity of 3.5 for each channel (depending upon the resulting image, chose lower intensity values to reduce excess background or higher intensity values if bands are too faint). Mark the position of the membrane on the grid, and proceed with the scan.
-
4.15
After completion of the scan, rotate the image as desired, use the ‘subtract’ option to reduce the background. Do not use the crop or filter options. Desired areas of the image can be analyzed separately by using the ‘crop’ function later. Quantitation of bands is invalid if the ‘filter’ option is used. Save the original image.
Results
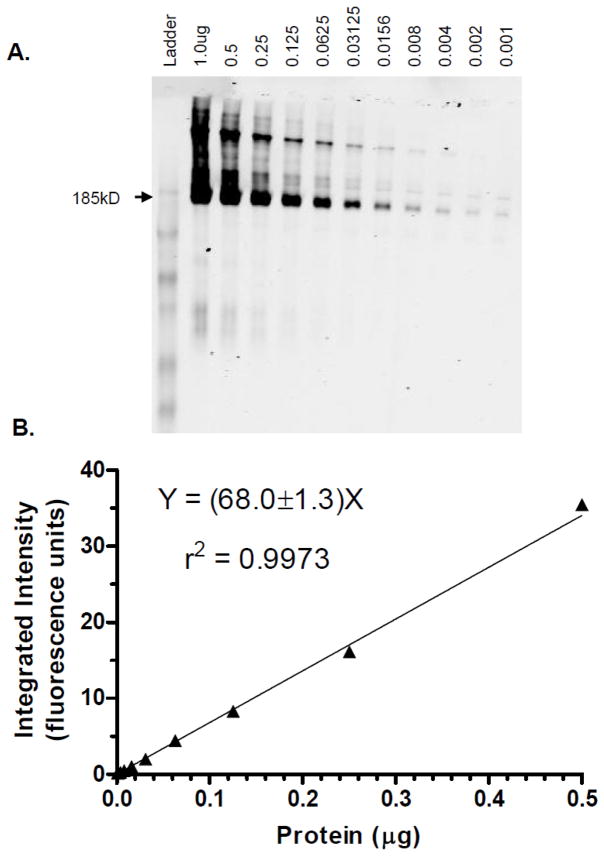
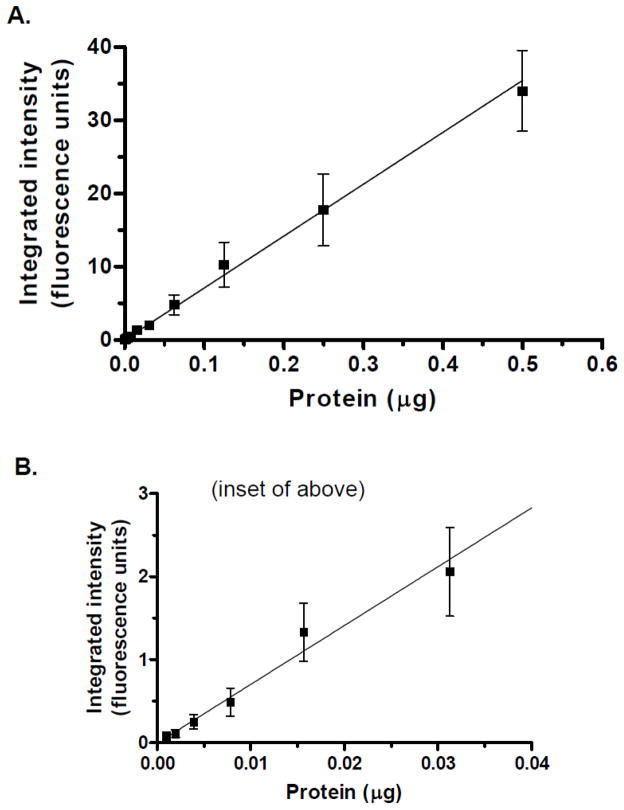
Figure 1A shows a representative image of a blot of membrane vesicles from MRP2-overexpressing Sf9 insect cells. MRP2 was detected over a 1000-fold range, with excellent linearity (Figure 1B). As shown in Figure 2, the combined calibration curves (n=4 at each protein level) The limits of quantitation were from 0.001μg to 0.5μg, the average slope (Y-intercept was not significant) from four independent experiments was 63.7±5.7 (CV=8.9%); and the r2 values were 0.986±0.012. Intraday and interday variabilities were below 15%. The integrated intensity of 0.0625μg protein from three different membrane preparations was 5.44±0.86 (mean, SD), giving a batch-to-batch coefficient of variability of 16%.
Figure 1.
Representative immunofluorescent blotting results for membrane vesicles isolated from MRP2-overexpressing Sf9 insect cells. A: Scanned image of blot converted to black and white; Sf9 cells lacking MRP2 are non-immunoreactive (data not shown); protein amounts (μg) as shown. B: Corresponding calibration curve weighted 1/Y, with linear regression results as shown.
Figure 2.
Averaged calibration curves. A: Averaged data (mean±SD) for independent experiments (n=4–7 at each protein level); linear regression results (weighted 1/Y) as shown. B: inset of upper graph showing lower data points.
