Abstract
Lead is a naturally-occurring element and industrially-produced metal that is highly toxic to children, causing intellectual and behavioral deficits, hyperactivity, fine motor function deficits, decreased intelligence quotient, hand-eye coordination, and problems in reaction time. Children’s exposure to lead occurs mainly through ingestion of contaminated food, water and soil. Few discussions have been held on the magnitude and potential risk associated with exposure from the consumption of breast milk. Hence, this paper was designed to systematically review the scientific literature on published epidemiologic studies, with an emphasis on the study design and analytical procedures used for Pb assessment in breast milk. From a total of 112 selected articles published since the 1980s, 11 met the inclusion criteria. A review of the data indicated that Pb levels varied from 0.15 to 6.1 μg L−1 in mature milk samples, from 0.48 to 14.6 μg L−1 in colostrums samples, and were non-detectable in some samples. The milk/blood ratio, which estimates the mean efficiency transfer of lead from blood to milk, varied between 0.01 and 0.48. The heterogeneity of methods revealed by our assessment of the published studies emphasizes the need for harmonization of study designs and sample collection and analysis protocols to reflect specific exposure scenarios. Human milk seems to be one of the feasible biological matrices for use as a biomarker for assessing children’s health risk to lead poisoning.
Keywords: lead, human milk, children, exposure biomarker, health risk assessment
INTRODUCTION
Lead, a known toxic element, is a public health problem due to its adverse effects, mainly those affecting the central nervous system in the most vulnerable populations, such as pregnant and lactating women and children. In the absence of a safe exposure limit of children to lead and because of its ability to accumulate in the body for a long time, a great interest in the evaluation of the adverse effects of this metal in low concentrations has emerged /1–7/. In general, children are the most vulnerable to lead exposure because they have greater gastrointestinal absorption and less effective renal excretion. Lead is extremely damaging to the developing brain in utero. Experimental studies provide evidence that when compared with a more mature brain, the fetal brain presents greater sensitivity to the toxic effects of lead. The investigations also suggest that the encephalic barrier of the fetus is immature and does not promote protection against the metal /1, 7–8/.
Lead is readily transferred to the fetus through the placenta /9/. In the absence of placental barrier efficacy, the fetus would be exposed to lead in a concentration very close to that of the mother. Fetus vulnerability can occur even if the mother’s exposure had ceased many years earlier /10–11/. The interference of lead can be observed on early embryonic development and during the last months of pregnancy /1,12/.
In epidemiologic studies of lead poisoning, biological indicators of lead exposure must be considered whenever possible. Many such studies have used human milk when assessing risk to children’s health. Measuring lead concentrations in this biological matrix would be the first step in establishing guidelines for safe breastfeeding.
The primary sources of lead in breast milk are maternal diet and bone lead. During pregnancy and lactation, up to 5% of bone mass is mobilized as a source of calcium /13–16/. Concomitantly, lead accumulated in bone from past exposures can be released into the blood and excreted into breast milk /4/; also/. Distinguishing how much of the blood lead derives from ongoing environmental exposure as opposed to the mobilization of bone lead is impossible. Although this phenomenon is of special concern in countries where environmental lead exposure is high, detectable levels of lead in breast milk have been documented in population studies of women with no current environmental or occupational exposures /17/.
A multinational study conducted by the World Health Organization /18/ in six countries comprising four continents, representing different degrees of industrial development, estimated lead levels in breast milk ranging from 2.0–17.8 μg L−1. The study concluded that lead concentrations in breast milk samples varying from 2.0–5.0 μg μg L−1 might be considered within reference values. A review by Abadin et al /17/ also concluded that in a population not occupationally exposed to lead, concentrations of this metal in milk would be within the same range.
The aim of this paper was to review the scientific literature on published epidemiologic studies, with an emphasis on the study design and analytical procedures used for lead assessment in breast milk. We also intended to apply a set of selection criteria to obtain a broader picture of lead assessment in human milk.
METHODS
A systematic review of the literature was conducted to retrieve studies published and indexed in MEDLINE (PubMed) and in Latin American and Caribbean Literature in Health Science (LILACS) databases using initially the key words lead, human milk, and blood lead. The inclusion criteria were studies published between 2000 and the first semester of 2010 (regardless of the sample collection period); those using biomarkers of lead levels in breast milk; human biomonitoring studies, and studies published in English or Portuguese. Exclusion criteria were studies that were not original, studies on animals; studies lacking data on lead exposure; studies lacking information for understanding the data (for example, units used), and studies with non-representative samples.