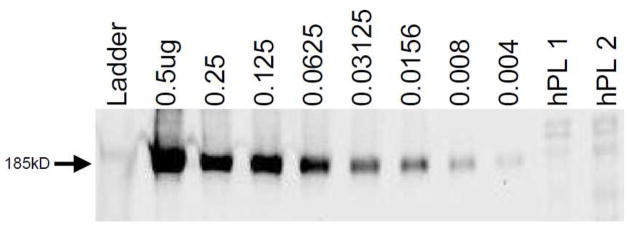
Figure 3 shows a determination of MRP2 protein expression in two placental villous tissue samples obtained from healthy term deliveries. The underglycosylated form of MRP2 expressed by Sf9 insect cells runs slightly faster (lower) than the fully glycosylated form expressed in human tissues. Interpolation of the image intensities from the calibration curves provided estimates of the expression of MRP2 as 6.63±0.26ng and 6.11±0.24ng Sf9-equivalent MRP2 protein in each 25μg sample of total protein in placental villous tissue homogenate.
Figure 3.
Application of the technique to detection of MRP2 in human placenta; MRP2 membrane vesicle protein amounts (μg) as indicated; human placental villous tissue homogenate (25μg protein) from two patients (hPL1 and hPL2); interpolated results are as indicated in the text.
Discussion
This technique represents a substantial improvement in immunoblotting, in many aspects. First, there are cost savings in reagents (including primary and secondary antibodies) as well as savings by avoiding chemiluminescence reagent kits. Second, since blots can be stored and rescanned days or even months later, much repeated blotting can be avoided since inappropriate film exposure does not occur. If desired exposure is not obtained, the scanning intensity can be easily adjusted as described above. Next, sensitivity is improved; by chemiluminescence we can routinely detect 0.1 micrograms of membrane protein from MRP2-overexpressing Sf9 cells (Gerk et al., 2007); here we report 100-fold increased sensitivity compared to chemiluminescence. Such enhanced sensitivity enables sample conservation, detection of low levels of expression, or detection in dilute samples. The technique was successfully applied to detect MRP2 in human placental homogenates, where the expression of this protein is reported to range from undetectable to comparable to hepatic expression levels (St-Pierre et al., 2000; Pascolo et al., 2003; Meyer Zu Schwabedissen et al., 2005; Evseenko et al., 2006). Additionally, we have successfully applied this technique to the analysis of other ATP-Binding Cassette transporters, including P-glycoprotein (ABCB1/MDR1) and the Breast Cancer Resistance Protein (ABCG2/BCRP) (data not shown). Finally, the Odyssey imager requires no fluid supply, very little electricity, and almost no maintenance, and no dark room, in contrast to film developers. Thus there may also be long-term cost savings regarding equipment and space utilization.
In conclusion, the described technique provides considerable advantages over conventional Western blotting, including protein quantitation, speed, reproducibility, sensitivity, and decreased cost of use.
Acknowledgments
The author gratefully acknowledges technical work by Deaette M. Smith and Soniya Vaidya in generating the data for this manuscript. The author acknowledges Dr. Ross Mikkelsen for the initial use of the Odyssey imager. Funding for this work was provided by the A.D. Williams Foundation, the Thomas F. and Kate Miller Jeffress Memorial Trust, the Virginia Commonwealth University School of Pharmacy, the National Institutes of Health National Center on Minority Health and Health Disparities [Grant 1P60-MD002256], and the Higher Education Equipment Trust Fund of Virginia. The study sponsors had no role in study design, data collection, analysis, interpretation, writing or deciding to submit this manuscript for publication.
Footnotes
Conflict of Interest Statement
The author declares no conflicts of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azzaroli F, Mennone A, Feletti V, Simoni P, Baglivo E, Montagnani M, Rizzo N, Pelusi G, DDEA, Lodato F, Festi D, Colecchia A, Roda E, Boyer JL, Mazzella G. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007;26:1139–1146. doi: 10.1111/j.1365-2036.2007.03462.x. [DOI] [PubMed] [Google Scholar]
- Baugher PJ, Richmond A. The Carboxyl-terminal PDZ Ligand Motif of Chemokine Receptor CXCR2 Modulates Post-endocytic Sorting and Cellular Chemotaxis. Journal of Biological Chemistry. 2008;283:30868–30878. doi: 10.1074/jbc.M804054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1357–1365. doi: 10.1152/ajpregu.00630.2005. [DOI] [PubMed] [Google Scholar]
- Gerk PM, Li W, Megaraj V, Vore M. Human multidrug resistance protein 2 transports the therapeutic bile salt tauroursodeoxycholate. J Pharmacol Exp Ther. 2007;320:893–899. doi: 10.1124/jpet.106.106922. [DOI] [PubMed] [Google Scholar]
- Gerk PM, Li W, Vore M. Estradiol 3-glucuronide is transported by the multidrug resistance-associated protein 2 but does not activate the allosteric site bound by estradiol 17-glucuronide. Drug Metab Dispos. 2004;32:1139–1145. doi: 10.1124/dmd.104.000372. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki H, Sugiyama Y. Single amino acid substitution of rat MRP2 results in acquired transport activity for taurocholate. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1034–1043. doi: 10.1152/ajpgi.2001.281.4.G1034. [DOI] [PubMed] [Google Scholar]
- Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent Interacting Proteins Mediate Immobilization and Degradation of Estrogen Receptor-α. Journal of Biological Chemistry. 2006;281:9607–9615. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Meyer Zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, Cascorbi I, Kroemer HK. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33:896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]
- Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun. 2003;303:259–265. doi: 10.1016/s0006-291x(03)00327-9. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1495–1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- Vaidya SS, Walsh SW, Gerk PM. Formation and Efflux of ATP-Binding Cassette Transporter Substrate 2,4-Dinitrophenyl-S-Glutathione from Cultured Human Term Placental Villous Tissue Fragments. Mol Pharm. 2009;6:1689–1702. doi: 10.1021/mp900019z. [DOI] [PubMed] [Google Scholar]