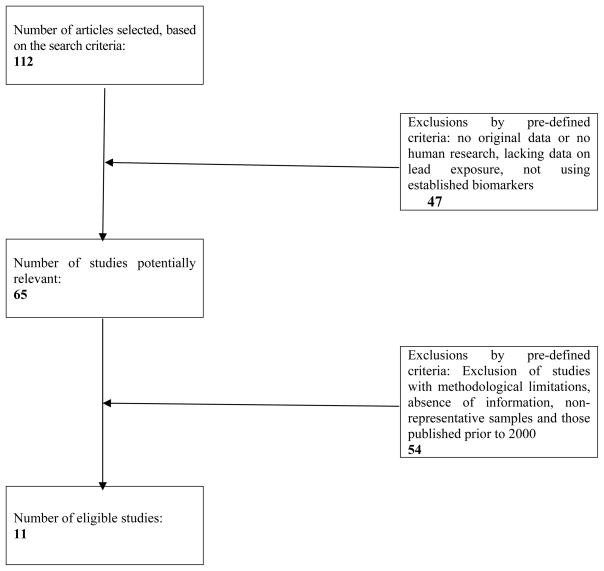
The flowchart presented in Figure 1 summarizes the search strategy adopted to identify and include studies. A total of 112 articles were selected as being relevant, based on the information provided in the titles and abstracts. According to the pre-established inclusion and exclusion criteria, a total of 11 articles were selected for in-depth evaluation in this review.
Fig. 1.
Study selection process flowchart
During the evaluation process, the selected articles were classified in chronological order, milk time (colostrum or mature milk, for instance), analytical technique applied, detection limits, and epidemiologic study design. The indexes compiled from different studies considered in this research were estimated based on the mean concentrations of lead in tissues of interest. For consistency, both milk and blood lead levels were converted to microgram per liter.
RESULTS
Scenarios of Lead Levels in Human Milk
Two review studies are worth mentioning. Dorea /18/ reviewed more than 30 studies reporting lead concentrations in mature milk and/or colostrum ranging from 0.11–791 μg L−1. Gulson et al /5/ analyzed studies conducted for over 15 years, with concentrations varying from 0.7–209 μg L−1.
In the present review, the mean concentrations of lead in breast milk varied, ranging over three levels of magnitude:
below 1.0 μg L−l: Leotsinidis et al /20/
between 1.0 and 10.0 μg L−l: Koyashiki et al, Ettinger et al, Chien et al, Kïrel et al, Ursinyova and Masanova, Anastacio et al, Hanning et al, Sowers et al, Gundacker et al /21,13,22–28/
between 10.0 and 100.0 μg L−l: Turan et al /29/.
In Brazil, only two studies have analyzed lead levels in breast milk. Koyashiki et al /21/, in a cross-sectional study, observed concentrations of this metal ranging from 1.0–8.0 L−1 in a city in the South of the country. Anastacio et al /25/ also conducted a cross-sectional study in the city of Rio de Janeiro, and reported lead concentrations between < 0.1 and 11.9 L−1. In South America, a longitudinal analysis in Mexico City conducted by Ettinger et al /13/ revealed mean concentrations of lead in mature milk ranging from 0.2 to 8.0 L−1, at 1, 4, and 7 months postpartum.
Lead Levels in Colostrum and Mature Milk
From the 112 studies analyzed, 11 meeting our inclusion criteria are summarized in Table 1. The key aspects of study quality (i.e., sample size, analytical technique, detection limit, milk time) were also reported. In colostrum samples, mean levels of the metal ranged from 0.48 to 14.60 μg L−1 for mothers who were not occupationally exposed to lead. Chien et al /22/, Kïrel et al /23/, Ursinyova and Masanova /24/, and Gundacker et al /28/ reported mean levels of lead in milk samples that were within WHO reference values (2–5 5 μg L−1) /18/. The values reported in a study by Leotsinidis et al /19/ were below this range. Turan et al /29/ found values much higher than those considered to be within the acceptable range. Overall, the mean lead levels in mature milk samples varied from 0.15 to 6.1 μg L−1. According to the reference values established by the WHO /18/, only concentrations observed by Sowers et al /27/ in unexposed populations in the United States were above 5 μg L−1.
Table 1.
Summary of epidemiologic studies on lead levels in human milk in general populations published after 2000.
| Author/Year | Period of sample collection | Country | Sample size (n) | Milk | Maternal blood | Mother milk time | Analytical Technique, Detection Limit μg L−1 | Objective of the study (Comments) | |
|---|---|---|---|---|---|---|---|---|---|
| Pb μg L−1 | |||||||||
| Koyashiki et al., 2010 /17/ | 2007 | Brazil | 92 | 2.90±1.1a | 28.2±9.4 | mature milk | ICP-MSe LD: 1.0 μg |
Evaluate lead levels in milk and blood from milk Bank donors | |
| 3.0b | 27.0b | ||||||||
| Chien et al., 2006 /19/ | 2001–2003 | China | 72 | 35 | 8.59±10.95a | NA | colostrum | GFASSf LD: not given |
Examine the relationship between lead concentrations in breast milk and the consumption of traditional Chinese herbs commonly used during pregnancy and lactation. Consumption vs control group. |
| 37 | 6.84±2.68a | NA | |||||||
| 16 | 9 | 2.34a | NA | mature milk | |||||
| 7 | 2.36a | NA | |||||||
| Ettinger et al., 2006 /18/ | 1994–1995 | Mexico | 310 | 1.40±1.1a | 93.0±45.0a | mature milk | ICP-MSe LD: 0.1 |
A longitudinal analysis conducted among lactating women at 1, 4, and 7 months postpartum to evaluate the relations among maternal blood, bone, and breast milk lead levels. | |
| 1.0b | 83.0b | ||||||||
| 224 | 1.20±1.0a | 90.0±40.0a | |||||||
| 0.9b | 82.0b | ||||||||
| 195 | 0.90±0.8a | 81.0±34.0a | |||||||
| 0.7b | 74.0b | ||||||||
| Leotsinidis et al., 2005 /16/ | 2000–2002 | Greece | 95 | 0.48±0.60a | NA | colostrum | GFAASf LD: 0.20 |
Measuring lead and cadmium concentrations in colostrum and transitory milk samples, from lactating mothers, to assess if the infant intakes are within recommended levels. | |
| 0.44b | |||||||||
| 0.15±0.25a | NA | mature milk | |||||||
| NDbc | |||||||||
| Author/Year | Period of sample collection | Country | Sample size (n) | Milk | Maternal blood | Samle type (time) | Analytical technique, DL μg L−1 | Objective of study (Comments) | |
|---|---|---|---|---|---|---|---|---|---|
| Pb μg L−1 | |||||||||
| Kïrel et al., 2005 /20/ | 2001–2002 | Turkey | 143 | 2.34±1.0a | 28.0±15.0a | colostrum | GFAASf LD: not given |
To determine the level of lead exposure in children and adults among the general population; and to determine the lead level in maternal-cord blood and breast-milk samples during prenatal and neonatal periods. | |
| Ursinyova & Masanova, 2005 /24/ | NA | Slovakia | 158 | 4.70±4.1a | NA | colostrum (4d) | GFAASf LD: 0.65 |
To determine lead, mercury and cadmium levels in breast milk of lactating women. | |
| 3.4b | |||||||||
| Anastacio et al., 2004 /25/ | NA | Brazil | 38 | 2.80±2.5a | 63.0±20.0a | mature milk | ICP-MSe LD: 0.1 |
To determine the concentration and distribution of lead, calcium, iron, zinc, and copper in major fractions (fat, casein, whey) of mature milk from nursing women with low environmental lead exposure. | |
| 1.20d | 60.0d | ||||||||
| Hanning et al, 2003 /26/ | NA | Canada | 25 | 2.08±1.67a | 22.9±12.5a | mature milk | GFAASf LD: 2.08 |
To describe lead in maternal and cord blood at birth and infant blood at four months of age. Also to analyze lead levels in breast milk at 2 and 4 months lactation. | |
| Sowers et al., 2002 /27/ | 1997–2000 | USA | 15 | 6.10±1.0a | 14.0a | mature milk | ICP-MSe LD: not given |
To determine whether breast milk lead levels are correlated with maternal blood lead levels, bone loss, or bone turnover during reproduction. Data were collected prospectively at 0, 1.5, 3, 6 and 12 months after delivery in lactating women. | |
| 5.60±1.1a | 16.0a | ||||||||
| 5.90±1.0a | 17.0a | ||||||||
| 4.30±1.6a | 14.0a | ||||||||
| Gundacker et al., 2002 /28/ | 1999 | Austria | 48 | 1.22±0.92a | NA | colostrum (2–14d) | GFAASf LD: 0.10 |
Measure possible changes in levels of lead in breast milk since earlier measurements in 1981 and 1993. | |
| 37 | 2.48±2.39a | NA | |||||||
| 27 | 1.29±1.12a | NA | |||||||
| Turan et al., 2001 /29/ | 1995–1996 | Turkey | 30 | 14.6±5.5a | NA | colostrum (4d) | GFAASf LD: 0.92 |
To determine the concentrations of toxic and essential elements in colostrum samples, to evaluate the exposure to environmentally toxic metals. | |
arithmetic mean;
median;
non detected;
geometric mean;
Inductively Coupled Plasma Mass Spectrometry;
Graphite Furnace Atomic Absorption Spectrophotometry;;
DL – detection limit; NA – not applicable
Four studies shown in Table 1 did not present information for the date on which the collections were made. The number of samples collected in these studies varied widely from 15 to 310 samples. Regarding the analytical technique applied, Chien et al /22/, Kïrel et al /23/, Ursinyova and Masanova /24/, and Hanning et al /26/ used graphite furnace atomic absorption spectrometry (GFAAS) to analyze lead in colostrum or mature milk samples. Koyashiki et al /21/, Ettinger et al /13/, Anastacio et al /25/, and Sowers et al /27/ used inductively coupled plasma mass spectrometry (ICPMS) for analyzing lead in mature milk samples. From the 11 papers analyzed, the detection limits for various analytical techniques ranged from 0.1 μg L−1 for ICP-MS to 2.08 μg L−1 for GFAAS, and in three studies, the data were not presented.
Correlation between Lead Concentrations in Milk and Blood
Several studies reported a significant correlation between the concentration of lead in maternal milk and blood, as it can be observed in Table 2. Kïrel et al /23/ found a good correlation between lead levels in maternal milk and blood during lactation. Koyashiki et al /21/, Ettinger et al /13/, Anastacio et al /25/, and Sowers et al /27/ found a significant but modest correlation between maternal blood lead levels and mature milk samples.
Table 2.
Studies on the correlation between lead concentrations in maternal milk and blood
| Reference | Correlation | Lactation stage |
|---|---|---|
| Kïrel et al (2005) /23/ | r = 0.6, p < .0001 | Colostrum |
| Anastacio et al (2004) /25/ | rs = 0.49, p = .02 | Mature milk |
| Ettinger et al (2006) /13/ | rs = 0.32, p < .0001 | Mature milk |
| Sowers et al (2002) /27/ | rs = 0.30* | Mature milk |
| Koyashiki (2010) /21/ | rs = 0.297, p = .048 | Mature milk |
rs – Sperman’s correlation;
at six weeks of lactation
Milk/Blood Lead Ratio
The ratio was obtained by dividing the means of lead level in milk by the means in lead concentration in maternal blood. This ratio establishes the transfer efficiency means of lead in maternal blood to milk and provides an indication of the concentration of this metal in milk when compared with the concentration presented in maternal blood. Among the 11 articles analyzed, only 7 determined the levels of lead in two biological matrices (milk and blood). Table 3 presents the milk/maternal blood ratio in these studies. A ratio ≥1 can be assumed to represent a significant dose to the child /18/. The milk/blood ratio in the analyzed studies ranged between 0.01 and 0.43. In a review performed by Dorea /19/, the blood/milk ratio varied between 0.02 and 8.3, and in a study conducted by Gulson et al /5/, the ratio from the revised studies ranged between 0.02 and 0.42. Only in the study conducted by Li et al /30/ was this ratio > 1.
Table 3.
Milk/blood ratio estimated in studies conducted in different countries
| Reference | Location | Pb (μg L−1) |
Milk/Blood | Ratio | |
|---|---|---|---|---|---|
| Milk | Maternal blood | ||||
| Koyashiki et al 2010 /21/ | Brazil | 2.9 | 28.0 | 0.11 | |
| 1.4 | 93.0 | 0.01 | |||
| Ettinger et al 2006 /13/ | Mexico | 1.2 | 90.0 | 0.01 | |
| 0.9 | 81.0 | 0.11 | |||
| Kïrel et al (2005) /23/ | Turkey | 2.3 | 28.0 | 0.08 | |
| Anastacio etal (2004) /25/ | Brazil | 2.8 | 63.0 | 0.04 | |
| Hanning et al (2003) /26/ | Canada | 2.1 | 22.9 | 0.09 | |
| 6.1 | 14.0 | 0.43 | |||
| 5.6 | 16.0 | 0.35 | |||
| Sowers et al (2002) /27/ | USA | 5.9 | 17.0 | 0.35 | |
| 4.3 | 14.0 | 0.31 | |||
Source: adapted from Koyashiki /32/
DISCUSSION
The objective of this systematic review was to gather and discuss the main studies available in literature, on lead levels in human milk. One difficulty faced in the selection of articles was the heterogeneity of methods used in the different studies. This difficulty, however, is common in systematic reviews. As new inclusion criteria are added, a gain in specificity is achieved but also a loss in sensitivity, compromising the objective of showing the scenario of publications on a specific subject. Because of the substantial heterogeneity and the limitations in methodology in the original studies, we considered a quantitative pooling inappropriate. Hence, the qualitative systematic review was conducted on the evidence available. Although multiple search strategies were used to identify relevant studies, some publications may have been missed.
Among the different studies, a diversity of epidemiologic design and analytic methodologies to measure lead in breast milk could be observed, making data comparison difficult and not allowing for a precise evaluation of estimates. Hence, determining whether these gaps are a result of analytic limitations or if the data truly reflect different types of exposure is difficult. Several studies are limited by the small number of samples, non-adjustment for confounding variables, and short follow-up periods. Other factors include differences in the age groups studied, parity, lactation stage, as well as bias in selecting the participants. Not every author followed international definitions and parameters as inclusion criteria, which also brought difficulty in comparing these studies. All these factors were fundamental for establishing the criteria for the selection of studies for this review.
The articles analyzed in the present review were characterized, in their majority, as cross-sectional studies. Note that only the study by Ettinger et al /13/ had a longitudinal design.
Detection levels are comparable, and most studies have reported these limits, which can be seen in Table 1. Nevertheless, the ways in which values lower than the detection limit are reported and used in statistical analysis were not presented in all papers. These numbers must be reported, either as non-detectable or by using half their values in statistic calculations. Another possibility would be to divide the limit of detection value by the square root of 2 or, in a more liberal way, use the detection limit value itself in these calculations /31/. However, these options must be carefully and clearly described and the studies have not presented the option used, with the exception of Koyashiki et al /32/, which used half the detection limit for statistics calculations.
Considering that the concentrations of lead in milk did not necessarily present a normal distribution, the best measures of central tendencies to be used would be the median and the geometric mean /33/, which are less influenced by high values than the arithmetic mean. Nevertheless, a great portion of the studies analyzed in the present review presented the arithmetic mean as their central measure.
In the articles conducting analyses of lead in milk, the highly sensitive techniques like ICP-MS and GFAAS could guarantee results that would present good accuracy and precision.
The sample preparation steps are usually the most common source of analytic error and usually increase the overall imprecision of the method /34/. We must also consider that because of the low levels of metal in milk, one problem was the contamination of samples during collection or analytical determination as milk is a substance that can be easily contaminated. Much of the older data and values > 3 parts per billion of lead in breast milk reported during the last 15 years have been questioned /4,5,18/. Using a comparisons of ratios expressed as percentage of lead concentration in milk to that in blood constitutes a method to validate results. Data showing a milk/blood ratio of > 15% should be treated with caution, however, because of possible contamination during the collection of the samples or during laboratorial processing and analysis /4–5/.
When determining lead in a biological matrix, fat content in milk is also a complicating factor because it changes during the course of lactation. A methodological challenge would be to establish the most appropriate time for the milk to be used as a good exposure biomarker. According to Needham and Wang /31/, for monitoring purposes, mature milk must be collected only when its fat content is set, which happens 2 weeks after birth. Therefore, mature milk would be more appropriate when compared to colostrum, which might explain the greater variability in lead concentrations found in the studies of colostrum presented in Table 1.
Lead in breast milk from mothers with current ongoing exposure to lead or mothers exposed by the redistribution of cumulative maternal bone lead stores could pose a potential health risk to the infant. Ettinger et al. /14/ found, however, that even among a population of women with relatively high cumulative lifetime exposures to lead as assessed by bone lead levels, breast milk levels were low. Nevertheless, breast milk was found to exert a strong influence on infant blood lead levels over and above the influence of maternal blood lead. Thus, the question arises of what exactly is the risk to child health? Maternal blood lead levels < 10 μg L−1 are considered no cause for concern as the amount of lead in breast milk is only ≤5% of that in her blood. Within the range of lead concentrations reported in the studies reviewed here, the effects are expected to be small /14,35/. Thus, breast-feeding should not be discouraged.
CONCLUSION
This review has shown that human milk could be a feasible biological matrix for use as a biomarker for lead exposure, with the goal of evaluating the risk to children’s health using a non-invasive biological procedure. Although the lead concentrations were highly variable in this review, the health risk for the infant from breast mild appears to be small at such low levels. To increase the accuracy, consistency, and reproducibility of future studies, we recommend that collection protocols and analytical methods for lead assessment in human milk be harmonized to provide a scientific basis for inter-laboratory comparisons and to improve risk assessment strategies to address different exposure scenarios.
Acknowledgments
This work was supported by the Health Science Center at the State University of Londrina in Parana, Brazil, and the RCMI-Center for Environmental Health (Grant No. 2G12RR013459) at Jackson State University in Jackson, Mississippi, USA.
References
- 1.Schnaas L, Rothenberg SJ, Flores MF, Martinez S, Hernandez C, Osorio E, Velasco SR, Perroni E. Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect. 2006;114:791–799. doi: 10.1289/ehp.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microgram per deciliter. N Engl J Med. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;13:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation - a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 5.Gulson BL, Jameson CW, Mahaffey KR, Mizon K, Patison N, Law AJ, Korsch MJ, Salter M. Relationships of lead in breast milk to lead in blood, urine, and diet of the infant and mother. Environ Health Perspect. 1998;106:667–674. doi: 10.1289/ehp.98106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong S, Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78(9):1068–1077. [PMC free article] [PubMed] [Google Scholar]
- 7.Oskarsson A, Hallén IP, Sundberg J, Grawé KP. Risk assessment in relation to neonatal metal exposure. Analyst. 1998;123:19–23. doi: 10.1039/a705136k. [DOI] [PubMed] [Google Scholar]
- 8.Goyer RA. Transplacental transport of lead. Environ Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossipal E, Krachler M, Li F, Micetic-Turk D. Investigation of the transport of trace elements across barriers in humans: studies of placental and mammary transfer. Acta Paedriatr. 2000;89:1190–1195. doi: 10.1080/080352500750027556. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger D. Teratogen update: lead and pregnancy. (Part A) Birth Defects Res A Clin Mol Teratol. 2005;73(6):409–420. doi: 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- 11.Hu H, Tellez-Rojo MM, Bellinger D. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agency for Toxic Substances and Disease Registry. Toxicological profile for lead. U.S. Department of Health and Human Services. Public Health Service; Atlanta, USA: 2007. [Accessed 18 April 2008.]. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp13.html. [Google Scholar]
- 13.Ettinger AS, Téllez-Rojo MM, Amarasiriwardena C, Peterson KE, Schwartz J, Aro A, Hu H, Hernández-Avila M. Influence of maternal bone lead burden and calcium intake on levels of lead in breast milk over the course of lactation. Am J Epidemiol. 2006;163:48–56. doi: 10.1093/aje/kwj010. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger AS, Téllez-Rojo MM, Amarasiriwardena C, Bellinger D, Peterson K, Schwartz J, Hu H, Hernández-Avila M. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environ Health Perspect. 2004;112(14):1381–85. doi: 10.1289/ehp.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and determinants of blood lead during pregnancy. Am J Epidemiol. 2000;52(9):829–37. doi: 10.1093/aje/152.9.829. [DOI] [PubMed] [Google Scholar]
- 16.Rothenberg SJ, Khan F, Manalo M, Jiang J, Cuellar R, Reyes S, et al. Maternal bone lead contribution to blood lead during and after pregnancy. Environ Res. 2000;82(1):81–90. doi: 10.1006/enrs.1999.4007. [DOI] [PubMed] [Google Scholar]
- 17.Abadin HG, Hibbs BF, Pohl HR. Breast-feeding exposure of infants to cadmium, lead and mercury: a public health viewpoint. Toxicol Ind Health. 1997;13:495–517. doi: 10.1177/074823379701300403. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Minor and trace elements in breast milk. Geneva: 1989. [Google Scholar]
- 19.Dorea JG. Mercury and lead during breast-feeding. Br J Nutr. 2004;92:21–40. doi: 10.1079/BJN20041163. [DOI] [PubMed] [Google Scholar]
- 20.Leotsinidis M, Alexopoulos A, Kostopoulou-Farri E. Toxic and essential trace elements in human milk from Greek lactating women: association with dietary habits and other factors. Chemosphere. 2005;61:238–247. doi: 10.1016/j.chemosphere.2005.01.084. [DOI] [PubMed] [Google Scholar]
- 21.Koyashiki GAK, Paoliello MMB, Matsuo T, Oliveira MMB, Mezzaroba L, Carvalho MF, Sakuma AM, Turini C, Oliveira Vannuchi MTO, Barbosa CSD. Lead levels in milk and blood from donors to the breast milk Bank in southern Brazil. Environ Res. 2010;110(3):265–271. doi: 10.1016/j.envres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Chien L, Yeh C, Lee H, Chao H, Shieh M, Han B. Effect of the mother’s consumption of traditional Chinese herbs on estimated infant daily intake of lead from breast milk. Sci Total Environ. 2006;354:120–126. doi: 10.1016/j.scitotenv.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Kïrel B, Akþt MA, Bulut H. Blood lead levels of maternal-cord pairs, children and adults who live in a central urban area in Turkey. Turk J Pediat. 2005;47:125–131. [PubMed] [Google Scholar]
- 24.Ursinyova M, Masanova V. Cadmium, lead and mercury in human milk from Slovakia. Food Addit Contam. 2005;22(6):579–589. doi: 10.1080/02652030500135201. [DOI] [PubMed] [Google Scholar]
- 25.Anastacio AS, Silveira CL, Miekeley N, Donangelo CM. Distribution of lead in human milk fractions: relationship with essential minerals and maternal blood lead. Biol Trace Elem Res. 2004;102:27–37. doi: 10.1385/bter:102:1-3:027. [DOI] [PubMed] [Google Scholar]
- 26.Hanning RM, Sandhu R, MacMillan A, Moss L, Tsuji LJS, Nieboer E. Impact on blood Pb levels of maternal and early infant feeding practices of First Nation Cree in the Mushkegowuk Territory of Northern Ontario, Canada. J Environ Monit. 2003;5:241–245. doi: 10.1039/b208220a. [DOI] [PubMed] [Google Scholar]
- 27.Sowers M, Scholl TO, Hall G, Jannausch M, Kemp F, Li X, Bogden J. Lead in breast milk and maternal bone turnover. Am J Obstet Gynecol. 2002;187:770–776. doi: 10.1067/mob.2002.125736. [DOI] [PubMed] [Google Scholar]
- 28.Gundacker C, Pietschnig B, Wittmann KJ, Lischka A, Salzer H, Hohenauer L, Schuster E. Lead and mercury in breast milk. Pediatrics. 2002;110:873–878. doi: 10.1542/peds.110.5.873. [DOI] [PubMed] [Google Scholar]
- 29.Turan S, Saygi S, Kiliç Z, Acar O. Determination of heavy metal contents in human colostrum samples by electrothermal atomic absorption spectrophotometry. J Trop Pediatr. 2001;47:81–5. doi: 10.1093/tropej/47.2.81. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Sheng Y, Wang Q, Gu L, Wang Y. Transfer of lead via placenta and breast milk in human. Biomed Environ Sci. 2000;13:85–89. [PubMed] [Google Scholar]
- 31.Needham LA, Wang RY. Analytic considerations for measuring environmental chemicals in breast milk. Environ Health Perspect. 2002;110:317–324. doi: 10.1289/ehp.021100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyashiki GAK. Dissertation. Master Degree in Public Health at State University of Londrina; Londrina-Brazil: 2008. Lead levels in milk and blood from donors to the breast milk Bank in Southern Brazil. [Google Scholar]
- 33.Lakind JS, Berlin CM, Naiman DQ. Infant exposure to chemicals in breast milk in The United States: what we need to learn from a breast milk monitoring program. Environ Health Perspect. 2001;109:75–88. doi: 10.1289/ehp.0110975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr DB, Wang RY, Needham LL. Biologic Monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study. Environ Health Perspect. 2005;113:1083–1091. doi: 10.1289/ehp.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulson BK. Lead in breast milk. Fact sheet for medical professionals. http://www.lead.org.au/lanv6n2/update014.html; Updated 10 Feb 2010.